Understanding of Depressive Symptomatology across Major Depressive Disorder and Bipolar Disorder: A Network Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Measurement
The Zung Self-Rating Depression Scale
2.3. Statistical Analysis
2.3.1. Network Construction and Community Detection
2.3.2. Centrality Estimation
2.3.3. Network Stability and Robustness
2.3.4. Network Comparisons between MDD and BD
3. Results
3.1. Demographic and Clinical Characteristics
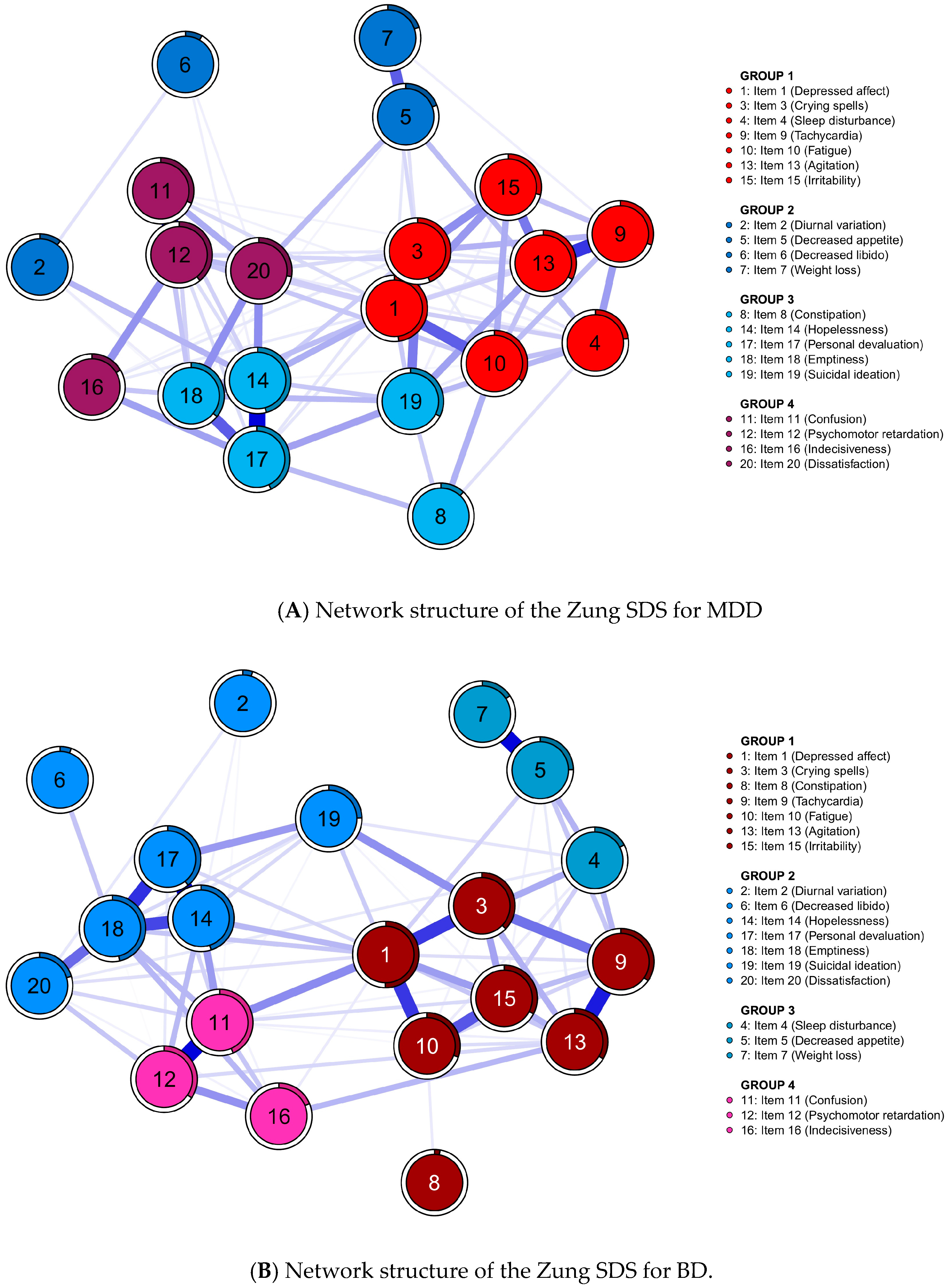
3.2. Network and Community Estimation
3.3. Principal Component Analysis
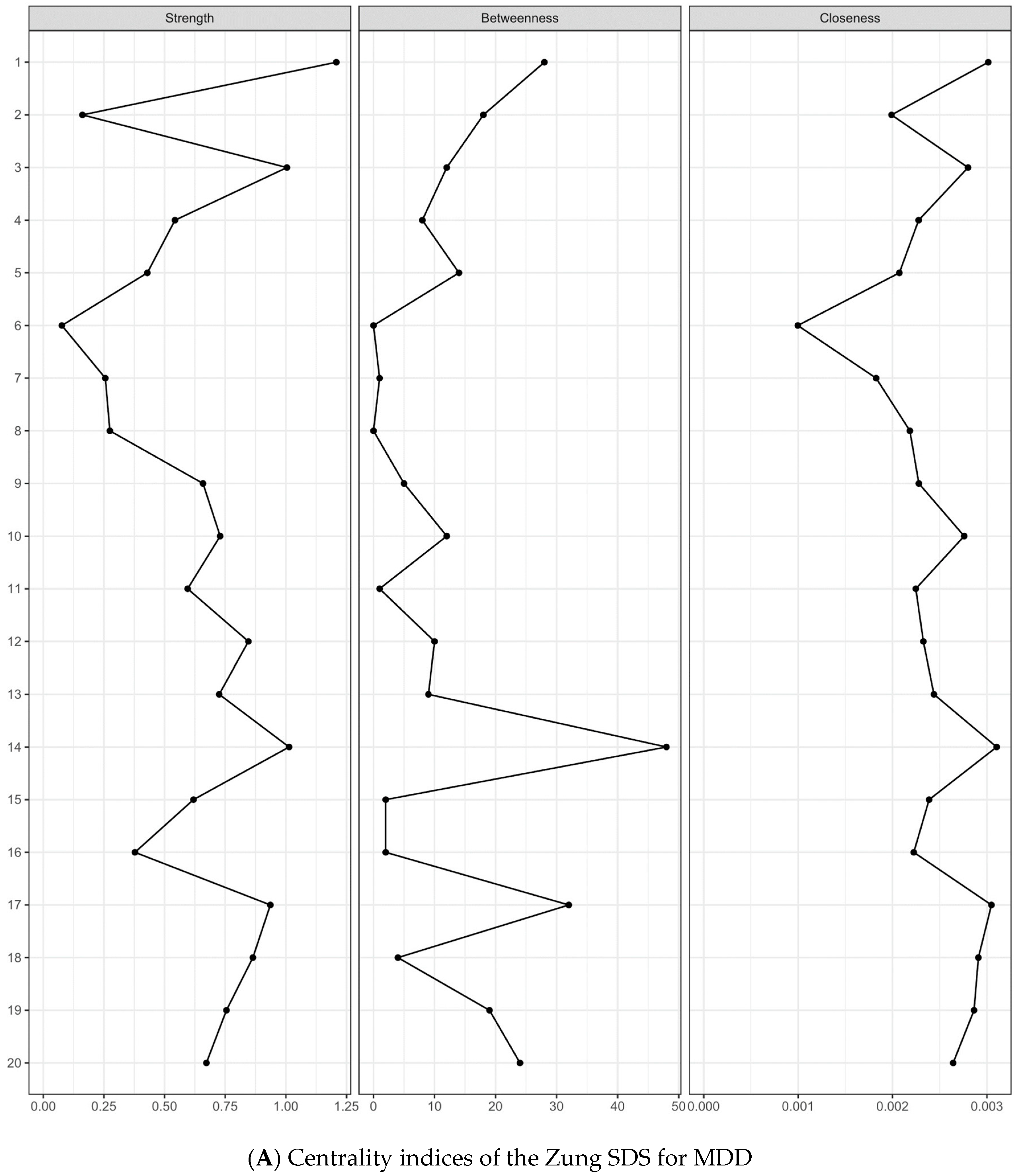
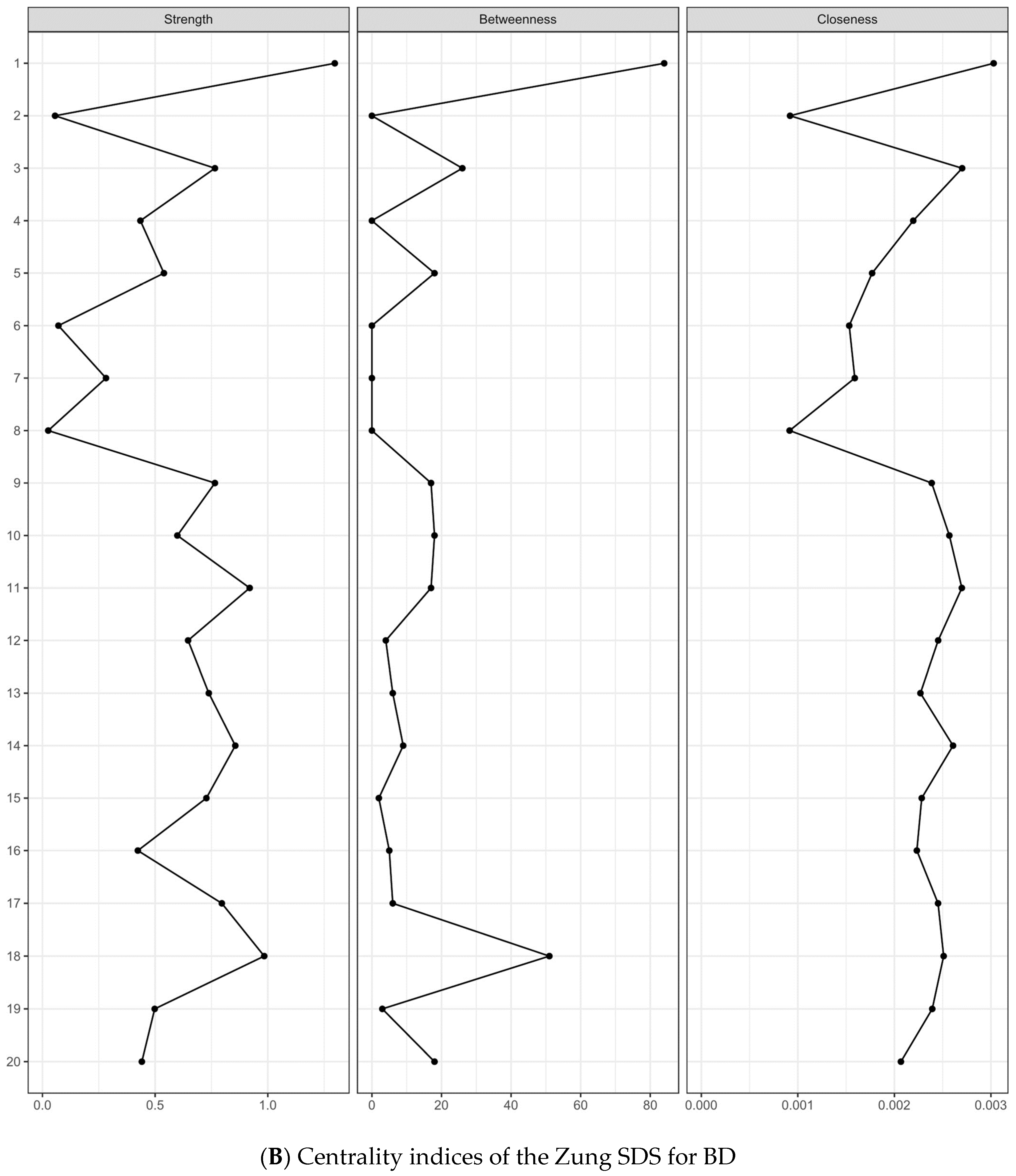
3.4. Centrality Indices and Edge Weights
3.5. Network stability
3.6. Network Comparisons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). Jama 2003, 289, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, K.R.; Akiskal, H.S.; Angst, J.; Greenberg, P.E.; Hirschfeld, R.M.; Petukhova, M.; Kessler, R.C. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry 2007, 64, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Petersen, T.; Mahal, Y.; Mischoulon, D.; Nierenberg, A.A.; Fava, M. Quality of life assessments in major depressive disorder: A review of the literature. Gen. Hosp. Psychiatry 2004, 26, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Michalak, E.E.; Yatham, L.N.; Lam, R.W. Quality of life in bipolar disorder: A review of the literature. Health Qual. Life Outcomes 2005, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Smit, F. Excess mortality in depression: A meta-analysis of community studies. J. Affect. Disord. 2002, 72, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, K.; Winkleby, M.A.; Sundquist, J. Comorbidities and mortality in bipolar disorder: A Swedish national cohort study. JAMA Psychiatry 2013, 70, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Rihmer, Z.; Döme, P. Major mood disorders and suicidal behavior. In The International Handbook of Suicide Prevention; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 74–92. [Google Scholar]
- Perlis, R.H.; Ostacher, M.J.; Patel, J.K.; Marangell, L.B.; Zhang, H.; Wisniewski, S.R.; Ketter, T.A.; Miklowitz, D.J.; Otto, M.W.; Gyulai, L. Predictors of recurrence in bipolar disorder: Primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am. J. Psychiatry 2006, 163, 217–224. [Google Scholar] [CrossRef]
- Mueller, T.I.; Leon, A.C.; Keller, M.B.; Solomon, D.A.; Endicott, J.; Coryell, W.; Warshaw, M.; Maser, J.D. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am. J. Psychiatry 1999, 156, 1000–1006. [Google Scholar] [CrossRef]
- Solomon, D.A.; Keitner, G.I.; Miller, I.W.; Shea, M.T.; Keller, M.B. Course of illness and maintenance treatments for patients with bipolar disorder. J. Clin. Psychiatry 1995, 56, 5–13. [Google Scholar]
- American Psychiatric Association, D.; Association, A.P. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; Volume 5. [Google Scholar]
- Perugi, G.; Angst, J.; Azorin, J.-M.; Bowden, C.L.; Mosolov, S.; Reis, J.; Vieta, E.; Young, A.H.; Group, B.-I.-M.S. Mixed features in patients with a major depressive episode: The BRIDGE-II-MIX study. J. Clin. Psychiatry 2015, 76, 12074. [Google Scholar] [CrossRef]
- Vieta, E.; Valentí, M. Mixed states in DSM-5: Implications for clinical care, education, and research. J. Affect. Disord. 2013, 148, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cuellar, A.K.; Johnson, S.L.; Winters, R. Distinctions between bipolar and unipolar depression. Clin. Psychol. Rev. 2005, 25, 307–339. [Google Scholar] [CrossRef] [PubMed]
- Abrams, R.; Taylor, M.A. A comparison of unipolar and bipolar depressive illness. Am. J. Psychiatry 1980, 137, 1084–1087. [Google Scholar] [PubMed]
- Brockington, I.F.; Altman, E.; Hillier, V.; Meltzer, H.Y.; Nand, S. The clinical picture of bipolar affective disorder in its depressed phase: A report from London and Chicago. Br. J. Psychiatry 1982, 141, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Forty, L.; Smith, D.; Jones, L.; Jones, I.; Caesar, S.; Cooper, C.; Fraser, C.; Gordon-Smith, K.; Hyde, S.; Farmer, A. Clinical differences between bipolar and unipolar depression. Br. J. Psychiatry 2008, 192, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, N.; Sachs, G.S.; Goodwin, F.K. What is to be done? Controversies in the diagnosis and treatment of manic-depressive illness. World J. Biol. Psychiatry 2000, 1, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N. Diagnosis and management of patients with bipolar II disorder. J. Clin. Psychiatry 2005, 66, 13–17. [Google Scholar]
- Goodwin, F.K.; Jamison, K.R. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression; Oxford University Press: Oxford, UK, 2007; Volume 2. [Google Scholar]
- Hirschfeld, R.M.; Lewis, L.; Vornik, L.A. Perceptions and impact of bipolar disorder: How far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J. Clin. Psychiatry 2003, 64, 161–174. [Google Scholar] [CrossRef]
- Perlis, R.H.; Ostacher, M.J.; Goldberg, J.F.; Miklowitz, D.J.; Friedman, E.; Calabrese, J.; Thase, M.E.; Sachs, G.S. Transition to mania during treatment of bipolar depression. Neuropsychopharmacology 2010, 35, 2545–2552. [Google Scholar] [CrossRef]
- Valentí, M.; Pacchiarotti, I.; Bonnín, C.M.; Rosa, A.R.; Popovic, D.; Nivoli, A.M.; Goikolea, J.M.; Murru, A.; Undurraga, J.; Colom, F. Risk factors for antidepressant-related switch to mania. J. Clin. Psychiatry 2012, 73, 18391. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Salvatore, P.; Khalsa, H.M.K.; Gebre-Medhin, P.; Imaz, H.; González-Pinto, A.; Perez, J.; Cruz, N.; Maggini, C.; Tohen, M. Morbidity in 303 first-episode bipolar I disorder patients. Bipolar Disord. 2010, 12, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.R.; Reinares, M.; Michalak, E.E.; Bonnin, C.M.; Sole, B.; Franco, C.; Comes, M.; Torrent, C.; Kapczinski, F.; Vieta, E. Functional impairment and disability across mood states in bipolar disorder. Value Health 2010, 13, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M. Bipolar depression and treatment with antidepressants. Br. J. Psychiatry 2012, 200, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Borsboom, D. Psychometric perspectives on diagnostic systems. J. Clin. Psychol. 2008, 64, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- Schmittmann, V.D.; Cramer, A.O.; Waldorp, L.J.; Epskamp, S.; Kievit, R.A.; Borsboom, D. Deconstructing the construct: A network perspective on psychological phenomena. New Ideas Psychol. 2013, 31, 43–53. [Google Scholar] [CrossRef]
- Borsboom, D. A network theory of mental disorders. World Psychiatry 2017, 16, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Saxe, G.N. Network psychiatry: Computational methods to understand the complexity of psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 639–641. [Google Scholar] [CrossRef]
- Barabási, A.-L. Network medicine—From obesity to the “diseasome”. N. Engl. J. Med. 2007, 357, 404–407. [Google Scholar] [CrossRef]
- van Borkulo, C.; Boschloo, L.; Borsboom, D.; Penninx, B.W.; Waldorp, L.J.; Schoevers, R.A. Association of symptom network structure with the course of depression. JAMA Psychiatry 2015, 72, 1219–1226. [Google Scholar] [CrossRef]
- Scott, J.; Crouse, J.J.; Ho, N.; Carpenter, J.; Martin, N.; Medland, S.; Parker, R.; Byrne, E.; Couvy-Duchesne, B.; Mitchell, B. Can network analysis of self-reported psychopathology shed light on the core phenomenology of bipolar disorders in adolescents and young adults? Bipolar Disord. 2021, 23, 584–594. [Google Scholar] [CrossRef]
- Corponi, F.; Anmella, G.; Verdolini, N.; Pacchiarotti, I.; Samalin, L.; Popovic, D.; Azorin, J.-M.; Angst, J.; Bowden, C.L.; Mosolov, S. Symptom networks in acute depression across bipolar and major depressive disorders: A network analysis on a large, international, observational study. Eur. Neuropsychopharmacol. 2020, 35, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar] [PubMed]
- Zung, W.W. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W. How normal is depression? Psychosom. J. Consult. Liaison Psychiatry 1972, 13, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Zung, W.W. Zung self-rating depression scale and depression status inventory. In Assessment of Depression; Springer: Berlin/Heidelberg, Germany, 1986; pp. 221–231. [Google Scholar]
- Perugi, G.; Fornaro, M.; Maremmani, I.; Canonico, P.L.; Carbonatto, P.; Mencacci, C.; Muscettola, G.; Pani, L.; Torta, R.; Vampini, C. Discriminative hypomania checklist-32 factors in unipolar and bipolar major depressive patients. Psychopathology 2012, 45, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics 2008, 9, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, S.L.; Wermuth, N. Graphical models for associations between variables, some of which are qualitative and some quantitative. Ann. Stat. 1989, 17, 31–57. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z. Extended Bayesian information criteria for model selection with large model spaces. Biometrika 2008, 95, 759–771. [Google Scholar] [CrossRef]
- Epskamp, S.; Borsboom, D.; Fried, E.I. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods 2018, 50, 195–212. [Google Scholar] [CrossRef]
- Golino, H.F.; Epskamp, S. Exploratory graph analysis: A new approach for estimating the number of dimensions in psychological research. PLoS ONE 2017, 12, e0174035. [Google Scholar] [CrossRef]
- Christensen, A.P.; Golino, H. Estimating the stability of psychological dimensions via bootstrap exploratory graph analysis: A Monte Carlo simulation and tutorial. Psych 2021, 3, 479–500. [Google Scholar] [CrossRef]
- Golino, H.; Shi, D.; Christensen, A.P.; Garrido, L.E.; Nieto, M.D.; Sadana, R.; Thiyagarajan, J.A.; Martinez-Molina, A. Investigating the performance of exploratory graph analysis and traditional techniques to identify the number of latent factors: A simulation and tutorial. Psychol. Methods 2020, 25, 292. [Google Scholar] [CrossRef] [PubMed]
- Blondel, V.D.; Guillaume, J.-L.; Lambiotte, R.; Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, 2008, P10008. [Google Scholar] [CrossRef]
- Golino, H.; Christensen, A.P.; Moulder, R. EGAnet: Exploratory graph analysis: A framework for estimating the number of dimensions in multivariate data using network psychometrics. R Package Version 0.9 2020, 5, 3–10. [Google Scholar]
- Fried, E.I.; Boschloo, L.; Van Borkulo, C.D.; Schoevers, R.A.; Romeijn, J.-W.; Wichers, M.; De Jonge, P.; Nesse, R.M.; Tuerlinckx, F.; Borsboom, D. Commentary: “Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression”. Front. Psychiatry 2015, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Epskamp, S.; Waldorp, L.J.; Mõttus, R.; Borsboom, D. The Gaussian graphical model in cross-sectional and time-series data. Multivar. Behav. Res. 2018, 53, 453–480. [Google Scholar] [CrossRef] [PubMed]
- Van Borkulo, C.D.; van Bork, R.; Boschloo, L.; Kossakowski, J.J.; Tio, P.; Schoevers, R.A.; Borsboom, D.; Waldorp, L.J. Comparing network structures on three aspects: A permutation test. Psychol. Methods 2022. [Google Scholar] [CrossRef]
- Fried, E.I.; Epskamp, S.; Nesse, R.M.; Tuerlinckx, F.; Borsboom, D. What are ‘good’ depression symptoms? Comparing the centrality of DSM and non-DSM symptoms of depression in a network analysis. J. Affect. Disord. 2016, 189, 314–320. [Google Scholar] [CrossRef]
- Beard, C.; Millner, A.J.; Forgeard, M.J.; Fried, E.I.; Hsu, K.J.; Treadway, M.T.; Leonard, C.V.; Kertz, S.; Björgvinsson, T. Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychol. Med. 2016, 46, 3359–3369. [Google Scholar] [CrossRef]
- Fried, E.I.; van Borkulo, C.D.; Epskamp, S.; Schoevers, R.A.; Tuerlinckx, F.; Borsboom, D. Measuring depression over time. Or not? Lack of unidimensionality and longitudinal measurement invariance in four common rating scales of depression. Psychol. Assess 2016, 28, 1354–1367. [Google Scholar] [CrossRef]
- Fried, E.I.; Nesse, R.M. The impact of individual depressive symptoms on impairment of psychosocial functioning. PLoS ONE 2014, 9, e90311. [Google Scholar] [CrossRef] [PubMed]
- Rosenström, T.; Elovainio, M.; Jokela, M.; Pirkola, S.; Koskinen, S.; Lindfors, O.; Keltikangas-Järvinen, L. Concordance between Composite International Diagnostic Interview and self-reports of depressive symptoms: A re-analysis. Int. J. Methods Psychiatr. Res. 2015, 24, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, N.; Jablensky, A.; Gulbinat, W.; Ernberg, G. WHO collaborative study: Assessment of depressive disorders. Psychol. Med. 1980, 10, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Lythe, K.E.; Gethin, J.A.; Green, S.; Deakin, J.F.; Young, A.H.; Moll, J. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J. Affect. Disord. 2015, 186, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Malgaroli, M.; Calderon, A.; Bonanno, G.A. Networks of major depressive disorder: A systematic review. Clin. Psychol. Rev. 2021, 85, 102000. [Google Scholar] [CrossRef] [PubMed]
- Keitner, G.I.; Solomon, D.A.; Ryan, C.E.; Miller, I.W.; Mallinger, A.; Kupfer, D.J.; Frank, E. Prodromal and residual symptoms in bipolar I disorder. Compr. Psychiatry 1996, 37, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Ferrier, I.N. Evolution of cognitive impairment in bipolar disorder: A systematic review of cross-sectional evidence. Bipolar Disord. 2006, 8, 103–116. [Google Scholar] [CrossRef]
- Abramson, L.Y.; Metalsky, G.I.; Alloy, L.B. Hopelessness depression: A theory-based subtype of depression. Psychol. Rev. 1989, 96, 358. [Google Scholar] [CrossRef]
- LaFarge, L. Emptiness as defense in severe regressive states. J. Am. Psychoanal. Assoc. 1989, 37, 965–995. [Google Scholar] [CrossRef]
- Miller, C.E.; Lewis, K.L.; Huxley, E.; Townsend, M.L.; Grenyer, B.F.S. A 1-year follow-up study of capacity to love and work: What components of borderline personality disorder most impair interpersonal and vocational functioning? Pers. Ment. Health 2018, 12, 334–344. [Google Scholar] [CrossRef]
- Ellison, W.D.; Rosenstein, L.; Chelminski, I.; Dalrymple, K.; Zimmerman, M. The Clinical Significance of Single Features of Borderline Personality Disorder: Anger, Affective Instability, Impulsivity, and Chronic Emptiness in Psychiatric Outpatients. J. Pers. Disord. 2016, 30, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Didonna, F.; Gonzalez, Y.R. Mindfulness and feelings of emptiness. In Clinical Handbook of Mindfulness; Springer: Berlin/Heidelberg, Germany, 2009; pp. 125–151. [Google Scholar]
- Hirschfeld, R. Differential diagnosis of bipolar disorder and major depressive disorder. J. Affect. Disord. 2014, 169, S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Vöhringer, P.A.; Perlis, R.H. Discriminating between bipolar disorder and major depressive disorder. Psychiatr. Clin. 2016, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McElroy, S.L.; Kotwal, R.; Keck Jr, P.E.; Akiskal, H.S. Comorbidity of bipolar and eating disorders: Distinct or related disorders with shared dysregulations? J. Affect. Disord. 2005, 86, 107–127. [Google Scholar] [CrossRef]
- Angst, J.; Azorin, J.-M.; Bowden, C.L.; Perugi, G.; Vieta, E.; Gamma, A.; Young, A.H.; Group, B.S. Prevalence and characteristics of undiagnosed bipolar disorders in patients with a major depressive episode: The BRIDGE study. Arch. Gen. Psychiatry 2011, 68, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.E.; Townsend, M.L.; Day, N.J.S.; Grenyer, B.F.S. Measuring the shadows: A systematic review of chronic emptiness in borderline personality disorder. PLoS ONE 2020, 15, e0233970. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T. Cognitive Therapy of Depression; Guilford Press: New York, NY, USA, 1979. [Google Scholar]
- Romera, I.; Delgado-Cohen, H.; Perez, T.; Caballero, L.; Gilaberte, I. Factor analysis of the Zung self-rating depression scale in a large sample of patients with major depressive disorder in primary care. BMC Psychiatry 2008, 8, 4. [Google Scholar] [CrossRef]
- Linehan, M.M.; Goodstein, J.L.; Nielsen, S.L.; Chiles, J.A. Reasons for staying alive when you are thinking of killing yourself: The reasons for living inventory. J. Consult Clin. Psychol. 1983, 51, 276–286. [Google Scholar] [CrossRef]
- Malone, K.M.; Oquendo, M.A.; Haas, G.L.; Ellis, S.P.; Li, S.; Mann, J.J. Protective factors against suicidal acts in major depression: Reasons for living. Am. J. Psychiatry 2000, 157, 1084–1088. [Google Scholar] [CrossRef]
- Ellis, T.E.; Rutherford, B. Cognition and suicide: Two decades of progress. Int. J. Cogn. Ther. 2008, 1, 47–68. [Google Scholar] [CrossRef]
- Barabasi, A.-L. The network takeover. Nat. Phys. 2011, 8, 14–16. [Google Scholar] [CrossRef]
- Epskamp, S.; Kruis, J.; Marsman, M. Estimating psychopathological networks: Be careful what you wish for. PLoS ONE 2017, 12, e0179891. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Nieto, I.; Valiente, C.; Espinosa, R.; Vazquez, C. The Study of Psychopathology from the Network Analysis Perspective: A Systematic Review. Psychother. Psychosom. 2019, 88, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Forbes, M.K.; Wright, A.G.C.; Markon, K.E.; Krueger, R.F. Evidence that psychopathology symptom networks have limited replicability. J. Abnorm. Psychol. 2017, 126, 969–988. [Google Scholar] [CrossRef]
- Vetter, J.S.; Spiller, T.R.; Cathomas, F.; Robinaugh, D.; Brühl, A.; Boeker, H.; Seifritz, E.; Kleim, B. Sex differences in depressive symptoms and their networks in a treatment-seeking population–a cross-sectional study. J. Affect. Disord. 2021, 278, 357–364. [Google Scholar] [CrossRef]
| Characteristics | Total Sample Mean ± SD, n or % | MDD Mean ± SD, n or % | BD Mean ± SD, n or % | Test Statistics | Bonferroni Corrected p-Value |
|---|---|---|---|---|---|
| Age (years) | 34.39 ± 12.55 | 38.21 ± 13.36 | 32.00 ± 11.38 | 7.18 | <0.001 *** |
| Gender (%) | 0.90 | 1.00 | |||
| Male | 27.8 | 29.8 | 26.6 | ||
| Female | 72.2 | 70.2 | 73.4 | ||
| Education (%) | 2.97 | 0.68 | |||
| High school or below | 31.7 | 35.4 | 29.5 | ||
| Others | 68.3 | 64.6 | 70.5 | ||
| Employment status (%) | 1.39 | 1.00 | |||
| Unemployed | 45.1 | 47.8 | 43.4 | ||
| Employed | 54.9 | 52.2 | 56.6 | ||
| Marital status (%) | 12.10 | <0.05 * | |||
| Married | 30.8 | 37.3 | 26.7 | ||
| Others (Single, divorced, or widowed) | 69.2 | 62.7 | 73.3 | ||
| Alcohol use status (%) | 11.93 | <0.01 ** | |||
| Former or current | 55.5 | 47.8 | 60.3 | ||
| Never | 44.5 | 52.2 | 39.7 | ||
| Smoking status (%) | 0.94 | 1.00 | |||
| Past or current | 32.6 | 47.8 | 33.9 | ||
| Never | 67.4 | 52.2 | 66.1 | ||
| Psychiatric familial history | 45.6 | 35.1 | 52.1 | 22.52 | <0.001 *** |
| Items | Mean (SD) | U_Statistic | Bonferroni Corrected | ||
|---|---|---|---|---|---|
| MDD (n = 322) | BD (n = 516) | p-Value | |||
| 1 | I feel down-hearted and blue. | 2.79 (0.93) | 2.78 (0.81) | 83,384 | 1.00 |
| 2 | Morning is when I feel the best.* | 3.58 (0.74) | 3.46 (0.82) | 89,542. | 0.49 |
| 3 | I have crying spells or feel like it. | 2.31 (0.90) | 2.26 (0.86) | 84,531 | 1.00 |
| 4 | I have trouble sleeping at night. | 2.58 (1.05) | 2.54 (1.06) | 84,691 | 1.00 |
| 5 | I eat as much as I used to.* | 2.50 (1.06) | 2.38 (1.06) | 88,546 | 1.00 |
| 6 | I still enjoy sex.* | 3.68 (0.66) | 3.58 (0.72) | 90,013 | 0.20 |
| 7 | I notice that I am losing weight. | 1.53 (0.79) | 1.45 (0.77) | 88,408 | 1.00 |
| 8 | I have trouble with constipation. | 1.65 (0.91) | 1.76 (1.0) | 78,888 | 1.00 |
| 9 | My heart beats faster than usual. | 2.25 (0.88) | 2.29 (0.91) | 80,663 | 1.00 |
| 10 | I get tired for no reason. | 2.90 (0.90) | 2.99 (0.93) | 78,298 | 1.00 |
| 11 | My mind is as clear as it used to be.* | 3.40 (0.82) | 3.51 (0.78) | 76,939 | 0.75 |
| 12 | I find it easy to do the things I used to.* | 3.33 (0.86) | 3.48 (0.77) | 75,421 | 0.22 |
| 13 | I am restless and can’t keep still. | 2.02 (0.87) | 2.13 (0.89) | 77,251 | 1.00 |
| 14 | I feel hopeful about the future.* | 3.36 (0.81) | 3.41 (0.74) | 81,242 | 1.00 |
| 15 | I am more irritable than usual. | 2.45 (0.96) | 2.40 (0.96) | 85,791 | 1.00 |
| 16 | I find it easy to make decisions.* | 3.11 (0.92) | 3.23 (0.86) | 77,804 | 1.00 |
| 17 | I feel that I am useful and needed.* | 3.17 (0.88) | 3.26 (0.85) | 78,033 | 1.00 |
| 18 | My life is pretty full.* | 3.55 (0.71) | 3.60 (0.65) | 80,991 | 1.00 |
| 19 | I feel that others would be better off if I were dead. | 1.62 (0.90) | 1.77 (0.99) | 76,624 | 0.74 |
| 20 | I still enjoy the things I used to do.* | 3.29 (0.84) | 3.41 (0.72) | 78,220 | 1.00 |
| Total score | 55.08 (8.69) | 55.67 (7.86) | 79,783 | 1.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Jang, J.; Kang, H.S.; Lee, J.; Lee, D.; Yu, H.; Ha, T.H.; Park, J.; Myung, W. Understanding of Depressive Symptomatology across Major Depressive Disorder and Bipolar Disorder: A Network Analysis. Medicina 2024, 60, 32. https://doi.org/10.3390/medicina60010032
Lee H, Jang J, Kang HS, Lee J, Lee D, Yu H, Ha TH, Park J, Myung W. Understanding of Depressive Symptomatology across Major Depressive Disorder and Bipolar Disorder: A Network Analysis. Medicina. 2024; 60(1):32. https://doi.org/10.3390/medicina60010032
Chicago/Turabian StyleLee, Hyukjun, Junwoo Jang, Hyo Shin Kang, Jakyung Lee, Daseul Lee, Hyeona Yu, Tae Hyon Ha, Jungkyu Park, and Woojae Myung. 2024. "Understanding of Depressive Symptomatology across Major Depressive Disorder and Bipolar Disorder: A Network Analysis" Medicina 60, no. 1: 32. https://doi.org/10.3390/medicina60010032
APA StyleLee, H., Jang, J., Kang, H. S., Lee, J., Lee, D., Yu, H., Ha, T. H., Park, J., & Myung, W. (2024). Understanding of Depressive Symptomatology across Major Depressive Disorder and Bipolar Disorder: A Network Analysis. Medicina, 60(1), 32. https://doi.org/10.3390/medicina60010032








