The Effect of Aviva Exercise Intervention on Pain Level and Body Awareness in Women with Primary Dysmenorrhea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Study Population
2.3. Sample Size and Randomization of the Study Groups
2.4. Exercise Intervention Program
2.5. Statistical Methods
2.6. Registry
3. Results
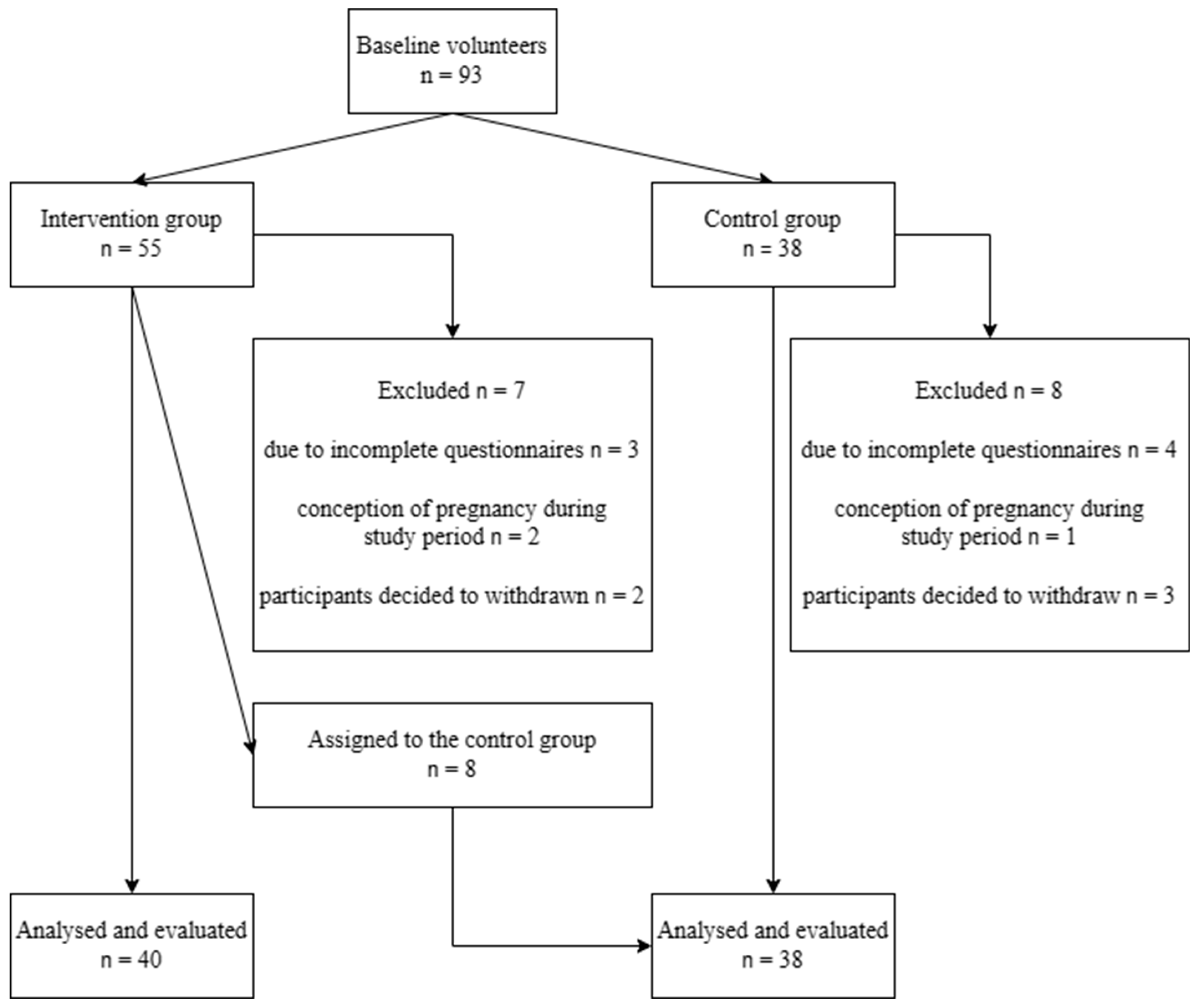
3.1. Study Population
3.2. Distribution of Demographic Data and Dysmenorrhea Scores (NRS) at Baseline
3.3. Primary Outcome Measures
3.4. Secondary Outcome Measures
3.4.1. The Difference between the IG and CG Regarding the Different Scales of the BAQ-H at T1, T2, and T3
3.4.2. Adherence to the Intervention
3.4.3. Results of the Borg Scale
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferries-Rowe, E.; Corey, E.; Archer, J.S. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet. Gynecol. 2020, 136, 1047. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Armour, M.; Parry, K.; Manohar, N.; Holmes, K.; Ferfolja, T.; Curry, C.; MacMillan, F.; Smith, C.A. The Prevalence and Academic Impact of Dysmenorrhea in 21,573 Young Women: A Systematic Review and Meta-Analysis. J. Women’s Health 2019, 28, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Burnett, M.; Lemyre, M. No. 345-Primary Dysmenorrhea Consensus Guideline. J. Obstet. Gynaecol. Can. 2017, 39, 7. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Farquhar, C.; Roberts, H.; Proctor, M. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst. Rev. 2009, 4, CD002120. [Google Scholar] [CrossRef] [PubMed]
- Armour, M.; Ee, C.C.; Naidoo, D.; Ayati, Z.; Chalmers, K.J.; Steel, K.A.; de Manincor, M.J.; Delshad, E. Exercise for dysmenorrhoea. Cochrane Database Syst. Rev. 2019, 2019, CD004142. [Google Scholar] [CrossRef]
- Zahradnik, H.-P.; Hanjalic-Beck, A.; Groth, K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: A review. Contraception 2010, 81, 185–196. [Google Scholar] [CrossRef]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory Markers in Dysmenorrhea and Therapeutic Options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef]
- Kannan, P.; Cheung, K.K.; Lau, B.W.-M. Does aerobic exercise induced-analgesia occur through hormone and inflammatory cytokine-mediated mechanisms in primary dysmenorrhea? Med. Hypotheses 2019, 123, 50–54. [Google Scholar] [CrossRef]
- Tamura, I.; Taketani, T.; Lee, L.; Kizuka, F.; Taniguchi, K.; Maekawa, R.; Asada, H.; Tamura, H.; Sugino, N. Differential Effects of Progesterone on COX-2 and Mn-SOD Expressions Are Associated with Histone Acetylation Status of the Promoter Region in Human Endometrial Stromal Cell. J. Clin. Endocrinol. Metab. 2011, 96, E1073–E1082. [Google Scholar] [CrossRef][Green Version]
- Ma, H. Altered Cytokine Gene Expression in Peripheral Blood Monocytes across the Menstrual Cycle in Primary Dysmenorrhea: A Case-Control Study. PLoS ONE 2013, 8, e55200. [Google Scholar] [CrossRef]
- iMedPub. Insight Medical Publishing|Kannan. Available online: https://www.imedpub.com/search-results.php?keyword=kannan&search= (accessed on 25 November 2021).
- Kannan, P.; Cheung, K.-K.; Lau, B.W.M.; Li, L.; Chen, H.; Sun, F. A mixed-methods study to evaluate the effectiveness and cost-effectiveness of aerobic exercise for primary dysmenorrhea: A study protocol. PLoS ONE 2021, 16, e0256263. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar] [CrossRef]
- Lima, L.V.; Abner, T.S.S.; Sluka, K.A. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J. Physiol. 2017, 595, 4141–4150. [Google Scholar] [CrossRef]
- Koltyn, K.F.; Brellenthin, A.G.; Cook, D.B.; Sehgal, N.; Hillard, C. Mechanisms of exercise-induced hypoalgesia. J. Pain. 2014, 15, 1294–1304. [Google Scholar] [CrossRef]
- Fisher, C.; Adams, J.; Hickman, L.; Sibbritt, D. The use of complementary and alternative medicine by 7427 Australian women with cyclic perimenstrual pain and discomfort: A cross-sectional study. BMC Complement. Altern. Med. 2016, 16, 129. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Kuo, F.C.; Kuo, H.C.; Liao, L.L. The prevalence of self-reported premenstrual symptoms and evaluation of regular exercise with premenstrual symptoms among female employees in Taiwan. Women Health 2018, 58, 247–259. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Armour, M.; Parry, K.; Al-Dabbas, M.A.; Curry, C.; Holmes, K.; MacMillan, F.; Ferfolja, T.; Smith, C.A. Self-care strategies and sources of knowledge on menstruation in 12,526 young women with dysmenorrhea: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0220103. [Google Scholar] [CrossRef]
- Carroquino-Garcia, P.; Jiménez-Rejano, J.J.; Medrano-Sanchez, E.; de la Casa-Almeida, M.; Diaz-Mohedo, E.; Suarez-Serrano, C. Therapeutic Exercise in the Treatment of Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 1371–1380. [Google Scholar] [CrossRef]
- Kovács, Z.; Hegyi, G.; Szőke, H. The Effect of Exercise on Pulsatility Index of Uterine Arteries and Pain in Primary Dysmenorrhea. J. Clin. Med. 2023, 12, 7021. [Google Scholar] [CrossRef]
- Aviva Method Information Center. Aviva Method—Home. Available online: https://avivamethod.info/ (accessed on 22 January 2023).
- Steiner, A. Aviva Method for Women and Men; Hungarian Aviva Foundation: Budapest, Hungary, 2019; ISBN 9786150047607. [Google Scholar]
- Aviva Method. Available online: https://avivamodszer.hu/ (accessed on 22 January 2023).
- Mehling, W.E.; Wrubel, J.; Daubenmier, J.J.; Price, C.J.; Kerr, C.E.; Silow, T.; Gopisetty, V.; Stewart, A.L. Body Awareness: A phenomenological inquiry into the common ground of mind-body therapies. Philos. Ethics Humanit. Med. 2011, 6, 6. [Google Scholar] [CrossRef]
- Mehling, W.E.; Gopisetty, V.; Daubenmier, J.; Price, C.J.; Hecht, F.M.; Stewart, A. Body Awareness: Construct and Self-Report Measures. PLoS ONE 2009, 4, e5614. [Google Scholar] [CrossRef]
- Unal, A.; Altug, F.; Erden, A.; Cavlak, U.; Senol, H. Validity and reliability of the Body Awareness Questionnaire in patients with non-specific chronic low back pain. Acta Neurol. Belg. 2021, 121, 3. [Google Scholar] [CrossRef]
- Erden, A.; Güner, S.G. Impact of exercise on quality of life, body awareness, kinesiophobia and the risk of falling among young older adults. Cukurova Med. J. 2018, 43, 941–950. [Google Scholar] [CrossRef]
- Kalkışım, Ş.N.; Erden, A.; Kanber Uzun, Ö.; Ertemoğlu Öksüz, C.; Zihni, N.B.; Çan, M.A. Relationship between body awareness level and musculoskeletal pain complaints, physical activity level and emotional status in healthy people. Acta Neurol. Belg. 2022, 123, 1789–1796. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Daubenmier, J.; Mehling, W.; Büssing, A.; Saha, F.J.; Dobos, G.; Shields, S.A. Being aware of the painful body: Validation of the German Body Awareness Questionnaire and Body Responsiveness Questionnaire in patients with chronic pain. PLoS ONE. 2018, 13, e0193000. [Google Scholar] [CrossRef]
- Köteles, F. Psychometric investigation of the Hungarian version of the Body Awareness Questionnaire (BAQ-H) among yoga practitioners and young adult controls. Mentálhigiéné Pszichoszomatika 2014, 15, 373–391. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar] [CrossRef]
- de Arruda, G.T.; Driusso, P.; Rodrigues, J.C.; de Godoy, A.G.; Avila, M.A. Numerical rating scale for dysmenorrhea-related pain: A clinimetric study. Gynecol Endocrinol. 2022, 38, 661–665. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Shields, S.A.; Mallory, M.E.; Simon, A. The Body Awareness Questionnaire: Reliability and Validity. J. Personal. Assess. 1989, 53, 802–815. [Google Scholar] [CrossRef]
- Hewit, G.D.; Karen, R.G. Dysmenorrhea and endometriosis in the adolescent. ACOG Committee Opinion No. 760. American College of Obstetricians and Gynecologists. Obs. Gynecol. 2018, 132, e249–e258. [Google Scholar] [CrossRef]
- Lumley, T.; Diehr, P.; Emerson, S.; Chen, L. The Importance of the Normality Assumption in Large Public Health Data Sets. Annu. Rev. Public Health 2002, 23, 151–169. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Ayeleke, R.O.; Farquhar, C.; Proctor, M. Nonsteroidal antiinflammatory drugs for dysmenorrhoea. Cochrane Database Syst. Rev. 2015, 2015, CD001751. [Google Scholar]
- Sharma, S.; Ali, K.; Narula, H.; Malhotra, N.; Rai, R.H.; Bansal, N.; Balasubramanian, K.; Kalra, S.; Sanjeevi, R.R.; Chahal, A. Exercise Therapy and Electrotherapy as an Intervention for Primary Dysmenorrhea: A Systematic Review and Meta-Analysis. J. Lifestyle Med. 2023, 13, 16–26. [Google Scholar] [CrossRef]
- Durand, H.; Monahan, K.; McGuire, B.E. Prevalence and Impact of Dysmenorrhea Among University Students in Ireland. Pain Med. 2021, 22, 2835–2845. [Google Scholar] [CrossRef]
- Matthewman, G.A.; Lee, J.; Kaur, G.; Daley, A.J. Physical activity for primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. Am. J. Obs. Gynecol. 2018, 219, 255.e1–255.e20. [Google Scholar] [CrossRef]
- Fuentes-Aparicio, L.; Cuenca-Martínez, F.; Muñoz-Gómez, E.; Mollà-Casanova, S.; Aguilar-Rodríguez, M.; Sempere-Rubio, N. Effects of therapeutic exercise in primary dysmenorrhea: An umbrella and mapping review. Pain Med. 2023, 24, 1386–1395. [Google Scholar] [CrossRef]
- Samy, A.; Zaki, S.S.; Metwaly, A. The Effect of Zumba Exercise on Reducing Menstrual Pain in Young Women with Primary Dysmenorrhea: A Randomized Controlled Trial. J. Pediatr. Adolesc. Gynecol. 2019, 32, 541–545. [Google Scholar] [CrossRef]
- Kannan, P.; Chapple, C.M.; Miller, D.; Claydon-Mueller, L.; Baxter, G.D. Effectiveness of a treadmill-based aerobic exercise intervention on pain, daily functioning, and quality of life in women with primary dysmenorrhea: A randomized controlled trial. Contemp. Clin. Trials. 2019, 81, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Armour, M.; Smith, C.A.; Steel, K.A.; Macmillan, F. The effectiveness of self-care and lifestyle interventions in primary dysmenorrhea: A systematic review and meta-analysis. BMC Complement. Altern. Med. 2019, 19, 22. [Google Scholar] [CrossRef]
- Dmitrović, R. Transvaginal color Doppler study of uterine blood flow in primary dysmenorrhea. Acta Obstet. Gynecol. Scand. 2000, 79, 1112–1116. [Google Scholar] [CrossRef]
- Guedes-Martins, L.; Gaio, R.; Saraiva, J.; Cerdeira, S.; Matos, L.; Silva, E.; Macedo, F.; Almeida, H. Reference Ranges for Uterine Artery Pulsatility Index during the Menstrual Cycle: A Cross-Sectional Study. PLoS ONE 2015, 10, e0119103. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H.; Davis, M.J.; Secher, N.H.; van Lieshout, J.J.; Arce-Esquivel, A.A.; Simmons, G.H.; Bender, S.B.; Padilla, J.; Bache, R.J.; Merkus, D.; et al. Peripheral Circulation. Compr. Physiol. 2012, 2, 321–447. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.; Kalliokoski, K.K.; Hannukainen, J.C.; Duncker, D.J.; Nuutila, P.; Knuuti, J. Organ-Specific Physiological Responses to Acute Physical Exercise and Long-Term Training in Humans. Physiology 2014, 29, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, G.; Abu Shaphe, M.; Khan, A.R.; Malhotra, D.; Khan, H.; Parveen, S.; Qasheesh, M.; Beg, R.A.; Chahal, A.; Ahmad, F.; et al. Effect of Exercises on Central and Endocrine System for Pain Modulation in Primary Dysmenorrhea. J. Lifestyle Med. 2022, 12, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chiu, P.C.; Ho, C.H. The Sprint-Interval Exercise Using a Spinning Bike Improves Physical Fitness and Ameliorates Primary Dysmenorrhea Symptoms through Hormone and Inflammation Modulations: A Randomized Controlled Trial. J. Sports Sci. Med. 2022, 21, 595–607. [Google Scholar] [CrossRef]
- Halliwill, J.R.; Buck, T.M.; Lacewell, A.N.; Romero, S.A. Postexercise hypotension and sustained postexercise vasodilatation: What happens after we exercise? Exp. Physiol. 2013, 98, 7–18. [Google Scholar] [CrossRef]
- Pearce, E.; Jolly, K.; Jones, L.L.; Matthewman, G.; Zanganeh, A.; Daley, A. Exercise for premenstrual syndrome: A systematic review and meta-analysis of randomised controlled trials. BJGP Open 2020, 4, bjgpopen20X101032. [Google Scholar] [CrossRef]
- Abdulraheem, S.; Bondemark, L. Hawthorne effect reporting in orthodontic randomized controlled trials: Truth or myth? Blessing or curse? Eur. J. Orthod. 2018, 40, 475–479. [Google Scholar] [CrossRef]
- Cronbach, L.J.; Shavelson, R.J. My current thoughts on coefficient alpha and successor procedures. Educ. Psychol. Meas. 2004, 64, 391–418. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Adolphs, R.; Cameron, O.G.; Critchley, H.D.; Davenport, P.W.; Feinstein, J.S.; Feusner, J.D.; Garfinkel, S.N.; Lane, R.D.; Mehling, W.E.; et al. Interoception and Mental Health: A Roadmap. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 501–513. [Google Scholar] [CrossRef]
- Erden, A.; Altuğ, F.; Cavlak, U. Investigation of the Relationship between Body Awareness, Pain, Emotional Status, and Quality of Life among Healthy People. J. Kartal Train. J. Kartal Train. Res. Med. 2013, 24, 145–150. [Google Scholar] [CrossRef]
- Doğan, H.; Eroğlu, S.; Akbayrak, T. The effect of kinesio taping and lifestyle changes on pain, body awareness and quality of life in primary dysmenorrhea. Complement. Ther. Clin. Pract. 2020, 39, 101120. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Günebakan, Ö.; Acar, M. The effect of tele-yoga training in healthy women on menstrual symptoms, quality of life, anxiety-depression level, body awareness, and self-esteem during COVID-19 pandemic. Ir. J. Med. Sci. 2023, 192, 467–479. [Google Scholar] [CrossRef]
- Daubenmier, J.J. The relationship of yoga, body awareness, and body responsiveness to self-objectification and disordered eating. Psychol. Women Q. 2005, 29, 207–219. [Google Scholar] [CrossRef]
- Mehling, W.E.; Price, C.; Daubenmier, J.; Acree, M.; Bartmess, E.; Stewart, A. The Multidimensional Assessment of Interoceptive Awareness (MAIA). PLoS ONE 2012, 7, e48230. [Google Scholar] [CrossRef]
- PEDro Scale—PEDro. Available online: https://pedro.org.au/english/resources/pedro-scale/ (accessed on 10 January 2024).
| K-S * | LTfEoV **** | t-Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Group | n | D | p | Me ** | Mean | S. D. *** | F | p | t | p |
| Dysmenorrhea scores | CG | 35 | 0.094 | 0.200 | 2.550 | 2.499 | 1.336 | 0.253 | 0.616 | 0.754 | 0.453 |
| IG | 40 | 0.126 | 0.200 | 2.000 | 2.270 | 1.295 | |||||
| Age distribution (year) | CG | 38 | 0.101 | 0.200 | 34.000 | 33.605 | 6.258 | 0.000 | 0.997 | 0.521 | 0.604 |
| IG | 40 | 0.098 | 0.200 | 33.000 | 32.875 | 6.115 | |||||
| BMI | CG | 38 | 0.132 | 0.157 | 22.315 | 23.389 | 4.812 | 2.399 | 0.126 | 1.682 | 0.097 |
| IG | 40 | 0.115 | 0.200 | 20.964 | 21.763 | 3.675 | M-W ***** | ||||
| z | p | ||||||||||
| Weight (kg) | CG | 38 | 0.124 | 0.200 | 63.000 | 65.158 | 13.060 | 0.733 | 0.395 | −1.901 | 0.057 |
| IG | 40 | 0.183 | 0.017 | 55.500 | 59.525 | 10.505 | |||||
| Duration of the first menstrual cycle (day) | CG | 38 | 0.264 | <0.001 | 28.000 | 30.500 | 9.058 | 1.916 | 0.170 | 1.394 | 0.167 |
| IG | 40 | 0.171 | 0.035 | 28.000 | 28.350 | 3.541 | |||||
| Duration of the second menstrual cycle (day) | CG | 37 | 0.230 | <0.001 | 29.000 | 31.243 | 6.238 | 3.956 | 0.050 | −1.038 | 0.299 |
| IG | 38 | 0.158 | 0.071 | 29.000 | 29.237 | 4.365 | |||||
| Age at onset of menstruation (year) | CG | 35 | 0.171 | 0.015 | 13.000 | 12.829 | 1.445 | 0.450 | 0.505 | −1.032 | 0.302 |
| IG | 35 | 0.199 | 0.006 | 13.000 | 13.171 | 1.317 | |||||
| Age at the first dysmenorrhea (year) | CG | 33 | 0.208 | 0.001 | 14.000 | 15.515 | 5.292 | 0.205 | 0.652 | −1.017 | 0.309 |
| IG | 29 | 0.247 | <0.001 | 15.000 | 15.966 | 4.040 | |||||
| Number of births | CG | 35 | 0.485 | <0.001 | 0.000 | 0.257 | 0.561 | 0.799 | 0.375 | −1.256 | 0.209 |
| IG | 35 | 0.539 | <0.001 | 0.000 | 0.171 | 0.618 | |||||
| Source | Type III Sum of Squares | df | Mean Square | F | p | Partial Eta Squared |
|---|---|---|---|---|---|---|
| Menst. Pain CHG a | 0.029 | 1.607 | 0.018 | 0.027 | 0.951 | <0.001 |
| Menst. Pain CHG × Age | 0.270 | 1.607 | 0.168 | 0.257 | 0.725 | 0.003 |
| Menst. Pain CHG × BMI | 0.730 | 1.607 | 0.454 | 0.694 | 0.471 | 0.009 |
| Menst. Pain CHG × Aviva b | 13.393 | 1.607 | 8.335 | 12.743 | <0.001 | 0.147 |
| Error (Menst. Pain CHG) | 77.771 | 118.907 | 0.654 |
| ToM * | CG | IG | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD ** | n | Mean | SD | |
| T1 | 35 | 2.43 | 1.325298 | 40 | 2.33 | 1.291325 |
| T2 | 35 | 2.42 | 1.498123 | 40 | 2.01 | 1.351697 |
| T3 | 35 | 3.25 | 1.649221 | 40 | 1.00 | 0.734987 |
| Type III | |||||||
|---|---|---|---|---|---|---|---|
| Scale | Source | Sum of Squares | df | Mean Square | F | p | Partial Eta Squared |
| Note responses or changes in body process | BAQ-H | 0.331 | 1.823 | 0.182 | 0.824 | 0.431 | 0.011 |
| BAQ-H × Age | 0.899 | 1.823 | 0.493 | 2.235 | 0.116 | 0.031 | |
| BAQ-H × BMI | 0.338 | 1.823 | 0.186 | 0.841 | 0.424 | 0.012 | |
| BAQ-H × AVIVA | 1.033 | 1.823 | 0.567 | 2.569 | 0.086 | 0.035 | |
| Error (BAQ-H) | 28.557 | 129.401 | 0.221 | ||||
| Predict of bodily reaction | BAQ-H | 0.024 | 1.845 | 0.013 | 0.064 | 0.926 | 0.001 |
| BAQ-H × Age | 0.549 | 1.845 | 0.298 | 1.488 | 0.230 | 0.021 | |
| BAQ-H × BMI | 1.654 | 1.845 | 0.897 | 4.483 | 0.015 | 0.059 | |
| BAQ-H × AVIVA | 0.339 | 1.845 | 0.184 | 0.920 | 0.394 | 0.013 | |
| Error (BAQ-H) | 26.193 | 130.970 | 0.200 | ||||
| Sleep-wake cycle | BAQ-H | 0.350 | 1.919 | 0.182 | 1.329 | 0.268 | 0.018 |
| BAQ-H × Age | 0.002 | 1.919 | 0.001 | 0.008 | 0.991 | 0.000 | |
| BAQ-H × BMI | 1.143 | 1.919 | 0.595 | 4.340 | 0.016 | 0.058 | |
| BAQ-H × AVIVA | 0.573 | 1.919 | 0.299 | 2.177 | 0.119 | 0.030 | |
| Error (BAQ-H) | 18.698 | 136.274 | 0.137 | ||||
| Onset of illness | BAQ-H | 0.174 | 1.692 | 0.103 | 0.289 | 0.712 | 0.004 |
| BAQ-H × Age | 0.262 | 1.692 | 0.155 | 0.434 | 0.615 | 0.006 | |
| BAQ-H × BMI | 0.162 | 1.692 | 0.096 | 0.268 | 0.728 | 0.004 | |
| BAQ-H × AVIVA | 0.665 | 1.692 | 0.393 | 1.104 | 0.327 | 0.015 | |
| Error (BAQ-H) | 42.788 | 120.102 | 0.356 |
| Note Responses or Changes in Body Process ** | Predict of Bodily Reaction | Sleep-Wake Cycle | Onset of Illness | |||||
|---|---|---|---|---|---|---|---|---|
| ToM * | CG | IG | CG | IG | CG | IG | CG | IG |
| T1 | 4.98 | 5.10 | 5.11 | 5.09 | 5.29 | 5.23 | 5.11 | 5.09 |
| T2 | 5.19 | 5.35 | 5.20 | 5.33 | 5.40 | 5.41 | 5.20 | 5.33 |
| T3 | 5.20 | 5.60 | 5.26 | 5.53 | 5.40 | 5.60 | 5.26 | 5.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, Z.; Atombosiye, E.; Hegyi, G.; Szőke, H. The Effect of Aviva Exercise Intervention on Pain Level and Body Awareness in Women with Primary Dysmenorrhea. Medicina 2024, 60, 184. https://doi.org/10.3390/medicina60010184
Kovács Z, Atombosiye E, Hegyi G, Szőke H. The Effect of Aviva Exercise Intervention on Pain Level and Body Awareness in Women with Primary Dysmenorrhea. Medicina. 2024; 60(1):184. https://doi.org/10.3390/medicina60010184
Chicago/Turabian StyleKovács, Zoltán, Ekine Atombosiye, Gabriella Hegyi, and Henrik Szőke. 2024. "The Effect of Aviva Exercise Intervention on Pain Level and Body Awareness in Women with Primary Dysmenorrhea" Medicina 60, no. 1: 184. https://doi.org/10.3390/medicina60010184
APA StyleKovács, Z., Atombosiye, E., Hegyi, G., & Szőke, H. (2024). The Effect of Aviva Exercise Intervention on Pain Level and Body Awareness in Women with Primary Dysmenorrhea. Medicina, 60(1), 184. https://doi.org/10.3390/medicina60010184






