Predictive Capabilities of Human Leukocyte Antigen-G and Galectin-13 Levels in the Amniotic Fluid and Maternal Blood for the Pregnancy Outcome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Conventional 2-Dimensional (2-D) Sonographic Examinations
2.3. Volume Acquisition
2.4. Determination of Power Doppler Indices
2.5. Amniocentesis Procedure
2.6. Samples
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Data and Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuffe, J.S.; Holland, O.; Salomon, C.; Rice, G.E.; Perkins, A.V. Review: Placental derived biomarkers of pregnancy disorders. Placenta 2017, 54, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zheng, J. Regulation of Placental Angiogenesis. Microcirculation 2014, 21, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Le Bouteiller, P. Human villous trophoblast and the lack of intron 4-retaining soluble HLA-G secretion: Beware of possible methodological biases. Mol. Hum. Reprod. 2005, 11, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.J.; Pace, J.L.; Platt, J.S.; Langat, D.K.; Hunt, J.S. Synthesis of β2-microglobulin-free, disulphide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunology 2007, 122, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, Y.; Wei, H. Roles of HLA-G in the Maternal-Fetal Immune Microenvironment. Front. Immunol. 2020, 11, 592010. [Google Scholar] [CrossRef] [PubMed]
- Yie, S.-M.; Li, L.-H.; Li, Y.-M.; Librach, C. HLA-G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. Am. J. Obstet. Gynecol. 2004, 191, 525–529. [Google Scholar] [CrossRef]
- Tantengco, O.A.G.; Richardson, L.; Lee, A.; Kammala, A.; Silva, M.D.C.; Shahin, H.; Sheller-Miller, S.; Menon, R. Histocompatibility antigen, class I, G (HLA-G)’s role during pregnancy and parturition: A systematic review of the literature. Life 2021, 11, 1061. [Google Scholar] [CrossRef]
- Emmer, P.M.; Joosten, I.; Schut, M.H.; Zusterzeel, P.L.M.; Hendriks, J.C.M.; Steegers, E.A.P. Shift in expression of HLA-G mRNA spliceforms in pregnancies complicated by preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 220–226. [Google Scholar] [CrossRef]
- Rizzo, R.; Andersen, A.S.; Lassen, M.R.; Sørensen, H.C.; Bergholt, T.; Larsen, M.H.; Melchiorri, L.; Stignani, M.; Baricordi, O.R.; Hviid, T.V.F. ORIGINAL ARTICLE: Soluble Human Leukocyte Antigen-G Isoforms in Maternal Plasma in Early and Late Pregnancy. Am. J. Reprod. Immunol. 2009, 62, 320–338. [Google Scholar] [CrossRef]
- Barbaro, G.; Inversetti, A.; Cristodoro, M.; Ticconi, C.; Scambia, G.; Di Simone, N. HLA-G and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2023, 24, 2557. [Google Scholar] [CrossRef]
- Than, N.; Sumegi, B.; Than, G.; Berente, Z.; Bohn, H. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new Lysophospholipase, homologue of human eosinophil Charcot-Leyden crystal protein. Placenta 1999, 20, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Pick, E.; Bellyei, S.; Szigeti, A.; Burger, O.; Berente, Z.; Janaky, T.; Boronkai, A.; Kliman, H.; Meiri, H.; et al. Functional analyses of placental protein 13/galectin-13. Eur. J. Biochem. 2004, 271, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Gadde, R.; Cd, D.; Sheela, S. Placental protein 13: An important biological protein in preeclampsia. J. Circ. Biomarkers 2018, 7, 1849454418786159. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H.; Bindra, R.; Turan, O.M.; Chefetz, I.; Sammar, M.; Meiri, H.; Tal, J.; Cuckle, H.S. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet. Gynecol. 2006, 27, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Cowans, N.J.; Spencer, K.; Goichman, S.; Meiri, H.; Harrington, K. First trimester maternal serum placental protein 13 for the prediction of pre-eclampsia in women with a priori high risk. Prenat. Diagn. 2009, 29, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Unverdorben, L.; Hüttenbrenner, R.; Knabl, J.; Jeschke, U.; Hutter, S. Galectin-13/PP-13 expression in term placentas of gestational diabetes mellitus pregnancies. Placenta 2015, 36, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kuc, S.; Wortelboer, E.J.; van Rijn, B.B.; Franx, A.; Visser, G.H.A.; Schielen, P.C.J.I. Evaluation of 7 serum biomarkers and uterine artery doppler ultrasound for first-trimester prediction of preeclampsia: A systematic review. Obstet. Gynecol. Surv. 2011, 66, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.S.; Jadhav, L.; Chu, W.; Geraghty, D.E.; Ober, C. Soluble HLA-G circulates in maternal blood during pregnancy. Am. J. Obstet. Gynecol. 2000, 183, 682–688. [Google Scholar] [CrossRef]
- Rebmann, V.; Pfeiffer, K.; Päßler, M.; Ferrone, S.; Maier, S.; Weiss, E.; Grosse-Wilde, H. Detection of soluble HLA-G molecules in plasma and amniotic fluid. Tissue Antigens 1999, 53, 14–22. [Google Scholar] [CrossRef]
- Chafetz, I.; Kuhnreich, I.; Sammar, M.; Tal, Y.; Gibor, Y.; Meiri, H.; Cuckle, H.; Wolf, M. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 2007, 197, 35.e1–35.e7. [Google Scholar] [CrossRef]
- Alegre, E.; Díaz-Lagares, A.; LeMaoult, J.; López-Moratalla, N.; Carosella, E.D.; González, A. Maternal antigen presenting cells are a source of plasmatic HLA-G during pregnancy: Longitudinal study during pregnancy. Hum. Immunol. 2007, 68, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Shaikly, V.R.; Morrison, I.E.G.; Taranissi, M.; Noble, C.V.; Withey, A.D.; Cherry, R.J.; Blois, S.M.; Fernández, N. Analysis of HLA-G in Maternal Plasma, Follicular Fluid, and Preimplantation Embryos Reveal an Asymmetric Pattern of Expression. J. Immunol. 2008, 180, 4330–4337. [Google Scholar] [CrossRef] [PubMed]
- Hackmon, R.; Hallak, M.; Krup, M.; Weitzman, D.; Sheiner, E.; Kaplan, B.; Weinstein, Y. HLA-G antigen and parturition: Maternal serum, fetal serum and amniotic fluid levels during pregnancy. Fetal Diagn. Ther. 2004, 19, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Hackmon, R.; Koifman, A.; Hyobo, H.; Glickman, H.; Sheiner, E.; Geraghty, D.E. Reduced third-trimester levels of soluble human leukocyte antigen G protein in severe preeclampsia. Am. J. Obstet. Gynecol. 2007, 197, 255.e1–255.e5. [Google Scholar] [CrossRef] [PubMed]
- Sammar, M.; Drobnjak, T.; Mandala, M.; Gizurarson, S.; Huppertz, B.; Meiri, H. Galectin 13 (PP13) Facilitates remodeling and structural stabilization of maternal vessels during pregnancy. Int. J. Mol. Sci. 2019, 20, 3192. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Joubert, K. Magyar születéskori testtömeg- és testhossz-standardok az 1990–96. évi országos élveszületési adatok alapján. Magy. Noorv. Lapja. 2000, 63, 155–163. [Google Scholar]
- Surányi, A.; Kozinszky, Z.; Molnár, A.; Nyári, T.; Bitó, T.; Pál, A. Placental three-dimensional power Doppler indices in mid-pregnancy and late pregnancy complicated by gestational diabetes mellitus. Prenat. Diagn. 2013, 33, 952–958. [Google Scholar] [CrossRef]
- Molnár, A.; Surányi, A.; Nyári, T.; Németh, G.; Pál, A. Examination of placental three-dimensional power Doppler indices and perinatal outcome in pregnancies complicated by intrauterine growth restriction. Int. J. Gynecol. Obstet. 2015, 129, 5–8. [Google Scholar] [CrossRef]
- Lai, P.K.; Wang, Y.A.; Welsh, A.W. Reproducibility of regional placental vascularity/perfusion measurement using 3D power Doppler. Ultrasound Obstet. Gynecol. 2010, 36, 202–209. [Google Scholar] [CrossRef]
- Sikovanyecz, J.; Vincze, M.; Földesi, I.; Németh, G.; Kozinszky, Z. Angiogenic factors measured in aspirated placental tissue between the 10 + 6 and 18 + 3 weeks of gestation. Reprod. Biol. 2021, 21, 100572. [Google Scholar] [CrossRef] [PubMed]
- Beneventi, F.; Locatelli, E.; De Amici, M.; Martinetti, M.; Spinillo, A. Soluble HLA-G concentrations in obese women during pregnancy and in cord blood. J. Reprod. Immunol. 2017, 119, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, P.; Saccone, G.; Morelli, M.; Interlandi, F.; Votino, C.; Zuccalà, V.; Di Carlo, C.; Zullo, F.; Venturella, R. Stillbirth, potentially preventable cases: An Italian retrospective study. Ital. J. Gynaecol. Obstet. 2022, 34, 89. [Google Scholar] [CrossRef]
- Krop, J.; Van Der Keur, C.; Kapsenberg, J.; Hollander, F.D.; Van Der Hoorn, M.; Heidt, S.; Claas, F.; Eikmans, M. Soluble HLA-G blood levels are not increased during ongoing pregnancy in women with a history of recurrent pregnancy loss. J. Reprod. Immunol. 2022, 153, 103665. [Google Scholar] [CrossRef]
- Steinborn, A.; Rebmann, V.; Scharf, A.; Sohn, C.; Grosse-Wilde, H. Placental abruption is associated with decreased maternal plasma levels of soluble HLA-G. J. Clin. Immunol. 2003, 23, 307–314. [Google Scholar] [CrossRef]
- Kusanovic, J.P.; Romero, R.; Jodicke, C.; Mazaki-Tovi, S.; Vaisbuch, E.; Erez, O.; Mittal, P.; Gotsch, F.; Chaiworapongsa, T.; Edwin, S.S.; et al. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. J. Matern. Neonatal Med. 2009, 22, 1151–1166. [Google Scholar] [CrossRef]
- Mercé, L.; Barco, M.; Bau, S. Reproducibility of the study of placental vascularization by three-dimensional power Doppler. Int. Sch. Res. Not. 2004, 32, 228–233. [Google Scholar] [CrossRef]
- Merce, L.T.; Barco, M.J.; Bau, S.; Kupesic, S.; Kurjak, A. Assessment of placental vascularization by three-dimensional power Doppler “vascular biopsy” in normal pregnancies. Croat. Med. J. 2005, 46, 765–771. [Google Scholar]
- Tuuli, M.; Houser, M.; Odibo, L.; Huster, K.; Macones, G.; Odibo, A. Validation of placental vascular sonobiopsy for obtaining representative placental vascular indices by three-dimensional power doppler ultrasonography. Placenta 2010, 31, 192–196. [Google Scholar] [CrossRef]
- Rizzo, G.; Capponi, A.; Pietrolucci, M.E.; Aiello, E.; Arduini, D. First trimester placental volume and three dimensional power doppler ultrasonography in type I diabetic pregnancies. Prenat. Diagn. 2012, 32, 480–484. [Google Scholar] [CrossRef]
- de Paula, C.; Ruano, R.; Campos, J.; Zugaib, M. Quantitative analysis of placental vasculature by three-dimensional power doppler ultrasonography in normal pregnancies from 12 to 40 weeks of gestation. Placenta 2009, 30, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Rahman, O.A.; Magenheim, R.; Nagy, B.; Fule, T.; Hargitai, B.; Sammar, M.; Hupuczi, P.; Tarca, A.L.; Szabo, G.; et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008, 453, 387–400. [Google Scholar] [CrossRef]
- Huppertz, B.; Sammar, M.; Chefetz, I.; Neumaier-Wagner, P.; Bartz, C.; Meiri, H. longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn. Ther. 2008, 24, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Sammar, M.; Nisemblat, S.; Fleischfarb, Z.; Golan, A.; Sadan, O.; Meiri, H.; Huppertz, B.; Gonen, R. Placenta-bound and body fluid PP13 and its mRNA in normal pregnancy compared to preeclampsia, HELLP and preterm delivery. Placenta 2011, 32 (Suppl. 1), S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, N.M.; Naghshvar, F.; Peyvandi, S.; Gheshlaghi, P.; Ehetshami, S. PP13 and PAPP-A in the First and Second Trimesters: Predictive Factors for Preeclampsia? ISRN Obstet. Gynecol. 2012, 2012, 263871. [Google Scholar] [CrossRef][Green Version]
- Burger, O.; Pick, E.; Zwickel, J.; Klayman, M.; Meiri, H.; Slotky, R.; Mandel, S.; Rabinovitch, L.; Paltieli, Y.; Admon, A.; et al. Placental protein 13 (PP-13): Effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta 2004, 25, 608–622. [Google Scholar] [CrossRef]
- Wu, P.; Berg, C.V.D.; Alfirevic, Z.; O’brien, S.; Röthlisberger, M.; Baker, P.N.; Kenny, L.C.; Kublickiene, K.; Duvekot, J.J. Early pregnancy biomarkers in pre-eclampsia: A systematic review and meta-analysis. Int. J. Mol. Sci. 2015, 16, 23035–23056. [Google Scholar] [CrossRef]
- Spencer, K.; Cowans, N.J.; Chefetz, I.; Tal, J.; Kuhnreich, I.; Meiri, H. Second-trimester uterine artery Doppler pulsatility index and maternal serum PP13 as markers of pre-eclampsia. Prenat. Diagn. 2007, 27, 258–263. [Google Scholar] [CrossRef]
- Sahraravand, M.; Järvelä, I.; Laitinen, P.; Tekay, A.; Ryynänen, M. The secretion of PAPP-A, ADAM12, and PP13 correlates with the size of the placenta for the first month of pregnancy. Placenta 2011, 32, 999–1003. [Google Scholar] [CrossRef]
| Maternal age (years) * | 34.52 ± 5.78 |
| Number of nulliparous women in the study ** | 23 (32.39) |
| BMI at the time of genetic consultation (kg/m2) * | 26.98 ± 5.90 |
| Gestational age at the time of amniocentesis (weeks) * | 18.25 ± 1.42 |
| Fetal weight at delivery (grams) * | 3424.08 ± 538.63 |
| Gestational age at delivery (weeks) * | 39.09 ± 1.38 |
| Fetal biometry | |
| Head circumference (mm) | 151.54 ± 16.18 |
| Head circumference (percentile) | 53.50 ± 30.79 |
| Abdominal circumference (mm) | 129.18 ± 17.07 |
| Abdominal circumference (percentile) | 51.63 ± 27.88 |
| Femur length (mm) | 27.04 ± 4.73 |
| Femur length (percentile) | 55.91 ± 30.52 |
| Estimated fetal weight (grams) | 243.20 ± 74.12 |
| Estimated fetal weight (percentile) | 52.92 ± 26.81 |
| Placental sonography | |
| Placental volume (mm3) | 227.36 ± 93.21 |
| VI | 14.11 ± 5.14 |
| FI | 44.97 ± 22.64 |
| VFI | 8.21 ± 3.63 |
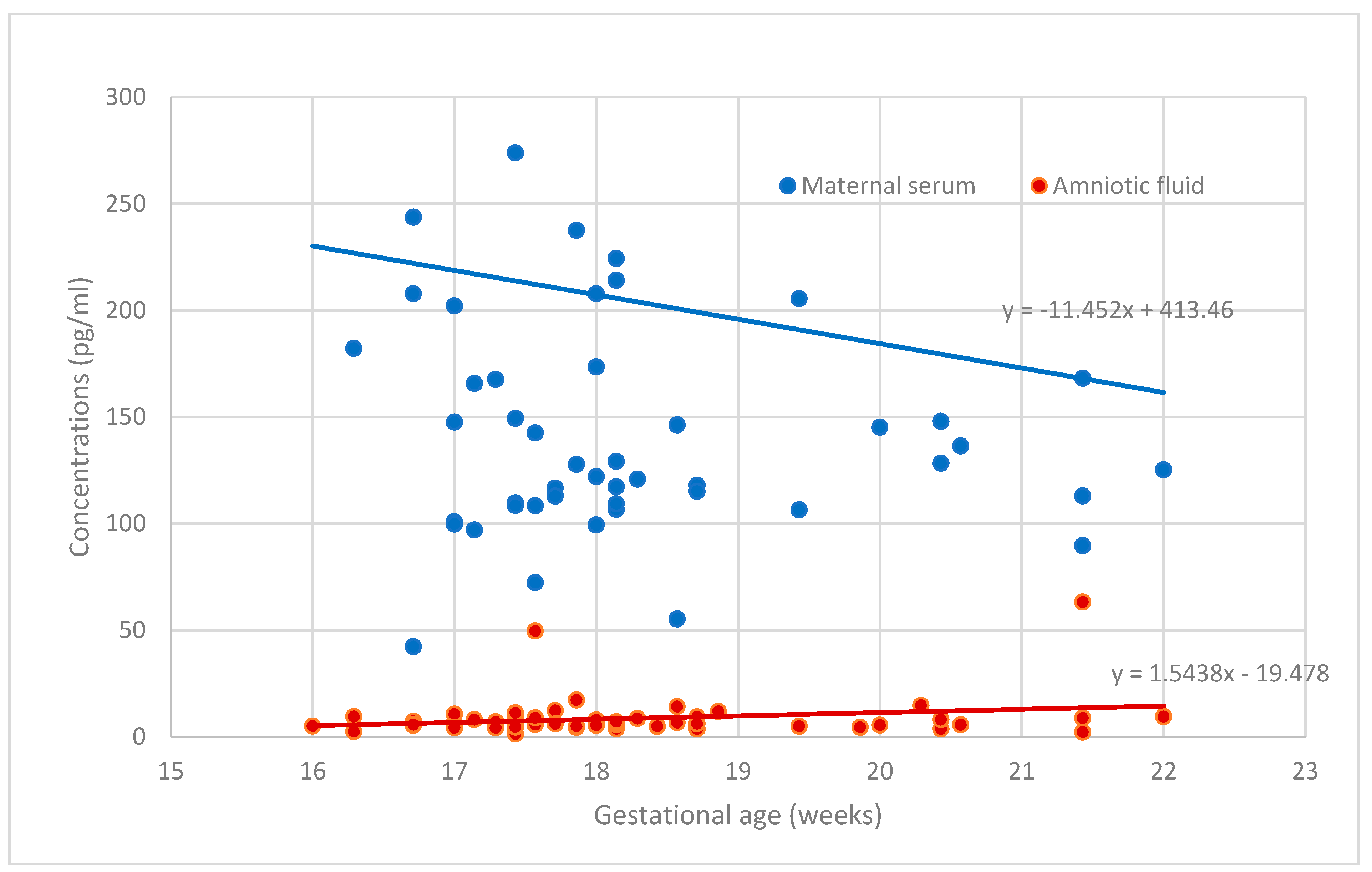
| PP-13 concentration in amniotic fluid (pg/mL) | 8.68 ± 9.85 |
| PP-13 concentration in serum (pg/mL) | 204.23 ± 171.34 |
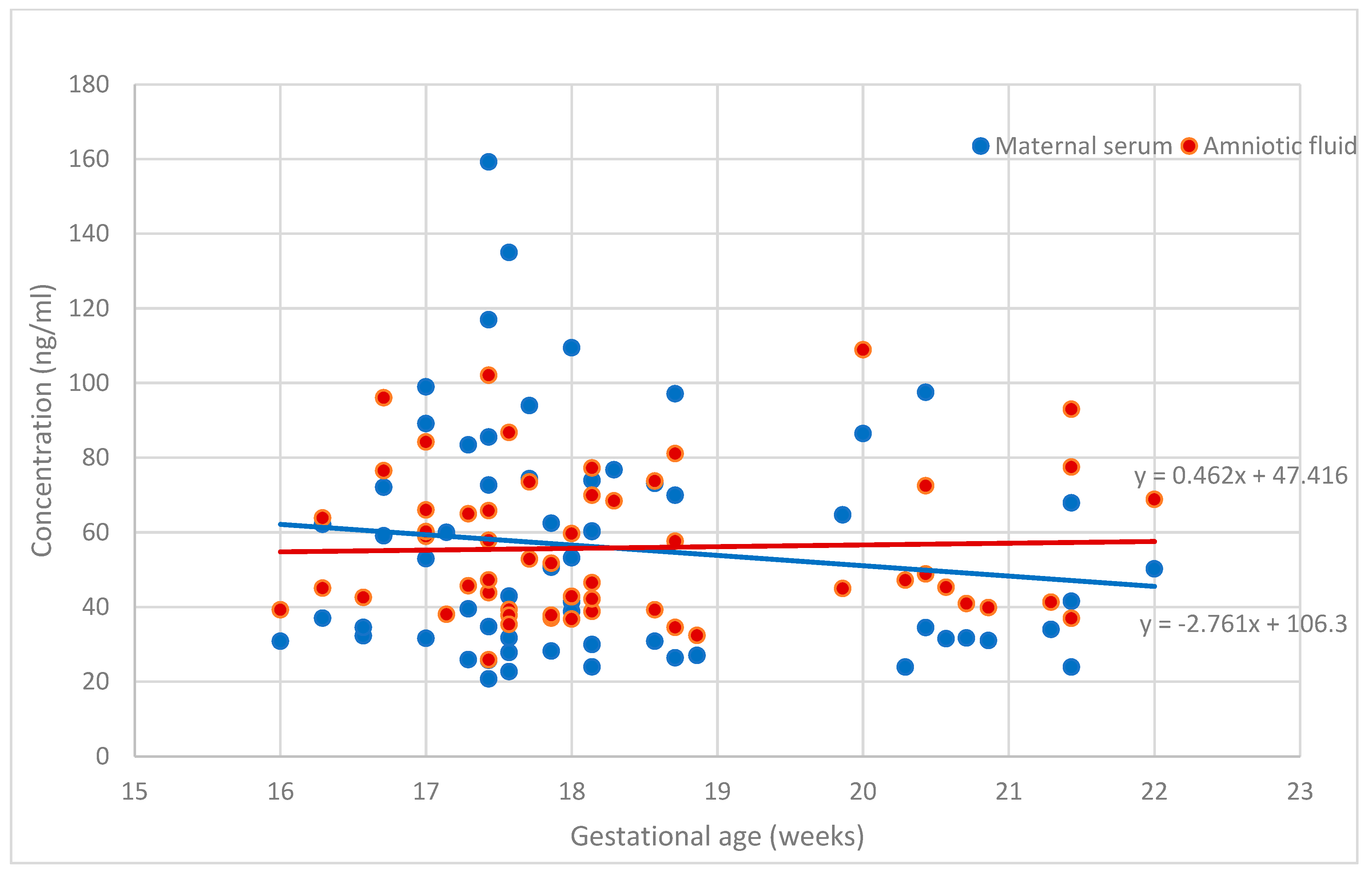
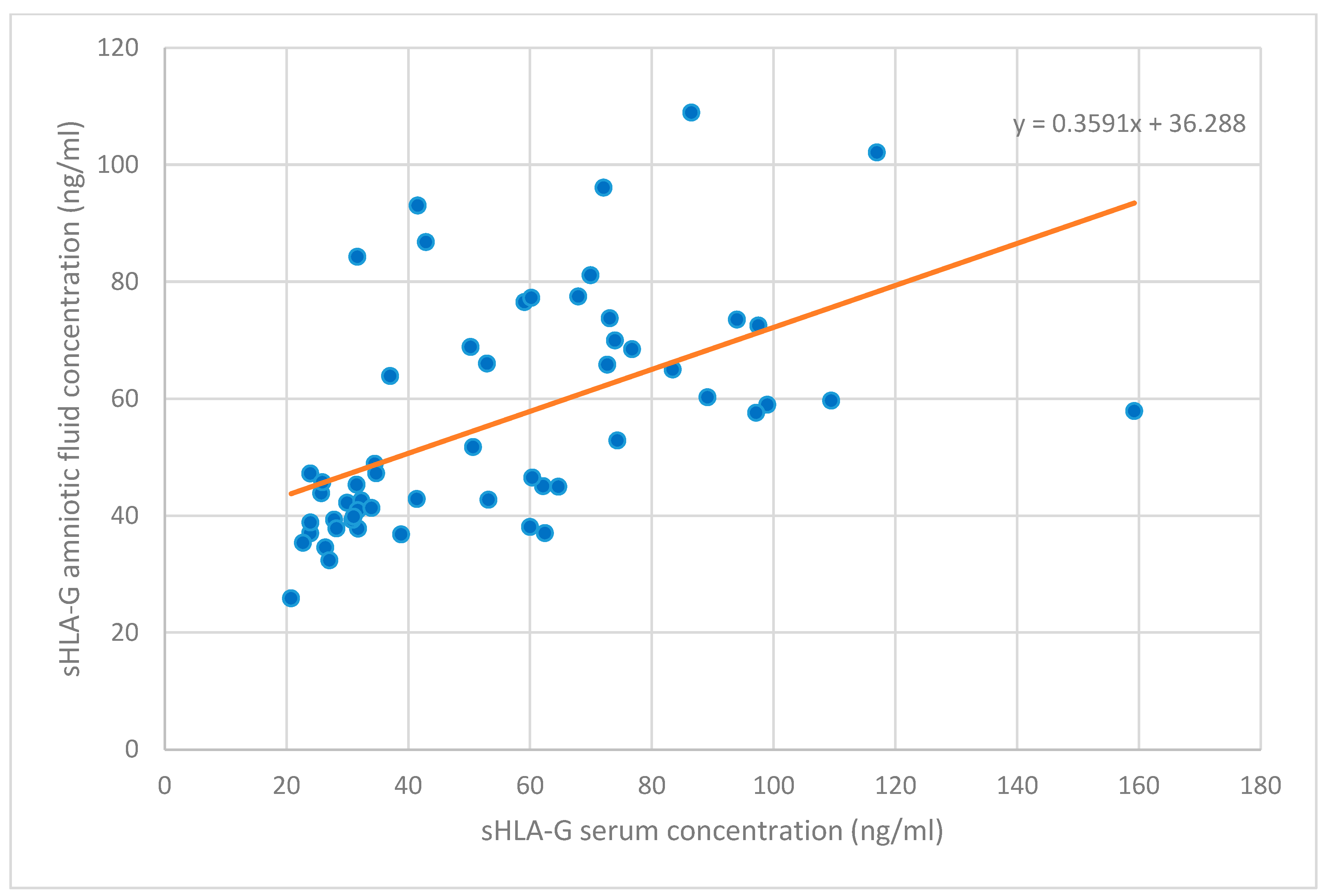
| sHLA-G concentration in amniotic fluid (ng/mL) | 55.89 ± 19.51 |
| sHLA-G concentration in serum (ng/mL) | 55.84 ± 30.50 |
| sHLA-G Serum Level | sHLA-G Amniotic Fluid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson r | Univariate Linear Regression | Multivariate Linear Regression | Pearson r | Univariate Linear Regression | Multivariate Linear Regression | |||||
| B | β2 | B | r2 | B | β2 | B | r2 | |||
| PP13 in serum | −0.186 ** | −0.07 ** | 0.14 ** | −0.07 ** | 0.16 ** | 0.052 | −0.03 | 0.08 | −0.30 | 0.07 |
| PP13 in amniotic fluid | −0.095 | −0.55 | 0.03 | −0.55 | 0.03 | 0.084 | 0.16 | 0.01 | 0.12 | 0.00 |
| sHLA-G in amniotic fluid | 0.662 ** | 0.36 ** | 0.29 ** | 0.37 ** | 0.30 ** | - | - | - | - | - |
| PP13 serum level | ||||||||||
| Pearson r | Univariate linear regression | Multivariate linear regression | ||||||||
| B | β2 | B | r2 | |||||||
| PP13 in amniotic fluid | 0.111 | 0.84 | 0.00 | 2.50 | 0.01 | |||||
| sHLA-G Serum Level | sHLA-G Amniotic Fluid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson r | Univariate Linear Regression | Multivariate Linear Regression | Pearson r | Univariate Linear Regression | Multivariate Linear Regression | |||||
| B | β2 | B | r2 | B | β2 | B | r2 | |||
| Clinical and obstetrical characteristics | ||||||||||
| Maternal age | 0.090 | 0.49 | 0.01 | 0.44 | 0.00 | −0.106 | −0.43 | 0.02 | −0.48 | 0.01 |
| BMI at the time of genetica consultation (kg/m2) | −0.012 | 0.01 | 0.00 | −0.11 | 0.00 | 0.010 | −0.17 | 0.00 | −0.16 | 0.00 |
| Fetal weight at delivery | 0.094 | 0.01 | 0.03 | 0.01 | 0.03 | 0.083 | 0.01 | 0.01 | 0.01 | 0.02 |
| GA at delivery | −0.163 | −2.41 | 0.01 | −2.15 | 0.01 | 0.056 | 0.90 | 0.00 | 0.73 | 0.00 |
| GA at the time of amniocentesis (weeks) | −0.102 | −2.76 | 0.02 | −2.32 | 0.01 | −0.041 | 0.46 | 0.00 | 0.26 | 0.00 |
| Fetal sonography at the time of amniocentesis | ||||||||||
| Head circumference (mm) | −0.047 | −0.17 | 0.01 | 0.16 | 0.00 | 0.045 | 0.08 | 0.00 | 0.20 | 0.01 |
| Abdominal circumference (mm) | 0.112 | 0.11 | 0.00 | 1.20 | 0.08 | 0.204 | 0.36 | 0.10 | 0.99 | 0.11 |
| Femur length (mm) | −0.120 | −0.69 | 0.01 | −0.30 | 0.00 | 0.119 | 0.85 | 0.05 | −0.31 | 0.00 |
| Estimated fetal weight (grams) | 0.042 | −0.00 | 0.00 | 0.17 | 0.02 | 0.175 | 0.06 | 0.06 | 0.18 | 0.05 |
| Placental sonography at the time of amniocentesis | ||||||||||
| Placental volume (mm3) | 0.142 * | 0.09 * | 0.08 * | 0.09 | 0.06 | −0.043 | 0.00 | 0.00 | 0.01 | 0.00 |
| VI | −0.114 | 0.12 | 0.00 | −0.02 | 0.00 | −0.256 | −0.65 | 0.03 | −0.62 | 0.02 |
| FI | 0.042 | 0.10 | 0.01 | 0.12 | 0.01 | −0.090 | 0.11 | 0.02 | 0.15 | 0.03 |
| VFI | −0.234 | −1.00 | 0.02 | −1.33 | 0.02 | −0.450 ** | −1.90 ** | 0.14 ** | −2.00 ** | 0.13 ** |
| PP13 Serum Level | PP13 Amniotic Fluid | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pearson r | Univariate Linear Regression | Multivariate Linear Regression | Pearson r | Univariate Linear Regression | Multivariate Linear Regression | |||||
| B | β2 | B | r2 | B | β2 | B | r2 | |||
| Clinical and obstetrical characteristics | ||||||||||
| Maternal age (years) | 0.057 | 3.30 | 0.01 | 2.44 | 0.00 | 0.076 | −0.11 | 0.00 | 0.06 | 0.00 |
| BMI at the time of genetic consultation (kg/m2) | 0.114 | 2.87 | 0.01 | 2.79 | 0.01 | 0.084 | −0.18 | 0.01 | −0.17 | 0.01 |
| Fetal weight at delivery (kg) | −0.174 | −0.03 | 0.01 | −0.04 | 0.01 | −0.102 * | −0.01 * | 0.07 * | −0.01 | 0.06 |
| GA at delivery (weeks) | 0.045 | 13.2 | 0.01 | 13.07 | 0.01 | −0.155 ** | −2.64 ** | 0.15 ** | −3.02 ** | 0.18 ** |
| GA at the time of amniocentesis (weeks) | −0.078 | −11.45 | 0.01 | −5.45 | 0.00 | 0.057 | 1.54 | 0.05 | 1.67 | 0.04 |
| Fetal sonography at the time of amniocentesis | ||||||||||
| Head circumference (mm) | −0.081 | −0.95 | 0.01 | −0.22 | 0.00 | 0.042 | 0.08 | 0.02 | −0.23 | 0.02 |
| Abdominal circumference (mm) | −0.089 | −2.18 | 0.03 | −1.14 | 0.00 | −0.098 * | 0.31 * | 0.15 * | 0.49 | 0.09 |
| Femur length (mm) | −0.010 | −3.63 | 0.01 | 19.39 | 0.05 | 0.157 | 0.32 | 0.02 | −0.635 | 0.01 |
| Estimated fetal weight (grams) | −0.067 | −0.43 | 0.02 | 0.12 | 0.00 | −0.002 | 0.06 | 0.10 | 0.07 | 0.02 |
| Placental sonography at the time of amniocentesis | ||||||||||
| Placental volume (mm3) | −0.044 | 0.15 | 0.01 | 0.10 | 0.00 | −0.162 | −0.01 | 0.00 | −0.00 | 0.00 |
| VI | −0.103 | 4.02 | 0.01 | 4.16 | 0.01 | 0.148 | −0.10 | 0.00 | −0.02 | 0.00 |
| FI | −0.227 | −0.60 | 0.01 | −0.79 | 0.01 | −0.161 | −0.05 | 0.01 | −0.06 | 0.02 |
| VFI | −0.210 | 1.18 | 0.00 | −0.01 | 0.00 | 0.011 | 0.02 | 0.00 | 0.01 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincze, M.; Sikovanyecz, J., Jr.; Molnár, A.; Földesi, I.; Surányi, A.; Várbíró, S.; Németh, G.; Sikovanyecz, J.; Kozinszky, Z. Predictive Capabilities of Human Leukocyte Antigen-G and Galectin-13 Levels in the Amniotic Fluid and Maternal Blood for the Pregnancy Outcome. Medicina 2024, 60, 85. https://doi.org/10.3390/medicina60010085
Vincze M, Sikovanyecz J Jr., Molnár A, Földesi I, Surányi A, Várbíró S, Németh G, Sikovanyecz J, Kozinszky Z. Predictive Capabilities of Human Leukocyte Antigen-G and Galectin-13 Levels in the Amniotic Fluid and Maternal Blood for the Pregnancy Outcome. Medicina. 2024; 60(1):85. https://doi.org/10.3390/medicina60010085
Chicago/Turabian StyleVincze, Márió, János Sikovanyecz, Jr., András Molnár, Imre Földesi, Andrea Surányi, Szabolcs Várbíró, Gábor Németh, János Sikovanyecz, and Zoltan Kozinszky. 2024. "Predictive Capabilities of Human Leukocyte Antigen-G and Galectin-13 Levels in the Amniotic Fluid and Maternal Blood for the Pregnancy Outcome" Medicina 60, no. 1: 85. https://doi.org/10.3390/medicina60010085
APA StyleVincze, M., Sikovanyecz, J., Jr., Molnár, A., Földesi, I., Surányi, A., Várbíró, S., Németh, G., Sikovanyecz, J., & Kozinszky, Z. (2024). Predictive Capabilities of Human Leukocyte Antigen-G and Galectin-13 Levels in the Amniotic Fluid and Maternal Blood for the Pregnancy Outcome. Medicina, 60(1), 85. https://doi.org/10.3390/medicina60010085





