Anastomotic Leak and Perioperative Outcomes of Esophagectomy for Esophageal Cancer during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
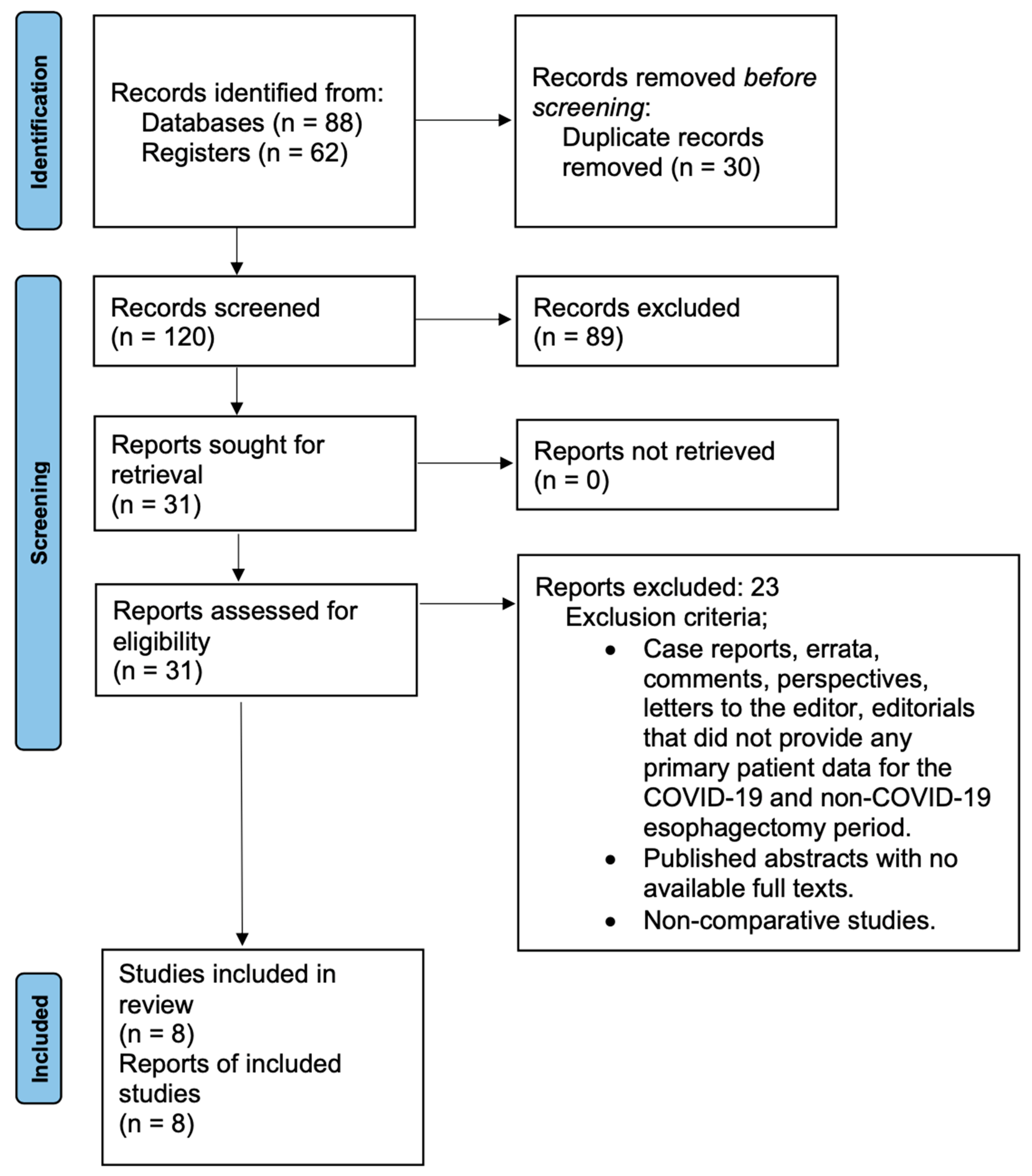
2. Materials and Methods
2.1. Study Design and Inclusion/Exclusion Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
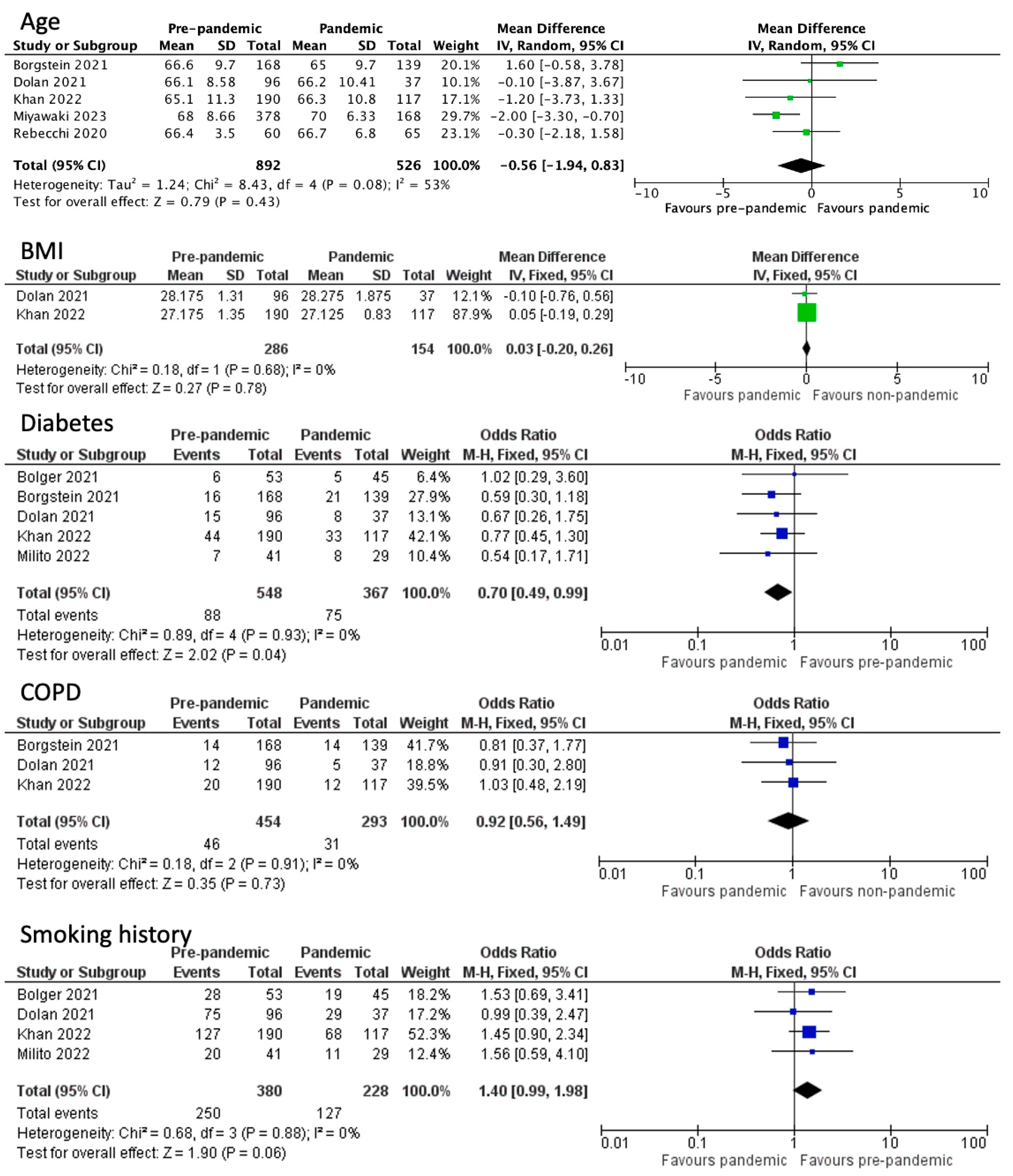
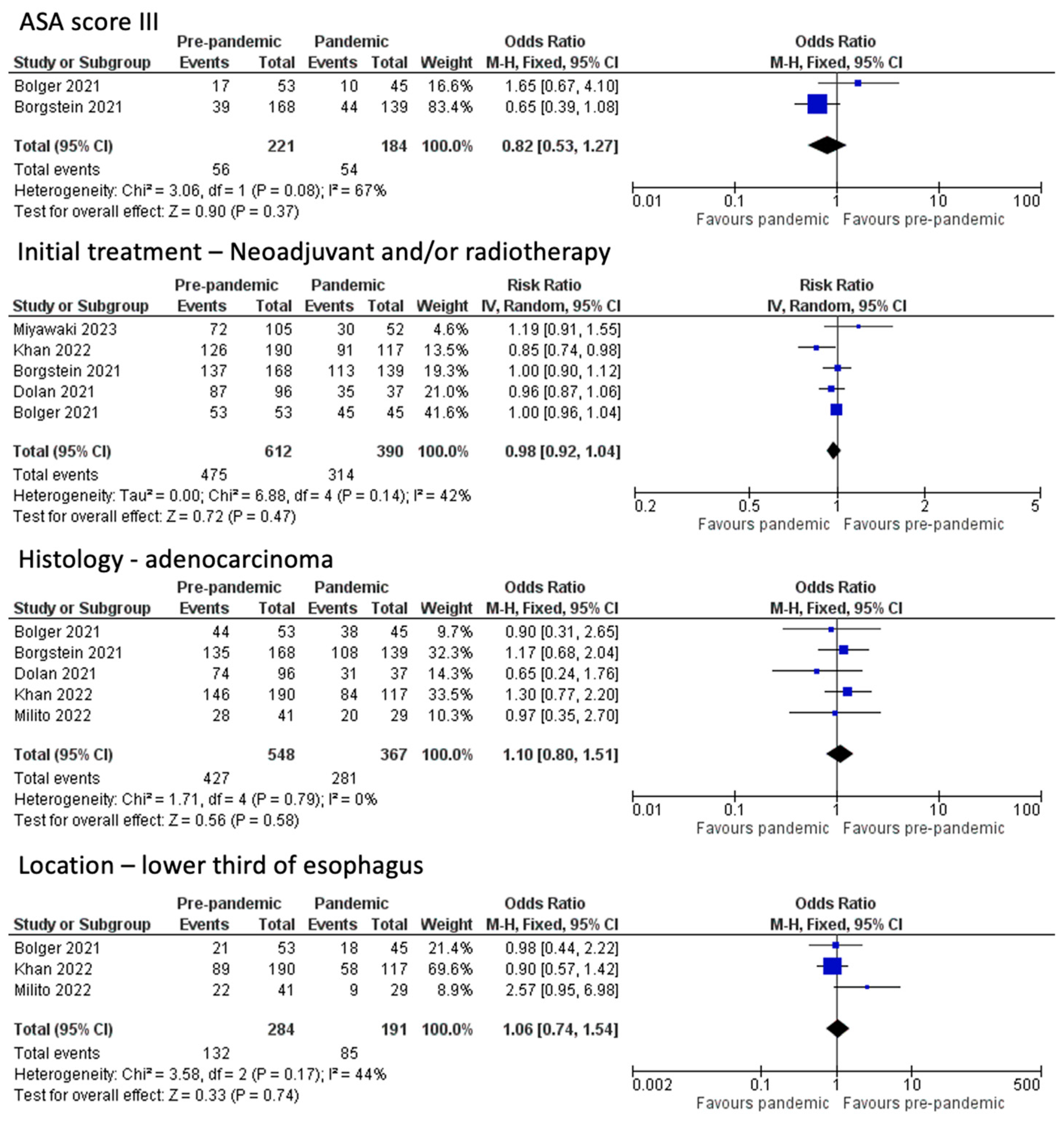
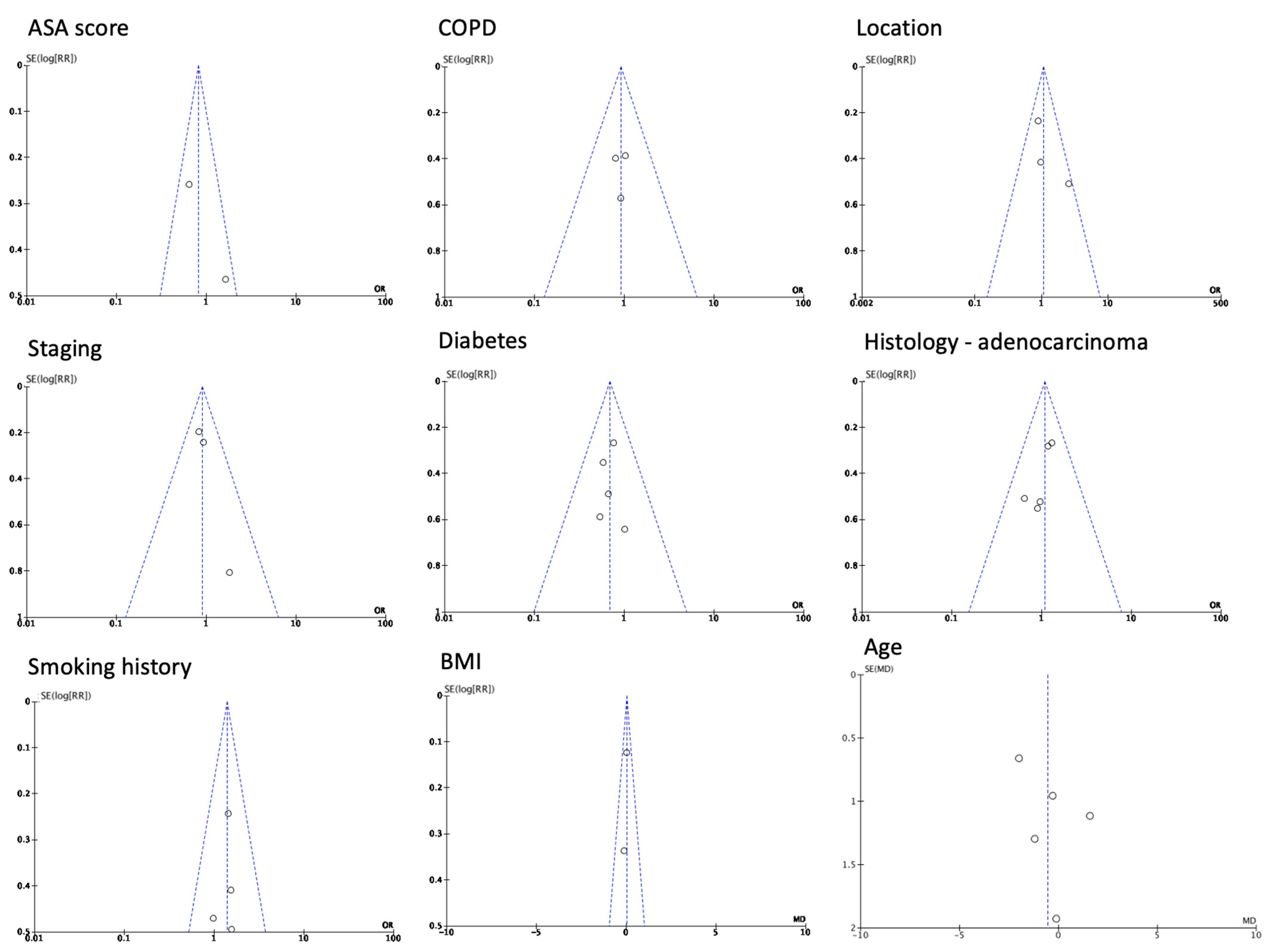
3.1. Baseline Patient Characteristics
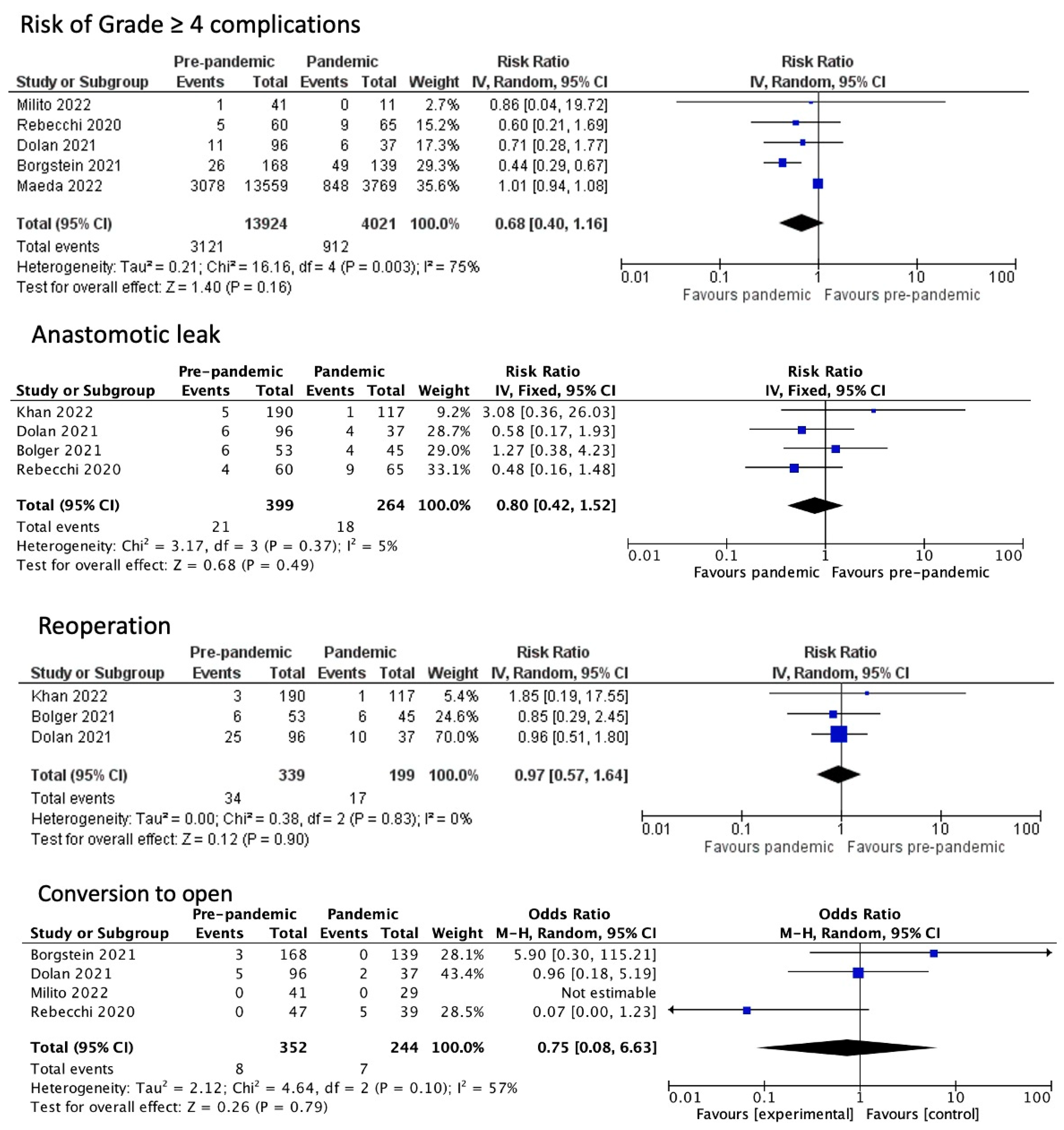
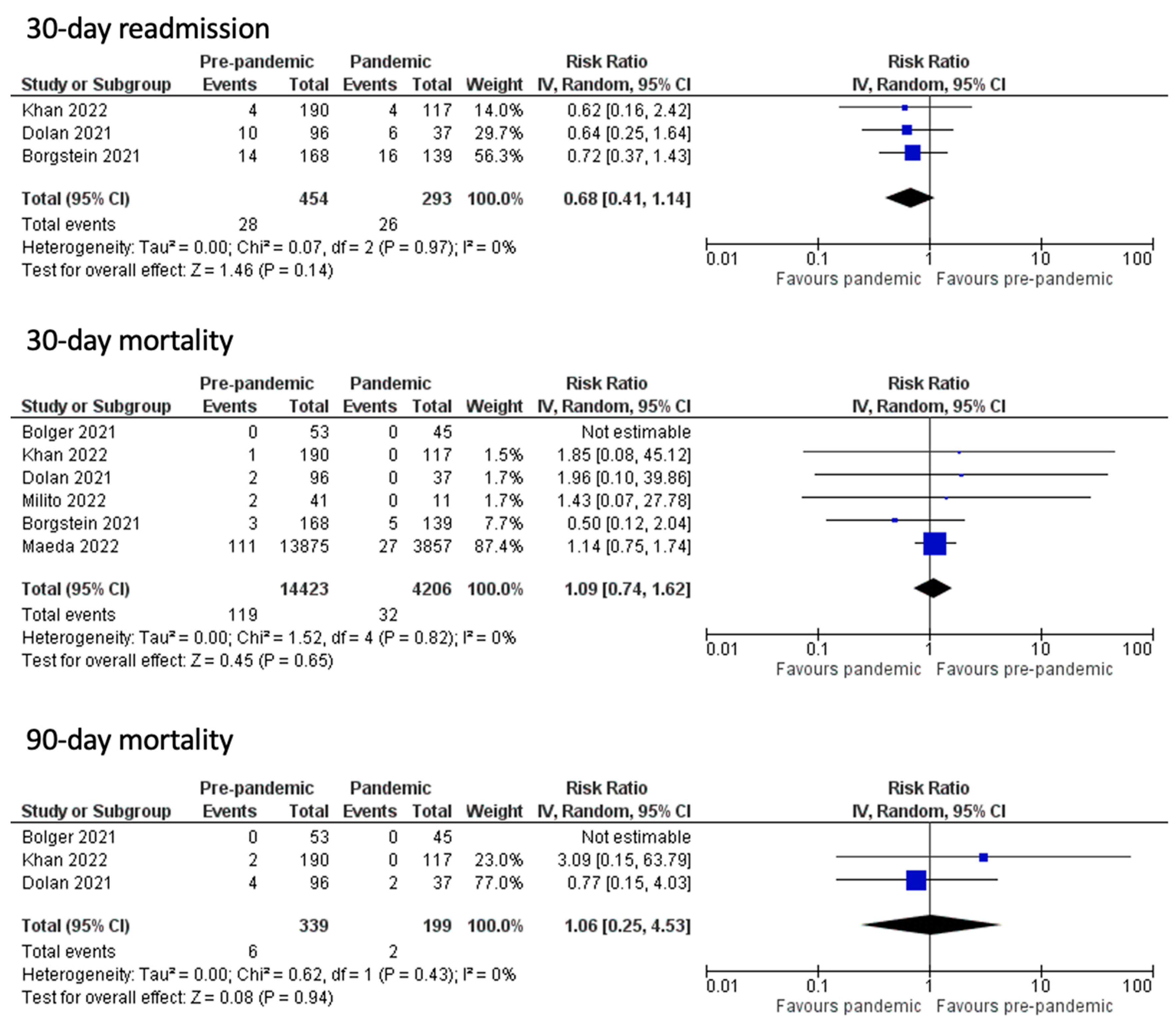
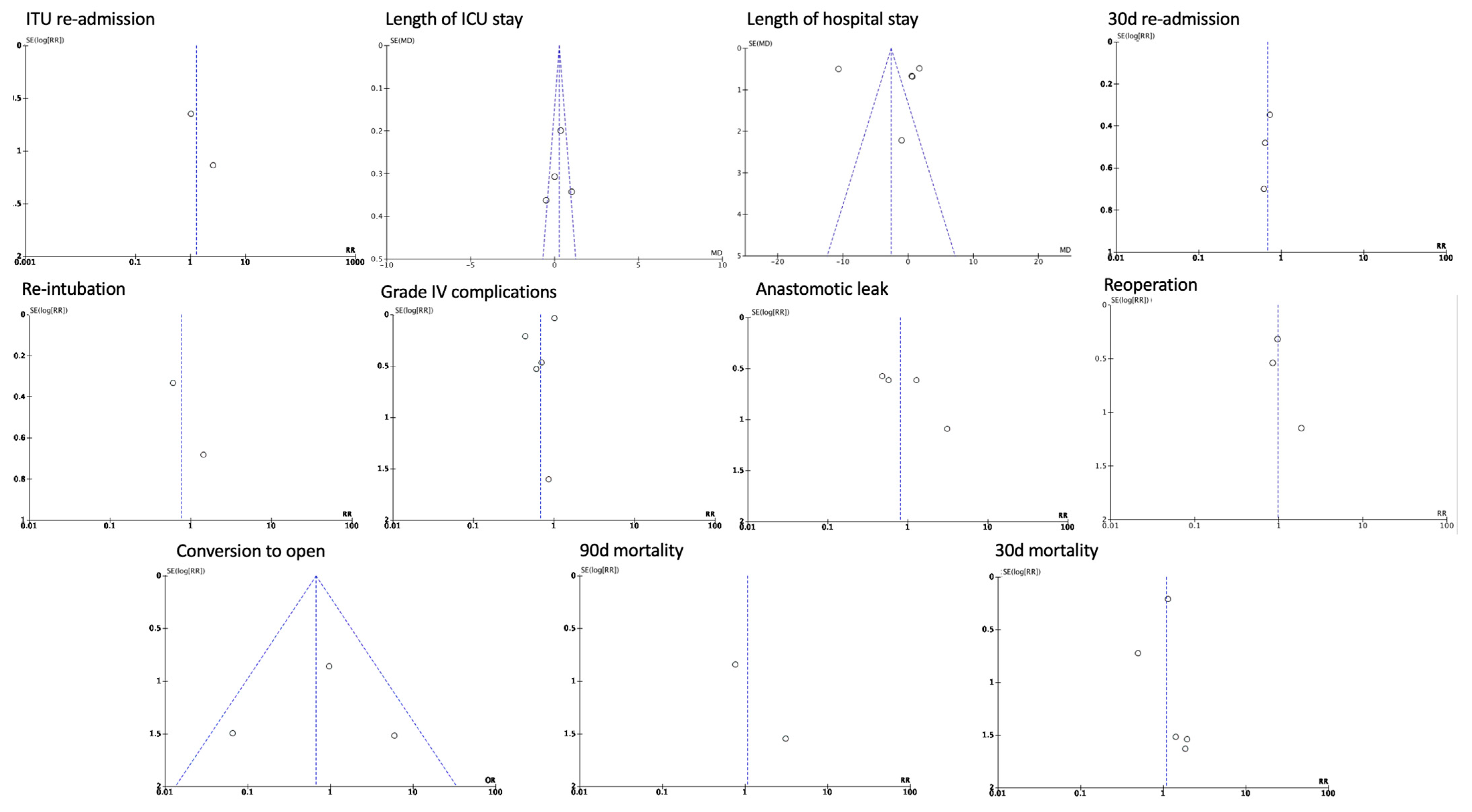
3.2. Post-Operative Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, H.; Johnson, C.; Malwankar, J.; Battafarano, R.; Yang, S.; Broderick, S.; Huang, P.; Lam, V.; Ha, J. The COVID-19 Era Is Associated With Delays in Esophageal Cancer Diagnosis and Treatment. J. Surg. Res. 2023, 285, 100–106. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 June 2023).
- Dong, E.; Du, H.; Gardner, L. An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Nepogodiev, D.; Abbott, T.E.; Ademuyiwa, A.O.; AlAmeer, E.; Bankhead-Kendall, B.K.; Biccard, B.M.; Chakrabortee, S.; Chaudhry, D.; Edwards, J.G.; El-Boghdadly, K.; et al. Projecting COVID-19 Disruption to Elective Surgery. Lancet 2022, 399, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Nepogodiev, D.; Bhangu, A.; Glasbey, J.C.; Li, E.; Omar, O.M.; Simoes, J.F.; Abbott, T.E.; Alser, O.; Arnaud, A.P.; Bankhead-Kendall, B.K.; et al. Mortality and Pulmonary Complications in Patients Undergoing Surgery with Perioperative SARS-CoV-2 Infection: An International Cohort Study. Lancet 2020, 396, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Glasbey, J.C.; Nepogodiev, D.; Simoes, J.F.; Omar, O.; Li, E.; Venn, M.L.; Abou Chaar, M.K.; Capizzi, V.; Chaudhry, D.; Desai, A.; et al. Elective Cancer Surgery in COVID-19–Free Surgical Pathways During the SARS-CoV-2 Pandemic: An International, Multicenter, Comparative Cohort Study. J. Clin. Oncol. 2021, 39, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, New York, NY, USA, 13 May 2014; pp. 1–10. [Google Scholar]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Dolan, D.P.; Swanson, S.J.; Lee, D.N.; Polhemus, E.; Kucukak, S.; Wiener, D.C.; Bueno, R.; Wee, J.O.; White, A. Esophagectomy for Esophageal Cancer Performed During the Early Phase of the COVID-19 Pandemic. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 1075–1080. [Google Scholar] [CrossRef]
- Borgstein, A.B.J.; Brunner, S.; Hayami, M.; Moons, J.; Fuchs, H.; Eshuis, W.J.; Gisbertz, S.S.; Bruns, C.J.; Nafteux, P.; Nilsson, M.; et al. Safety of Esophageal Cancer Surgery During the First Wave of the COVID-19 Pandemic in Europe: A Multicenter Study. Ann. Surg. Oncol. 2021, 28, 4805–4813. [Google Scholar] [CrossRef]
- Bolger, J.C.; Donlon, N.E.; Butt, W.; Neary, C.; Al Azzawi, M.; Brett, O.; King, S.; Downey, E.; Arumugasamy, M.; Murphy, T.; et al. Successful Maintenance of Process and Outcomes for Oesophageal Cancer Surgery in Ireland during the First Wave of the COVID-19 Pandemic. Ir. J. Med. Sci. 2022, 191, 831–837. [Google Scholar] [CrossRef]
- Rebecchi, F.; Arolfo, S.; Ugliono, E.; Morino, M.; Asti, E.; Bonavina, L.; Borghi, F.; Coratti, A.; Cossu, A.; De Manzoni, G.; et al. Impact of COVID-19 Outbreak on Esophageal Cancer Surgery in Northern Italy: Lessons Learned from a Multicentric Snapshot. Dis. Esophagus 2021, 34, doaa124. [Google Scholar] [CrossRef] [PubMed]
- Milito, P.; Asti, E.; Resta, M.; Bonavina, L. Minimally Invasive Esophagectomy for Cancer in COVID Hospitals and Oncological Hubs: Are the Outcomes Different? Eur. Surg. 2022, 54, 98–103. [Google Scholar] [CrossRef]
- Maeda, H.; Endo, H.; Yamamoto, H.; Miyata, H.; Munekage, M.; Taketomi, A.; Kakeji, Y.; Seto, Y.; Yoshida, K.; Yamaue, H.; et al. Effects of the COVID-19 Pandemic on Gastroenterological Surgeries in 2020: A Study Using the National Clinical Database of Japan. Ann. Gastroenterol. Surg. 2023, 7, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, Y.; Sato, H.; Lee, S.; Fujita, S.; Oya, S.; Sugita, H.; Hirano, Y.; Okamoto, K.; Koyama, I.; Sakuramoto, S. Impact of the Coronavirus Disease 2019 Pandemic on First-Visit Patients with Oesophageal Cancer in the First Infection Wave in Saitama Prefecture near Tokyo: A Single-Centre Retrospective Study. Jpn. J. Clin. Oncol. 2022, 52, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Rogers, J.E.; Iwatsuki, M.; Yamashita, K.; Baba, H.; Ajani, J.A. Recent Advances in Treating Oesophageal Cancer. F1000Research 2020, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- NHS England. Specialty Guides for Patient Management during the Coronavirus Pandemic. Clinical Guide for the Management of Essential Cancer Surgery for Adults during the Coronavirus Pandemic; NHS England and NHS Improvement: London, UK, 2020.
- Diaz, A.; Sarac, B.A.; Schoenbrunner, A.R.; Janis, J.E.; Pawlik, T.M. Elective Surgery in the Time of COVID-19. Am. J. Surg. 2020, 219, 900–902. [Google Scholar] [CrossRef]
- Torzilli, G.; Galvanin, J.; Viganò, L.; Donadon, M.; Montorsi, M. COVID-19: Emerging Challenges for Oncological Surgery. Glob. Health Med. 2020, 2, 197–199. [Google Scholar] [CrossRef]
- Alvarado, C.E.; Kapcio, K.C.; Lada, M.J.; Linden, P.A.; Towe, C.W.; Worrell, S.G. The Effect of Diabetes on Pathologic Complete Response Among Patients with Esophageal Cancer. Semin. Thorac. Cardiovasc. Surg. 2023, 35, 429–436. [Google Scholar] [CrossRef]
- Yeates, E.O.; Grigorian, A.; Schellenberg, M.; Owattanapanich, N.; Barmparas, G.; Margulies, D.; Juillard, C.; Garber, K.; Cryer, H.; Tillou, A.; et al. Decreased Hospital Length of Stay and Intensive Care Unit Admissions for Non-COVID Blunt Trauma Patients during the COVID-19 Pandemic. Am. J. Surg. 2022, 224, 90–95. [Google Scholar] [CrossRef]
- Farroha, A. Reduction in Length of Stay of Patients Admitted to a Regional Burn Centre during COVID-19 Pandemic. Burns 2020, 46, 1715. [Google Scholar] [CrossRef]
- Dexter, F.; Epstein, R.H.; Shi, P. Proportions of Surgical Patients Discharged Home the Same or the Next Day Are Sufficient Data to Assess Cases’ Contributions to Hospital Occupancy. Cureus 2021, 13, e13826. [Google Scholar] [CrossRef] [PubMed]
- Dexter, F.; Parra, M.C.; Brown, J.R.; Loftus, R.W. Perioperative COVID-19 Defense: An Evidence-Based Approach for Optimization of Infection Control and Operating Room Management. Anesth. Analg. 2020, 131, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Mujagic, E.; Marti, W.R.; Coslovsky, M.; Soysal, S.D.; Mechera, R.; Von Strauss, M.; Zeindler, J.; Saxer, F.; Mueller, A.; Fux, C.A.; et al. Associations of Hospital Length of Stay with Surgical Site Infections. World J. Surg. 2018, 42, 3888–3896. [Google Scholar] [CrossRef] [PubMed]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The Impact of the COVID-19 Pandemic on Cancer Deaths Due to Delays in Diagnosis in England, UK: A National, Population-Based, Modelling Study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]
| Author (Year) | Centre | Country | Enrolment Dates | Number of Non-Pandemic Patients (n) | Number of COVID-19 Patients (n) | Male/Female | Age (Mean ± SD) |
|---|---|---|---|---|---|---|---|
| Khan et al. (2022) [1] | The Johns Hopkins Hospital | USA | 3/2019–12/2019 (Control) 3/2020–12/2020 (COVID-19) | 190 | 117 | 147/43 (Control) 100/17 (COVID-19) | 65.1 ± 11.3 (Control) 66.3 ± 10.8 (COVID-19) |
| Dolan et al. (2021) [10] | Brigham and Women’s Hospital | USA | 1/2019–12/2019 (Control) 3/2020–6/2020 (COVID-19) | 96 | 37 | 78/18 (Control) 32/5 (COVID-19) | - |
| Borgstein et al. (2021) [11] | Multicenter | Netherlands, Germany, Belgium, and Sweden | 10/2019–2/2020 (Control) 3/2020–5/2020 (COVID-19) | 168 | 139 | 141/27 (Control) 116/23 (COVID-19) | - |
| Bolger et al. (2021) [12] | Irish esophageal cancer centers | Ireland | 4/2019–6/2019 (Control) 4/2020–6/2020 (COVID-19) | 53 | 45 | 37/16 (Control) 37/8 (COVID-19) | - |
| Rebecchi et al. (2020) [13] | Multicenter | Italy | 3/2019–5/2019 (Control) 3/2020–5/2020 (COVID-19) | 60 | 65 | - | 66.4 ± 3.5 (Control) 67.7 ± 6.8 (COVID-19) |
| Milito et al. (2022) [14] | Policlinico San Donato | Italy | 3/2019–2/2020 (Control) 3/2020–10/2020 (COVID-19 first cohort) 11/2020–3/2021 (COVID-19 second cohort) | 41 | 29 | 29/11 (Control) 22/7 (COVID-19) | - |
| Maeda et al. (2022) [15] | National Clinical Database of Japan | Japan | 1/2018–4/2020 (Control) 5/2020–12/2020 (COVID-19) | 13,588 | 3763 | - | - |
| Miyawaki et al. (2022) [16] | National Clinical Database of Japan | Japan | 4/2018–3/2020 (Control) 4/2020–3/2021 (COVID-19) | 105 | 52 | 94/11 (Control) 44/8 (COVID-19) | 65.9 ± 8 (Control) 69.2 ± 9.2 (COVID-19) |
| Author, Year | AIM | Inclusion of Consecutive Patients | Prospective Collection of Data | Endpoints Appropriate to the Aim of the Study | Unbiased Assessment of the Study Endpoint | Follow-Up Period Appropriate to the Aim of the Study | Loss to Follow-Up Less than 5% | Prospective Calculation of the Study Size | Total | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Khan, 2022 [1] | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 11/16 | Low |
| Maeda, 2022 [15] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14/16 | Low |
| Milito, 2022 [14] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 12/16 | Low |
| Miyawaki. 2022 [16] | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 11/16 | Low |
| Bolger, 2021 [12] | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 0 | 10/16 | Low |
| Borgstein, 2021 [11] | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 11/16 | Low |
| Dolan, 2021 [10] | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 11/16 | Low |
| Rebecchi, 2020 [13] | 1 | 1 | 0 | 2 | 1 | 2 | 2 | 0 | 9/16 | Some concerns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geropoulos, G.; Moschonas, S.; Fanariotis, G.; Koltsida, A.; Madouros, N.; Koumadoraki, E.; Katsikas Triantafyllidis, K.; Kechagias, K.S.; Koimtzis, G.; Giannis, D.; et al. Anastomotic Leak and Perioperative Outcomes of Esophagectomy for Esophageal Cancer during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Medicina 2024, 60, 31. https://doi.org/10.3390/medicina60010031
Geropoulos G, Moschonas S, Fanariotis G, Koltsida A, Madouros N, Koumadoraki E, Katsikas Triantafyllidis K, Kechagias KS, Koimtzis G, Giannis D, et al. Anastomotic Leak and Perioperative Outcomes of Esophagectomy for Esophageal Cancer during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Medicina. 2024; 60(1):31. https://doi.org/10.3390/medicina60010031
Chicago/Turabian StyleGeropoulos, Georgios, Stavros Moschonas, Georgios Fanariotis, Aggeliki Koltsida, Nikolaos Madouros, Evgenia Koumadoraki, Kontantinos Katsikas Triantafyllidis, Konstantinos S. Kechagias, Georgios Koimtzis, Dimitrios Giannis, and et al. 2024. "Anastomotic Leak and Perioperative Outcomes of Esophagectomy for Esophageal Cancer during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis" Medicina 60, no. 1: 31. https://doi.org/10.3390/medicina60010031
APA StyleGeropoulos, G., Moschonas, S., Fanariotis, G., Koltsida, A., Madouros, N., Koumadoraki, E., Katsikas Triantafyllidis, K., Kechagias, K. S., Koimtzis, G., Giannis, D., Notopoulos, A., Pavlidis, E. T., & Psarras, K. (2024). Anastomotic Leak and Perioperative Outcomes of Esophagectomy for Esophageal Cancer during the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Medicina, 60(1), 31. https://doi.org/10.3390/medicina60010031