Factors Affecting Daily Functioning in Turkish Patients with Obstructive Sleep Apnea
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Group
2.3. Study Layout
2.4. Tools
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Sample
3.2. The Psychometrics Properties of the FOSQ-10
3.3. The Levels of Daily Functioning, Quality of Life, Depression, and Anxiety According to OSA Category
3.4. The Relationships between Daily Functioning and Participant Characteristics, PSG Measures, Quality of Life, Depression, and Anxiety
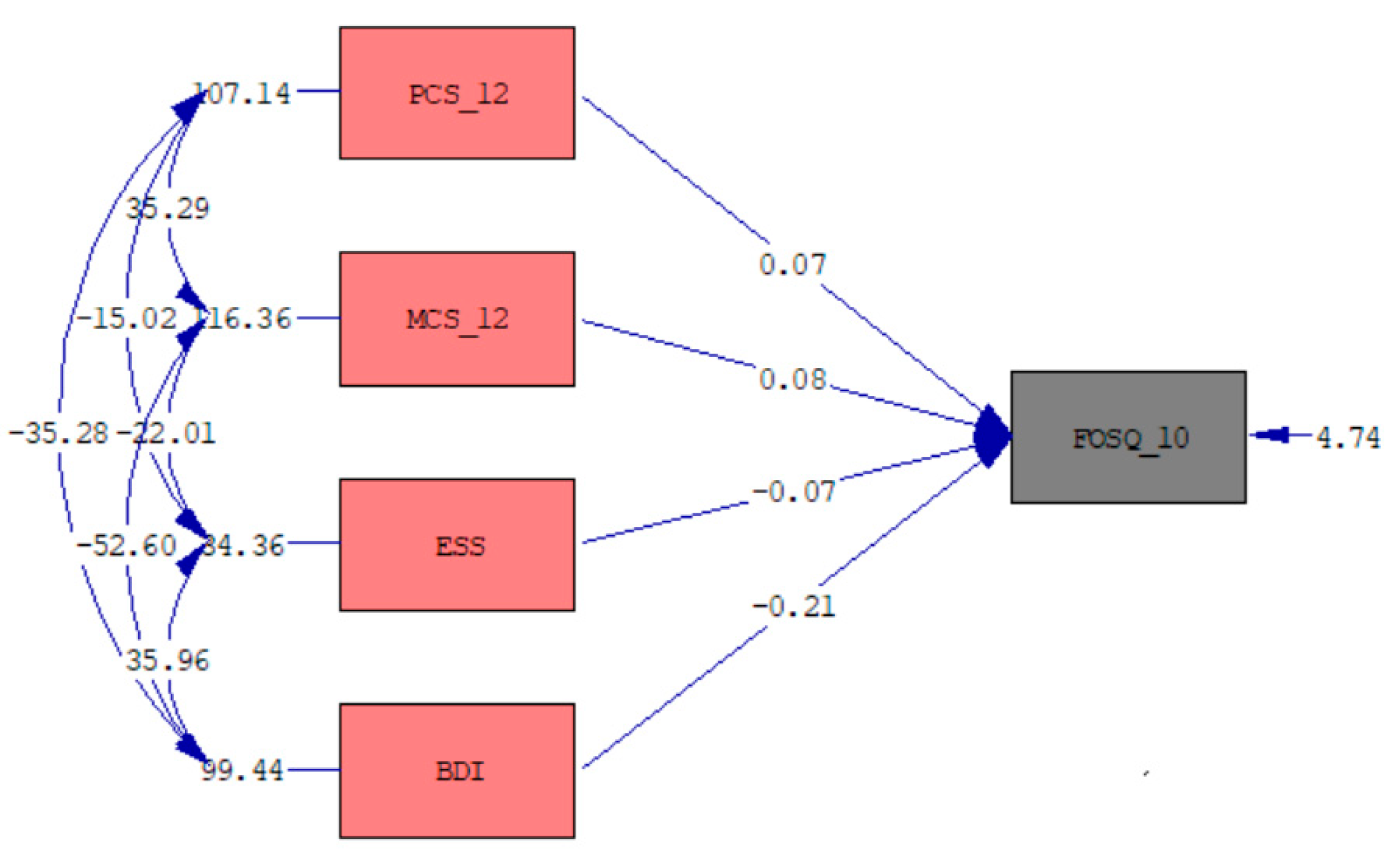
3.5. Multiple Regression Model of the FOSQ-10 Total Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999, 22, 667–689. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Ten Brink, M.; Dietch, J.R.; Tutek, J.; Suh, S.A.; Gross, J.J.; Manber, R. Sleep and affect: A conceptual review. Sleep Med. Rev. 2022, 65, 101670. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B.; Ham, S.A.; Gozal, D. The effect of sex and age on the comorbidity burden of OSA: An observational analysis from a large nationwide US health claims database. Eur. Respir. J. 2019, 54, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N.; Palagini, L. Association of anxiety and depression in obstructive sleep apnea patients: A systematic review and meta-analysis. Behav. Sleep. Med. 2020, 18, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Mehrtash, M.; Bakker, J.P.; Ayas, N. Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea. Lung 2019, 197, 115–121. [Google Scholar] [CrossRef]
- Weaver, T.E.; Laizner, A.M.; Evans, L.K.; Maislin, G.; Chugh, D.K.; Lyon, K.; Smith, P.L.; Schwartz, A.R.; Redline, S.; Pack, A.I.; et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 1997, 20, 835–843. [Google Scholar] [CrossRef]
- Chasens, E.R.; Ratcliffe, S.J.; Weaver, T.E. Development of the FOSQ-10: A short version of the functional outcomes of sleep questionnaire. Sleep 2009, 32, 915–919. [Google Scholar] [CrossRef]
- Silva, G.E.; Goodwin, J.L.; Vana, K.D.; Quan, S.F. Obstructive sleep apnea and quality of life: Comparison of the SAQLI, FOSQ, and SF-36 questionnaires. Southwest J. Pulm. Crit. Care. 2016, 13, 137–149. [Google Scholar] [CrossRef]
- Rey de Castro, J.; Rosales-Mayor, E.; Weaver, T.E. Reliability and validity of the functional outcomes of sleep questionnaire–Spanish short version (FOSQ-10SV) in Peruvian patients with obstructive sleep apnea. J. Clin. Sleep Med. 2018, 14, 615–621. [Google Scholar] [CrossRef]
- Björnsdóttir, E.; Janson, C.; Gíslason, T.; Sigurdsson, J.F.; Pack, A.I.; Gehrman, P.; Benediktsdóttir, B. Insomnia in untreated sleep apnea patients compared to controls. J. Sleep Res. 2012, 21, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Banhiran, W.; Assanasen, P.; Metheetrairut, C.; Nopmaneejumruslers, C.; Chotinaiwattarakul, W.; Kerdnoppakhun, J. Functional outcomes of sleep in Thai patients with obstructive sleep-disordered breathing. Sleep Breath. 2012, 16, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Han, N.E.; Kim, D.Y.; Lee, S.A. Validity of Korean version of functional outcomes of sleep questionnaire in patients with simple snoring and obstructive sleep apnea. Sleep Med. Res. 2014, 5, 5–14. [Google Scholar] [CrossRef]
- Rahavi-Ezabadi, S.; Amali, A.; Sadeghniiat-Haghighi, K.; Montazeri, A. Adaptation of the 10-item functional outcomes of sleep questionnaire to Iranian patients with obstructive sleep apnea. Qual. Life Res. 2016, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Dashzeveg, S.; Oka, Y.; Dashzeveg, S.; Oka, Y.; Purevtogtokh, M.; Tumurbaatar, E.; Lkhagvasuren, B.; Luvsannorov, O.; Boldbaatar, D. Obstructive sleep apnea in a clinical population: Prevalence, predictive factors, and clinical characteristics of patients referred to a sleep center in Mongolia. Int. J. Environ. Res. Public Health 2021, 18, 12032. [Google Scholar] [CrossRef]
- Che-Morales, J.L.; Carrillo-Alduenda, J.L. Functional outcomes in patients with obstructive sleep apnea residing in Southeastern Mexico. Sleep Med. Res. 2022, 13, 132–139. [Google Scholar] [CrossRef]
- Johns, M.W. Reliability and factor analysis of the Epworth sleepiness scale. Sleep 1992, 15, 376–381. [Google Scholar] [CrossRef]
- Agargun, M.Y.; Cilli, A.S.; Kara, H.; Bilici, M.; Telcioglu, M.; Semiz, Ü.B.; Basoglu, C. Validity and reliability of the Epworth Sleepiness Scale. Turk Psikiyatri Derg. 1999, 10, 261–267. Available online: https://psikiyatridizini.net/viewarticle.aspx?articleid=2530 (accessed on 15 March 2024).
- Ware, J.E.; Kosinski, M.; Keller, S.D. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales, 2nd ed.; The Health Institute, New England Medical Center: Boston, MA, USA, 1995; pp. 21–23. [Google Scholar]
- Soylu, C.; Kutuk, B. SF-12 Reliability and validity of the Turkish version of SF-12 health survey. Turk Psikiyatri Derg. 2022, 33, 108–117. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Garbin, M.G. Psychometric properties of the Beck Depression Inventory Twenty-five years of evaluation. Clin. Psychol. Rev. 1988, 8, 77–100. [Google Scholar] [CrossRef]
- Hisli, N. Beck depresyon envanterinin üniversite ögrencileri için geçerliligi ve guvenirliligi. Psikoloji Derg. 1989, 7, 3–13. Available online: https://toad.halileksi.net/wp-content/uploads/2022/07/beck-depresyon-envanteri-toad.pdf (accessed on 15 March 2024).
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, M.; Sahin, N.; Erkmen, H. Turkish version of the Beck anxiety inventory: Psychometric properties. J. Cogn. Psychother. 1998, 12, 163–172. Available online: https://www.proquest.com/openview/497f858f2abc75f77bc74df6f4e7f0c4/1?pq-origsite=gscholar&cbl=28723 (accessed on 15 March 2024).
- Lacasse, Y.; Godbout, C.; Series, F. Health-related quality of life in obstructive sleep apnoea. Eur. Respir. J. 2002, 19, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Field, A.P. Kendall’s coefficient of concordance. In Encyclopedia of Statistics in Behavioral Science; Everitt, B.S., Howell, D.C., Eds.; John Wiley & Sons: Chichester, UK, 2005; Volume 2, pp. 1010–1011, ISBN-13: 978-0-470-86080-9. [Google Scholar]
- Cangur, S.; Ercan, I. Comparison of model fit indices used in structural equation modeling under multivariate normality. JMASM 2015, 14, 152–167. [Google Scholar] [CrossRef]
- Hahs-Vaughn, D.L. Applied Multivariate Statistical Concepts; Routledge: New York, NY, USA, 2016; pp. 441–504, e-ISBN 9781315816685. [Google Scholar]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Almanasreh, E.; Moles, R.; Chen, T.F. Evaluation of methods used for estimating content validity. Res. Social Adm. Pharm. 2019, 15, 214–221. [Google Scholar] [CrossRef]
- Feißt, M.; Hennigs, A.; Heil, J.; Moosbrugger, H.; Kelava, A.; Stolpner, I.; Kieser, M.; Rauch, G. Refining scores based on patient reported outcomes–statistical and medical perspectives. BMC Med. Res. Methodol. 2019, 19, 167. [Google Scholar] [CrossRef]
- Izci, B.; Firat, H.; Ardic, S.; Kokturk, O.; Gelir, E.; Altinors, M. Adaptation of functional outcomes of sleep questionnaire (FOSQ) to Turkish population. Tuberk. Toraks. 2004, 52, 224–230. [Google Scholar]
- Vidal, S.; Ferrer, M.; Masuet, C.; Somoza, M.; Ballarín, J.I.M.; Monasterio, C. Spanish version of the Functional Outcomes of Sleep Questionnaire: Scores of healthy individuals and of patients with sleep apnea-hypopnea syndrome. Arch. Bronconeumol. 2007, 43, 256–261. [Google Scholar] [CrossRef]
- Tippin, J.; Aksan, N.; Dawson, J.; Anderson, S.W.; Rizzo, M. Sleep remains disturbed in patients with obstructive sleep apnea treated with positive airway pressure: A three-month cohort study using continuous actigraphy. Sleep Med. 2016, 24, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zheng, Y.J.; Cheng, C.M.; Strohl, K.P.; Mason, A.E.; Chang, J.L. Impact of Hypoglossal nerve stimulation on consumer sleep technology metrics and patient symptoms. Laryngoscope 2024, 134, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Pei, C.; Hao, L.; Xu, K.; Liu, F.; Ding, Z. The study of the relationship between moderate to severe sleep obstructive apnea and cognitive impairment, anxiety, and depression. Front. Neurol. 2024, 15, 1363005. [Google Scholar] [CrossRef]
- Korpe, L.; Lundgren, J.; Dahlström, L. Psychometric evaluation of a Swedish version of the Functional Outcomes of Sleep Questionnaire, FOSQ. Acta Odontol. Scand. 2013, 71, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Billings, M.E.; Rosen, C.L.; Auckley, D.; Benca, R.; Foldvary-Schaefer, N.; Iber, C.; Zee, P.C.; Redline, S.; Kapur, V.K. Psychometric performance and responsiveness of the functional outcomes of sleep questionnaire and sleep apnea quality of life instrument in a randomized trial: The HomePAP study. Sleep 2014, 37, 2017–2024. [Google Scholar] [CrossRef]
- Stavem, K.; Kjelsberg, F.N.; Ruud, E.A. Reliability and validity of the Norwegian version of the Functional Outcomes of Sleep Questionnaire. Qual. Life Res. 2004, 13, 541–549. [Google Scholar] [CrossRef]
| Mild OSA (n = 83) | Moderate OSA (n = 82) | Severe OSA (n = 163) | Negative OSA (n = 33) | p | ||
|---|---|---|---|---|---|---|
| Age (years) & | 46.42 ± 11.85 | 47.76 ± 10.02 | 49.42 ± 10.86 | 44.85 ± 11.91 | 0.069 | |
| Gender * | Male | 59 (71.1%) | 55 (67.1%) | 115 (70.6%) | 19 (57.6%) | 0.479 |
| Female | 24 (28.9%) | 27 (32.9%) | 48 (29.4%) | 14 (42.4%) | ||
| BMI (kg/m2) & | 30.37 ± 6.29 | 32.02 ± 6.32 | 34.85 ± 6.33 | 30.27 ± 7.05 | <0.001 | |
| AHI (events/h) # | 9.80 [4.90] | 21.20 [6.75] | 49 [33.5] | 3 [2.55] | <0.001 | |
| T90 % # | 2.5 [12.2] | 15.1 [44.6] | 70.5 [136.2] | 0 [0.7] | <0.001 | |
| TST (min) & | 377 ± 45.4 | 378 ± 46.7 | 377.6 ± 54.2 | 377.9 ± 50.5 | 0.999 | |
| SE (%) & | 83.1 ± 10.4 | 84.4 ± 10 | 83.2 ± 11.7 | 82 ± 11.2 | 0.733 | |
| REM (%TST) & | 13.6 ± 6.8 | 13.3 ± 6.6 | 11.1 ± 7.1 | 14.6 ± 3.9 | 0.003 | |
| N1(%TST) & | 9.9 ± 7.3 | 11.7 ± 8.9 | 13.2 ± 9.2 | 12.5 ± 10 | 0.048 | |
| N2 (%TST) & | 49.9 ± 10.9 | 49.4 ± 9.7 | 56.3 ± 14.1 | 46.8 ± 10.5 | <0.001 | |
| N3 (%TST) & | 26.6 ± 12.1 | 25.6 ± 11.9 | 19.4 ± 12.4 | 25.4 ± 9.8 | <0.001 | |
| ESS & | 9.13 ± 6.12 | 10.72 ± 5.41 | 10.01 ± 5.97 | 7.61 ± 5.03 | 0.027 | |
| Chronic diseases | ||||||
| Hypertension (+) * | 41 (49.4%) | 41 (50%) | 88 (54.3%) | 10 (30.3%) | 0.096 | |
| DM (+) * | 19 (22.9%) | 18 (22%) | 45 (27.6%) | 4 (12.1%) | 0.264 | |
| Lung disease (+) * | 9 (10.8%) | 12 (14.6%) | 27 (16.6%) | 3 (9.1%) | 0.523 | |
| Heart disease (+) * | 12 (14.5%) | 16 (19.5%) | 32 (19.6%) | 3 (9.1%) | 0.408 | |
| Neurological disease (+) * | 3 (3.6%) | 5 (6.1%) | 12 (7.4%) | 2 (6.1%) | 0.715 | |
| Chronic diseases (+) * | 46 (55.4%) | 54 (65.9%) | 109 (66.9%) | 15 (45.5%) | 0.057 | |
| Agreement, Validity, and Reliability | Results and Criteria | Decision |
|---|---|---|
| Translation λ | Kendall’s W concordance = 100%, ICC = 0.98 | Good agreement |
| Face validity * | 1.7 ≤ the impact score of each item ≤ 4.1 ⇒ All impact score ≥ 1.5 | Valid |
| Content validity κ | 0.88 ≤ Item-CVI ≤ 1.00 ⇒ Item-CVI ≥ 0.78 Overall average CVI = 0.98 ≥ 0.90 Universal CVI = 0.88 | Valid |
| Concurrent validity | r = −0.56 p < 0.001 for the ESS and FOSQ-10 Total r = 0.47 p < 0.001 for the PCS-12 and FOSQ-10 Total r = 0.58 p < 0.001 for the MCS-12 and FOSQ-10 Total | Valid |
| Construct validity #£ | Minres EFA Kaiser–Meyer–Olkin test = 0.81 Bartlett’s sphericity test χ2 = 1347.3 p < 0.001 The total explained variance rate of the two-factor model = 58.1%. 0.50 ≤ Factor loadings ≤ 0.93 | Valid |
| CFA for two-factor model χ2 = 46.87, p = 0.026, df = 30, χ2/df = 1.56 < 2, RMSEA = 0.041 < 0.05, SRMR = 0.038 < 0.05, CFI = 0.99 > 0.97, NNFI = 0.99 > 0.97, GFI = 0.97 > 0.95, AGFI = 0.95 > 0.90 0.49 ≤ Standardized Factor loadings ≤ 0.75 | Valid Good fit | |
| Reliability ϒ¥ | Cronbach’s alpha = 0.82 > 0.80, McDonald’s omega = 0.89 > 0.80, ICC = 0.90 > 0.75 | Good reliability |
| Mild OSA (n = 83) Mean ± SD | Moderate OSA (n = 82) Mean ± SD | Severe OSA (n = 163) Mean ± SD | Negative OSA (n = 33) Mean ± SD | p | |
|---|---|---|---|---|---|
| FOSQ-10 | |||||
| FOSQ-10 Total | 13.9 ± 3.95 | 13 ± 3.61 | 13.68 ± 4.07 | 15.27 ± 2.75 | 0.040 |
| General Productivity | 2.71 ± 0.96 | 2.65 ± 0.91 | 2.68 ± 0.93 | 2.91 ± 0.8 | 0.561 |
| Activity Level | 2.72 ± 0.88 | 2.41 ± 0.81 | 2.62 ± 0.86 | 2.98 ± 0.62 | 0.007 |
| Vigilance | 2.7 ± 0.96 | 2.61 ± 0.87 | 2.65 ± 1 | 3.08 ± 0.61 | 0.091 |
| Social Outcomes | 2.89 ± 1 | 2.72 ± 1.08 | 2.98 ± 1.07 | 3.36 ± 0.6 | 0.022 |
| Sexual Relationship | 2.8 ± 1.05 | 2.63 ± 1.07 | 2.63 ± 1.09 | 2.96 ± 1.16 | 0.401 |
| Quality of Life (SF-12) | |||||
| PCS-12 | 41.08 ± 9.85 | 39.93 ± 10.33 | 39.49 ± 10.63 | 41.56 ± 9.69 | 0.576 |
| MCS-12 | 41.16 ± 10.1 | 42.01 ± 10.68 | 42.66 ± 11.2 | 42.62 ± 10.5 | 0.768 |
| Depression and Anxious | |||||
| BDI | 18.30 ± 6.92 | 20.12 ± 8.92 | 22.64 ± 11.40 | 17.70 ± 5.75 | 0.002 |
| BAI | 22.59 ± 6.45 | 24.23 ± 8.84 | 26.79 ± 12.02 | 20.24 ± 4.62 | <0.001 |
| FOSQ-10 Total | General Productivity | Activity Level | Vigilance | Social Outcomes | Sexual Relationship | ||
|---|---|---|---|---|---|---|---|
| ESS | r | −0.56 | −0.45 | −0.50 | −0.55 | −0.42 | −0.42 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| AHI | r | 0.01 | 0.03 | −0.01 | −0.02 | 0.08 | −0.03 |
| p | 0.806 | 0.641 | 0.819 | 0.788 | 0.189 | 0.666 | |
| T90 | r | 0.04 | 0.06 | 0.04 | 0.06 | 0.07 | −0.04 |
| p | 0.448 | 0.302 | 0.523 | 0.316 | 0.245 | 0.504 | |
| TST (min) | r | −0.08 | −0.07 | −0.07 | −0.05 | −0.08 | −0.10 |
| p | 0.133 | 0.214 | 0.190 | 0.425 | 0.164 | 0.105 | |
| SE (%) | r | −0.09 | −0.11 | −0.09 | −0.04 | −0.06 | −0.09 |
| p | 0.107 | 0.056 | 0.117 | 0.533 | 0.298 | 0.163 | |
| PCS-12 | r | 0.47 | 0.40 | 0.45 | 0.38 | 0.33 | 0.47 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| MCS-12 | r | 0.58 | 0.56 | 0.53 | 0.37 | 0.45 | 0.49 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| BDI | r | −0.77 | −0.65 | −0.69 | −0.63 | −0.61 | −0.59 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| BAI | r | −0.70 | −0.59 | −0.62 | −0.58 | −0.53 | −0.52 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| B | Std. Error | p | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Constant | 12.36 | 0.89 | <0.001 | 10.61 | 14.11 |
| BDI | −0.21 | 0.02 | <0.001 | −0.24 | −0.19 |
| PCS-12 | 0.07 | 0.01 | <0.001 | 0.05 | 0.10 |
| MCS-12 | 0.08 | 0.01 | <0.001 | 0.05 | 0.11 |
| ESS | −0.07 | 0.03 | 0.008 | −0.12 | −0.02 |
| Model Significance | F = 182.344, p < 0.001, R2 = 0.693 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cangur, S.; Balbay, E.G.; Weaver, T.E. Factors Affecting Daily Functioning in Turkish Patients with Obstructive Sleep Apnea. Medicina 2024, 60, 1652. https://doi.org/10.3390/medicina60101652
Cangur S, Balbay EG, Weaver TE. Factors Affecting Daily Functioning in Turkish Patients with Obstructive Sleep Apnea. Medicina. 2024; 60(10):1652. https://doi.org/10.3390/medicina60101652
Chicago/Turabian StyleCangur, Sengul, Ege Gulec Balbay, and Terri E. Weaver. 2024. "Factors Affecting Daily Functioning in Turkish Patients with Obstructive Sleep Apnea" Medicina 60, no. 10: 1652. https://doi.org/10.3390/medicina60101652
APA StyleCangur, S., Balbay, E. G., & Weaver, T. E. (2024). Factors Affecting Daily Functioning in Turkish Patients with Obstructive Sleep Apnea. Medicina, 60(10), 1652. https://doi.org/10.3390/medicina60101652





