Gender Predicts Differences in Acute Ischemic Cardioembolic Stroke Profile: Emphasis on Woman-Specific Clinical Data and Early Outcome—The Experience of Sagrat Cor Hospital of Barcelona Stroke Registry
Abstract
1. Introduction
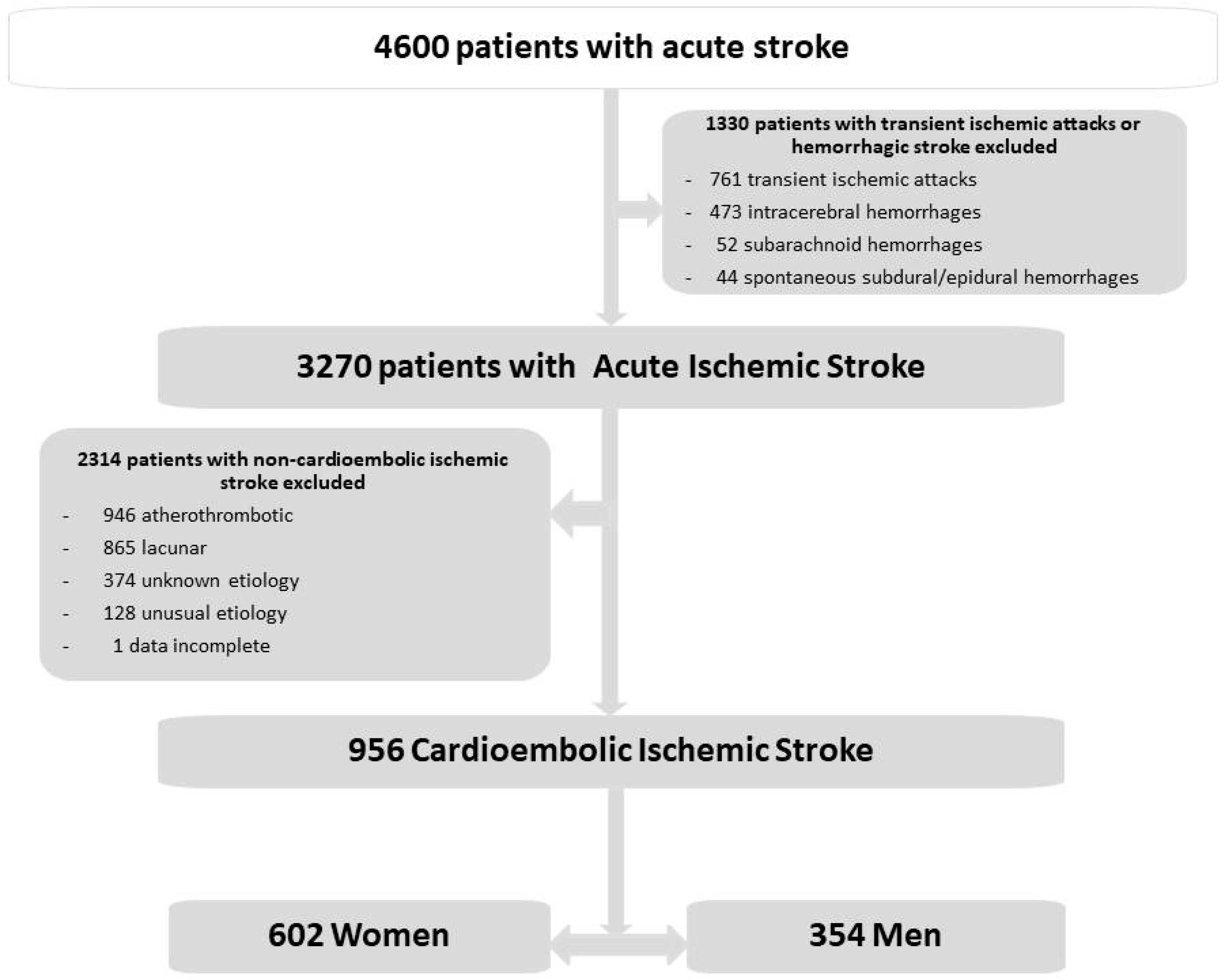
2. Materials and Methods
Statistical Analysis
3. Results
3.1. General Data
3.2. Differences between Cardioembolic Cerebral Infarct in Women and in Men
3.2.1. Univariate Analysis
3.2.2. Multivariate Analysis
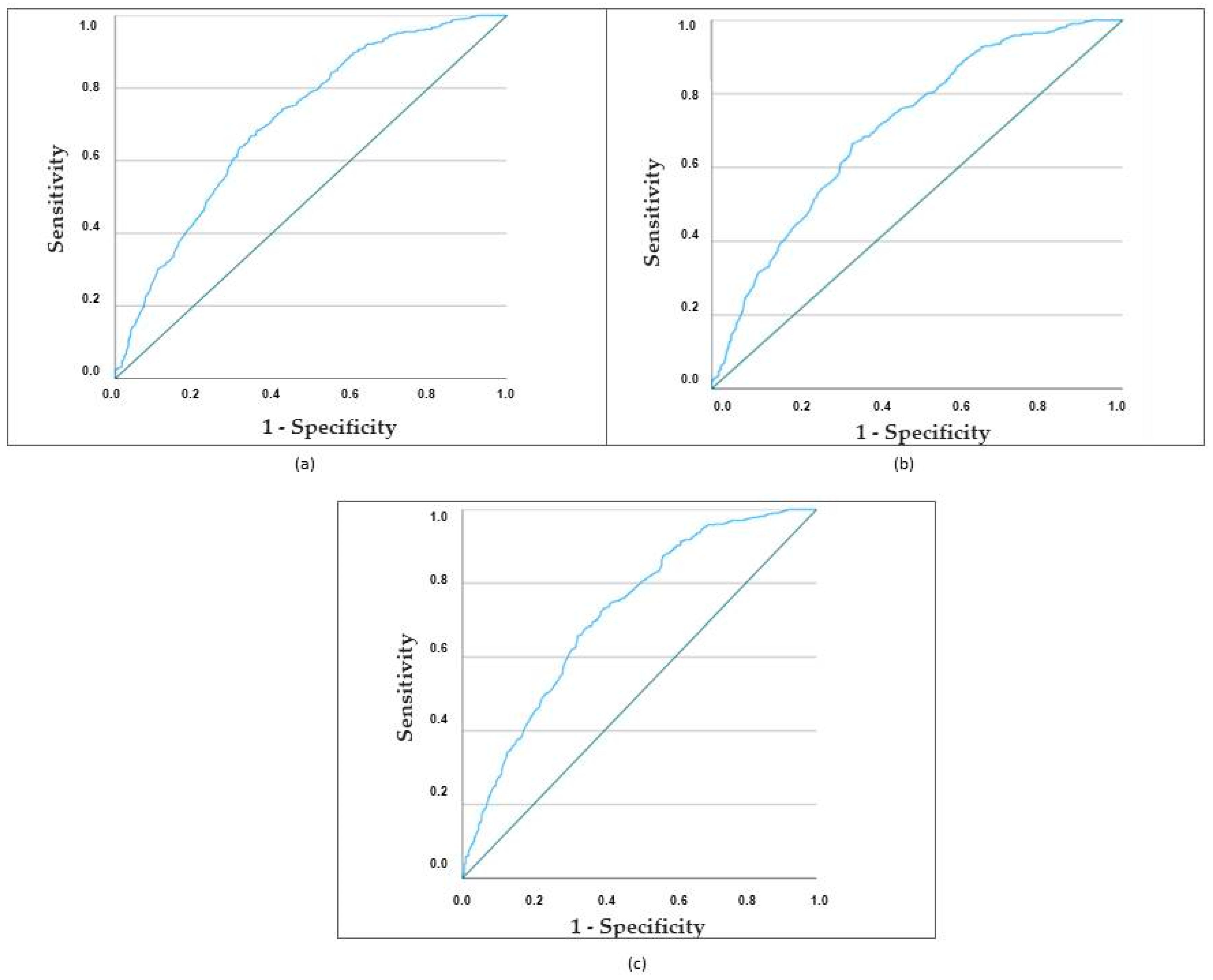
3.3. Predictors of In-Hospital Mortality in Ischemic Cardioembolic Stroke in Women
3.3.1. Univariate Analysis
3.3.2. Multivariate Analysis
4. Discussion
4.1. General Considerations
4.2. Biomarkers and Molecular Biological Features
4.3. Cardioembolic Cerebral Infarct in Women and in Men: Differences and Outcome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrera, E.; Maeder-Ingvar, M.; Rossetti, A.O.; Devuyst, G.; Bogousslavsky, J. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc. Dis. 2007, 24, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Paulus, J.K.; Lai, L.Y.; Lundquist, C.; Daneshmand, A.; Buettner, H.; Lutz, J.S.; Raman, G.; Wessler, B.S.; Kent, D.M. Field Synopsis of the Role of Sex in Stroke Prediction Models. J. Am. Heart Assoc. 2016, 5, e002809. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Castro, E.; Rodríguez-Yáñez, M.; Arias, S.; Santamaría, M.; López-Dequidt, I.; López-Loureiro, I.; Rodríguez-Pérez, M.; Hervella, P.; Sobrino, T.; Campos, F.; et al. Influence of Sex on Stroke Prognosis: A Demographic, Clinical, and Molecular Analysis. Front. Neurol. 2019, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- The European Stroke Organization (ESO) Executive Committee and the ESO Writing Committee. ESO Guidelines for management of ischaemic stroke 2008. Cerebrovasc. Dis. 2008, 25, 457–507. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.D. Epidemiology of stroke recurrence. Cerebrovasc. Dis. 1997, 7 (Suppl. S1), 2–4. [Google Scholar] [CrossRef]
- Gasull, T.; Arboix, A. Molecular mechanisms and pathophysiology of acute stroke: Emphasis on biomarkers in the different stroke subtypes. Int. J. Mol. Sci. 2022, 23, 9476. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. Potential Biomarkers for Post-Stroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 602. [Google Scholar] [CrossRef] [PubMed]
- Cullell, N.; Gallego-Fábrega, C.; Cárcel-Márquez, J.; Muiño, E.; Llucià-Carol, L.; Lledós, M.; Martín-Campos, J.M.; Molina, J.; Casas, L.; Almería, M.; et al. ICA1L is associated with small vessel disease: A proteome-wide association study in small vessel stroke and intracerebral haemorrhage. Int. J. Mol. Sci. 2022, 23, 3161. [Google Scholar] [CrossRef]
- Wang, W.X.; Springer, J.E.; Hatton, K.W. MicroRNAs as Biomarkers for Predicting Complications Following Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2021, 22, 9492. [Google Scholar] [CrossRef]
- Giralt-Steinhauer, E.; Jiménez-Baladó, J.; Fernández-Pérez, I.; Rey, L.A.; Rodríguez-Campello, A.; Ois, A.; Cuadrado-Godia, E.; Jiménez-Conde, J.; Roquer, J. Genetics and epigenetics of spontaneous intracerebral hemorrhage. Int. J. Mol. Sci. 2022, 23, 6479. [Google Scholar] [CrossRef]
- Ferro, J.M. Cardioembolic stroke: An update. Lancet Neurol. 2003, 2, 177–188. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, N.J.J.; Amarasinghe, S.; Muir, K.W. Secondary prevention of stroke. Expert. Rev. Neurother. 2009, 7, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.W.; Lip, G.Y.H. Clinical outcomes of acute stroke patients with atrial fibrillation. Expert Rev. Cardiovasc. Ther. 2009, 7, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Weir, N.U. An update on cardioembolic stroke. Postgrad. Med. J. 2008, 84, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M. Brain embolism. Answers to practical questions. J. Neurol. 2003, 250, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Abott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Massons, J.; Oliveres, M.; García, L.; Titus, F. An analysis of 1000 consecutive patients with acute cerebrovascular disease. The registry of cerebrovascular disease of La Alianza-Hospital Central of Barcelona. Med. Clin. 1993, 101, 281–285. [Google Scholar]
- Bogousslavsky, J.; Van Melle, G.; Regli, F. The Lausanne Registry: Analysis of 1000 consecutive patients with first stroke. Stroke 1988, 19, 1083–1092. [Google Scholar] [CrossRef]
- Timsit, S.G.; Sacco, R.L.; Mohr, J.P.; Foulkes, M.A.; Tatemichi, T.K.; Wolf, P.A.; Price, T.R.; Hier, D.B. Brain infarction severity differs according to cardiac or arterial embolic source. Neurology 1993, 43, 728–733. [Google Scholar] [CrossRef]
- Vázquez, J.; Gendre, J.; Martí-Vilalta, J.L. Manifestaciones clínicas del infarto cerebral embólico de origen cardíaco. In Isquemia Cerebral; Matías-Guiu, J., Martínez-Vila, E., Martí-Vilalta, J.L., Eds.; MCR: Barcelona, Spain, 1990; pp. 185–202. [Google Scholar]
- Al-Rajeh, S.; Larbi, E.; Bademosi, O.; Awada, A.; Ismail, H.; al-Freihi, H.; al-Ghassab, G. Stroke in a tertiary hospital in Saudi Arabia: A study of 372 cases. Eur. Neurol. 1991, 31, 251–256. [Google Scholar] [CrossRef]
- Rothrock, J.F.; Lyden, P.D.; Brody, M.L.; Taft-Alvarez, B.; Kelly, N.; Mayer, J.; Wiederholt, W.C. An analysis of ischemic stroke in an urban southern California population. The University of California, San Diego, Stroke Data Bank. Arch. Intern. Med. 1993, 153, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Norrving, B.; Löwenhielm, P. Epidemiology of first stroke in Lund-Orup, Sweden, 1983–1985. Incidence of first stroke and aged-related changes in subtypes. Acta Neurol. Scand. 1988, 78, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Vericat, M.C.; Pujades, R.; Massons, J.; García-Eroles, L.; Oliveres, M. Cardioembolic infarction in the Sagrat Cor-Alianza Hospital of Barcelona Stroke Registry. Acta Neurol. Scand. 1997, 96, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G.; Duffis, E.J.; Fisher, M. Cardiac workup of ischemic stroke. Can we improve our diagnostic yield? Stroke 2009, 40, 2893–2898. [Google Scholar] [CrossRef] [PubMed]
- Doufekias, E.; Segal, A.Z.; Kizer, J.R. Cardiogenic and aortogenic brain embolism. J. Am. Coll. Cardiol. 2008, 51, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.R. Brain embolism, revisited. Neurology 1993, 43, 1281–1287. [Google Scholar] [CrossRef]
- Chamorro, A.; Vila, N.; Saiz, A.; Alday, M.; Tolosa, E. Early anticoagulation after large cerebral embolic infarction: A safety study. Neurology 1995, 45, 861–865. [Google Scholar] [CrossRef]
- Ustrell, X.; Pellisé, A. Cardiac workup of ischemic stroke. Curr. Cardiol. Rev. 2010, 6, 175–183. [Google Scholar] [CrossRef]
- Dylla, L.; Higgins, H.M.; Piper, C.; Poisson, S.N.; Herson, P.S.; Monte, A.A. Sex as a biological variable in determining the metabolic changes influencing acute ischemic stroke outcomes—Where is the data: A systematic review. Front. Neurol. 2022, 13, 1026431. [Google Scholar] [CrossRef]
- Arboix, A.; Alvarez-Sabin, J.; Soler, L. Stroke. Classification and diagnostic criteria. Ad hoc Editorial Committee of the Task Force on Cerebrovascular Diseases of SEN. Neurologia 1998, 13 (Suppl. S3), S3–S10. [Google Scholar]
- Special Report from the National Institute of Neurological Disorders and Stroke: Classification of cerebrovascular diseases III. Stroke 1990, 21, 637–676. [CrossRef] [PubMed]
- Silver, F.L.; Norris, J.W.; Lewis, A.J.; Hachinski, V.C. Early mortality following stroke: A prospective review. Stroke 1984, 15, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Bamford, J.M.; Sandercock, P.A.; Warlow, C.P.; Slattery, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989, 20, 828. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemershow, S. Applied Logistic Regression; John Wiley & Sons: New York, NY, USA, 1989; pp. 25–37. [Google Scholar]
- Hosmer, D.W.; Lemershow, S. Goodness of fit tests for the multiple logistic regression model. Commun. Stat. 1980, A9, 1043–1069. [Google Scholar] [CrossRef]
- Norusis, M.J. SPSS Advances Statistics Student Guide; SPSS: Chicago, IL, USA, 1990. [Google Scholar]
- Dixon, W.J. BMDP Statistical Software Manual; University of California Press: Berkeley, CA, USA, 1981; pp. 330–344. [Google Scholar]
- Hart, R.G. Cardiogenic embolism to the brain. Lancet 1992, 339, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Caplan, L.R. Clinical diagnosis of brain embolism. Cerebrovasc. Dis. 1995, 5, 79–88. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Olindo, S.; Cabre, P.; Deschamps, R.; Chatot-Henry, C.; Rene-Corail, P.; Fournerie, P.; Saint-Vil, M.; May, F.; Smadja, D. Acute stroke in the very elderly. Epidemiological features, stroke subtypes, management, and outcome in Martinique, French West Indies. Stroke 2003, 34, 1593–1597. [Google Scholar] [CrossRef]
- Luy, M.; Gast, K. Do women live longer or do men die earlier? Reflections on the causes of sex differences in life expectancy. Gerontology 2014, 60, 143–153. [Google Scholar] [CrossRef]
- Kobayashi, L.C.; Beeken, R.J.; Meisel, S.F. Biopsychosocial predictors of perceived life expectancy in a national sample of older men and women. PLoS ONE 2017, 12, e0189245. [Google Scholar] [CrossRef]
- Gallego-Fabrega, C.; Muiño, E.; Cullell, N.; Cárcel-Márquez, J.; Lazcano, U.; Soriano-Tárraga, C.; Lledós, M.; Llucià-Carol, L.; Aguilera-Simón, A.; Marín, R.; et al. Biological Age Acceleration Is Lower in Women with Ischemic Stroke Compared to Men. Stroke 2022, 53, 2320–2330. [Google Scholar] [CrossRef] [PubMed]
- Eaker, E.D.; Chesebro, J.H.; Sacks, F.M.; Wenger, N.K.; Whisnant, J.P.; Winston, M. Cardiovascular disease in women. Circulation 1993, 88, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Bousser, M.G. Stroke in women. Circulation 1999, 99, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.W.; Giuliani, M.J.; Haley, E.C., Jr. Cerebrovascular disease and stroke in women. Cardiology 1990, 77 (Suppl. S2), 80–90. [Google Scholar] [CrossRef]
- Tansey, M.J.B.; Opie, L.H.; Kenelly, B.M. High mortality in obese women diabetics with acute myocardial infarction. BMJ 1977, 1, 1624–1626. [Google Scholar] [CrossRef][Green Version]
- Roquer, J.; Rodríguez, A.C.; Gomis, M. Sex differences in first-ever acute stroke. Stroke 2003, 34, 1581–1585. [Google Scholar] [CrossRef]
- Ayala, C.; Croft, J.B.; Greenlund, K.J.; Keenan, N.L.; Donehoo, R.S.; Malarcher, A.M.; Mensah, G.A. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995 to 1998. Stroke 2002, 33, 1197–1201. [Google Scholar] [CrossRef]
- Ho, J.E.; Paultre, F.; Mosca, L. Is diabetes mellitus a cardiovascular disease risk equivalent for fatal stroke in women? Data from the Women’s Pooling Project. Stroke 2003, 34, 2812–2816. [Google Scholar] [CrossRef]
- Soriano-Reixach, M.M.; Vivanco Hidalgo, R.M.; Ois, A.; Rodríguez Campello, A.; Roquer, J. Interaction of Sex and Diabetes on Outcome After Ischemic Stroke. Front. Neurol. 2018, 9, 250. [Google Scholar] [CrossRef]
- Gaillard, N.; Deltour, S.; Vilotijevic, B.; Hornych, A.; Crozier, S.; Leger, A.; Frank, R.; Samson, Y. Detection of paroxysmal atrial fibrillation with transtelephonic EKG in TIA or stroke patients. Neurology 2010, 74, 1666–1670. [Google Scholar] [CrossRef]
- Serena, J.; Jiménez-Nieto, M.; Silva, Y.; Castellanos, M. Patent foramen ovale in cerebral infarction. Current. Cardiol. Rev. 2010, 6, 162–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Latum, J.C.; Koudstaal, P.J.; Venables, G.S.; van Gijn, J.; Kappelle, L.J.; Algra, A. Predictors of major vascular events in patients with a transient ischemic attack or minor ischemic stroke and with nonrheumatic atrial fibrillation. Stroke 1995, 26, 801–806. [Google Scholar] [CrossRef]
- Andersen, K.K.; Andersen, Z.J.; Olsen, T.S. Age- and gender-specific prevalence of cardiovascular risk factors in 40,102 patients with first-ever ischemic stroke: A Nationwide Danish Study. Stroke 2010, 41, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Tseng, M.C.; Weng, H.H.; Lin, Y.H.; Liou, C.W.; Tan, T.Y. Prediction of length of stay of first-ever ischemic stroke. Stroke 2002, 33, 2670–2674. [Google Scholar] [CrossRef] [PubMed]
- Bejot, Y.; Catteau, A.; Caillier, M.; Rouaud, O.; Durier, J.; Marie, C.; Di Carlo, A.; Osseby, G.V.; Moreau, T.; Giroud, M. Trends in incidence, risk factors, and survival in symptomatic lacunar stroke in Dijon, France, from 1989 to 2006: A population-based study. Stroke 2008, 39, 1945–1951. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rudilosso, S.; Rodríguez-Vázquez, A.; Urra, X.; Arboix, A. The Potential Impact of Neuroimaging and Translational Research on the Clinical Management of Lacunar Stroke. Int. J. Mol. Sci. 2022, 23, 1497. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Marini, S.; Pera, J.; Norrving, B.; Jimenez-Conde, J.; Roquer, J.; Fernandez-Cadenas, I.; Tirschwell, D.L.; Selim, M.; Brown, D.L.; et al. Genome-Wide Association Study of Cerebral Small Vessel Disease Reveals Established and Novel Loci. Brain 2019, 142, 3176–3189. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Åsberg, S.; Sunnerhagen, K.S.; von Euler, M. Sex Differences in Stroke Care and Outcome 2005–2018: Observations From the Swedish Stroke Register. Stroke 2021, 52, 3233–3242. [Google Scholar] [CrossRef]
- Kumar, S.; Selim, M.; Caplan, L. Medical complications after stroke. Lancet. Neurol. 2010, 9, 105–118. [Google Scholar] [CrossRef]
- Tu, J.V.; Gong, Y. Trends in treatment and outcomes for acute stroke patients in Ontario, 1992–1998. Arch. Intern. Med. 2003, 163, 293–297. [Google Scholar] [CrossRef][Green Version]
- Junttola, U.; Lahtinen, S.; Liisanantti, J.; Vakkala, M.; Kaakinen, T.; Isokangas, J.M. Medical complications and outcome after endovascular therapy for acute ischemic stroke. Acta Neurol. Scand. 2021, 144, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.H.; Ebaid, D.; Kramer, S.; Werden, E.; Baxter, H.; Campbell, B.C.; Brodtmann, A. Pre-stroke physical activity and admission stroke severity: A systematic review. Int. J. Stroke 2021, 16, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; De Angelis, M.V.; Montemitro, C.; Russo, M.; Carrini, C.; di Giannantonio, M.; Brighina, F.; Onofrj, M.; Werring, D.J.; Simister, R. Clinical presentation of strokes confined to the insula: A systematic review of literature. Neurol. Sci. 2021, 42, 1697–1704. [Google Scholar] [CrossRef]
- Wise, F.M.; Harris, D.W.; Olver, J.H.; Davis, S.M.; Disler, P.B. Acute Predictors of Social Integration Following Mild Stroke. J. Stroke Cerebrovasc. Dis. 2018, 27, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Leventis, I.; Perlepe, K.; Sagris, D.; Sirimarco, G.; Strambo, D.; Georgiopoulos, G.; Eskandari, A.; Karagkiozi, E.; Vemmou, A.; Koroboki, E.; et al. Characteristics and outcomes of Embolic Stroke of Undetermined Source according to stroke severity. Int. J. Stroke 2020, 15, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, B.; Smalling, R.W. Cardioembolic stroke. Curr. Atherosclr. Rep. 2006, 8, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hornig, C.R.; Brainin, M.; Mast, H. Cardioembolic stroke: Results from three current stroke data banks. Neuroepidemiology 1994, 13, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Egashira, S.; Koga, M.; Toyoda, K. Intravenous Thrombolysis for Acute Ischemic Stroke in Patients with End-Stage Renal Disease on Hemodialysis: A Narrative Review. J. Cardiovasc. Dev. Dis. 2022, 9, 446. [Google Scholar] [CrossRef]
- Karelis, G.; Micule, M.; Klavina, E.; Haritoncenko, I.; Kikule, I.; Tilgale, B.; Polaka, I. The Riga East University Hospital Stroke Registry-An Analysis of 4915 Consecutive Patients with Acute Stroke. Medicina 2021, 57, 632. [Google Scholar] [CrossRef]
- Arboix, A.; Massons, J.; García-Eroles, L.; Targa, C.; Parra, O.; Oliveres, M. Trends in clinical features and early outcome in patients with acute cardioembolic stroke subtype over a 19-year period. Neurol. India 2012, 60, 288–293. [Google Scholar] [CrossRef]
- Grau-Olivares, M.; Arboix, A.; Junqué, C.; Arenaza-Urquijo, E.M.; Rovira, M.; Bartrés-Faz, D. Progressive gray matter atrophy in lacunar patients with vascular mild cognitive impairment. Cerebrovasc. Dis. 2010, 30, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; López-Grau, M.; Casasnovas, C.; García-Eroles, L.; Massons, J.; Balcells, M. Clinical study of 39 patients with atypical lacunar syndrome. J. Neurol. Neurosurg. Psychiatry 2006, 77, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Rexrode, K.M.; Madsen, T.E.; Yu, A.Y.X.; Carcel, C.; Lichtman, J.H.; Miller, E.C. The Impact of Sex and Gender on Stroke. Circ. Res. 2022, 130, 512–528. [Google Scholar] [CrossRef] [PubMed]

| Variables | Men (n = 354) | Women (n = 602) | p Value |
|---|---|---|---|
| Cerebrovascular risk factors | |||
| Hypertension | 173 (48.9) | 340 (56.5) | 0.023 |
| Diabetes mellitus | 71 (20.1) | 106 (17.6) | 0.347 |
| Valvular heart disease | 40 (11.3) | 117 (19.4) | 0.001 |
| Ischemic heart disease | 108 (30.5) | 91 (15.1) | 0.000 |
| Atrial fibrillation | 248 (70.1) | 462 (76.7) | 0.022 |
| Heart failure | 27 (7.6) | 73 (12.1) | 0.028 |
| Previous ischemic stroke | 74 (20.9) | 100 (16.6) | 0.097 |
| Chronic obstructive pulmonary disease | 50 (14.1) | 34 (5.6) | 0.000 |
| Peripheral vascular disease | 31 (8.8) | 37 (6.1) | 0.129 |
| Obesity | 6 (1.7) | 22 (3.7) | 0.083 |
| Alcohol abuse (>80 gr/day) | 10 (2.8) | 0 (0.0) | 0.000 |
| Anticoagulants | 36 (10.2) | 69 (11.5) | 0.537 |
| Heavy smoking (>20 cigarettes/day) | 38 (10.7) | 4 (0.7) | 0.000 |
| Hyperlipidemia | 59 (16.7) | 71 (11.8) | 0.034 |
| Clinical findings | |||
| Sudden onset | 234 (66.1) | 390 (64.8) | 0.679 |
| Acute onset (hours) | 68 (19.2) | 133 (22.1) | 0.291 |
| Subacute onset (>24 h) | 24 (6.8) | 27 (4.5) | 0.127 |
| Headache | 32 (9.0) | 41 (6.8) | 0.210 |
| Vertigo | 12 (3.4) | 15 (2.5) | 0.418 |
| Seizures | 5 (1.4) | 12 (2.0) | 0.512 |
| Nausea, vomiting | 29 (8.2) | 33 (5.5) | 0.100 |
| Altered consciousness | 92 (26.0) | 172 (28.6) | 0.388 |
| Limb weakness | 266 (75.1) | 495 (82.2) | 0.009 |
| Sensory deficit | 123 (34.7) | 223 (37.0) | 0.475 |
| Hemianopia | 74 (20.9) | 128 (21.3) | 0.896 |
| Speech disorders (dysarthria, aphasia) | 206 (58.2) | 383 (63.6) | 0.096 |
| Extrapyramidal symptoms | 7 (2.0) | 10 (1.7) | 0.721 |
| Neuroimaging finding topography | |||
| Frontal lobe | 74 (20.9) | 139 (23.1) | 0.433 |
| Parietal lobe | 116 (32.8) | 235 (39.0) | 0.052 |
| Temporal lobe | 139 (39.3) | 246 (40.9) | 0.627 |
| Occipital lobe | 43 (12.1) | 70 (11.6) | 0.810 |
| Internal capsule | 36 (10.2) | 95 (15.8) | 0.015 |
| Thalamus | 12 (3.4) | 23 (3.8) | 0.732 |
| Basal ganglia | 44 (12.4) | 96 (15.9) | 0.137 |
| Centrum semiovale | 7 (2.0) | 5 (0.8) | 0.216 |
| Anterior cerebral artery | 20 (5.6) | 25 (4.2) | 0.291 |
| Middle cerebral artery | 219 (61.9) | 401 (66.6) | 0.138 |
| Posterior cerebral artery | 30 (8.5) | 47 (7.8) | 0.714 |
| Basilar artery | 10 (2.8) | 13 (2.2) | 0.517 |
| Vertebral artery | 13 (3.7) | 6 (1.0) | 0.004 |
| Outcome | |||
| Neurological complications | 44 (12.4) | 82 (13.6) | 0.599 |
| Respiratory complications | 51 (14.4) | 87 (14.5) | 0.985 |
| Gastrointestinal complications | 11 (3.1) | 17 (2.8) | 0.802 |
| Urinary tract complications | 33 (9.3) | 53 (8.8) | 0.787 |
| Cardiac complications | 33 (9.3) | 53 (8.8) | 0.787 |
| Vascular complications | 11 (3.1) | 16 (2.7) | 0.685 |
| Hemorrhagic complications | 11 (3.1) | 16 (2.7) | 0.685 |
| Infectious complications * | 57 (16.1) | 115 (19.1) | 0.243 |
| Symptom free at discharge | 57 (16.1) | 67 (11.1) | 0.027 |
| Poor outcome (mRS grades 4–5) | 43 (12.1) | 105 (17.4) | 0.029 |
| In-hospital death | 79 (22.3) | 139 (23.1) | 0.783 |
| Length of hospital stay—days, mean (SD) | 17.89 (14.88) | 18.21 (14.38) | 0.709 |
| Regression Models | Coefficient (β) | Standard Error | Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|
| First model: demographics and risk factors | ||||
| Age | 0.044 | 0.008 | 1.05 (1.03–1.06) | 0.000 |
| Hypertension | 0.372 | 0.146 | 1.45 (1.09–1.93) | 0.011 |
| Valvular heart disease | 0.790 | 0.216 | 2.20 (1.44–3.36) | 0.000 |
| Ischemic heart disease | −0.764 | 0.174 | 0.47 (0.33–0.66) | 0.000 |
| Chronic obstructive pulmonary disease | −1.004 | 0.254 | 0.37 (0.22–0.60) | 0.000 |
| Obesity | 1.213 | 0.528 | 3.36 (1.19–9.47) | 0.022 |
| Heavy smoking (>20 cigarettes/day) | −2.477 | 0.544 | 0.08 (0.03–0.24) | 0.000 |
| Second model: demographics, risk factors, and clinical features | ||||
| Age | 0.045 | 0.008 | 1.05 (1.03–1.06) | 0.000 |
| Hypertension | 0.369 | 0.146 | 1.45 (1.09–1.93) | 0.012 |
| Valvular heart disease | 0.771 | 0.217 | 2.16 (1.41–3.31) | 0.000 |
| Ischemic heart disease | −0.773 | 0.174 | 0.46 (0.33–0.65) | 0.000 |
| Chronic obstructive pulmonary disease | −1.019 | 0.254 | 0.36 (0.22–0.60) | 0.000 |
| Obesity | 1.184 | 0.530 | 3.27 (1.16–9.23) | 0.025 |
| Heavy smoking (>20 cigarettes/day) | −2.506 | 0.544 | 0.08 (0.03–0.24) | 0.000 |
| Subacute onset | −0.635 | 0.302 | 0.53 (0.29–0.96) | 0.036 |
| Third model: demographics, risk factors, clinical features, location, vascular topography, and complications | ||||
| Age | 0.045 | 0.008 | 1.05 (1.03–1.06) | 0.000 |
| Hypertension | 0.409 | 0.148 | 1.51 (1.13–2.01) | 0.006 |
| Valvular heart disease | 0.734 | 0.218 | 2.08 (1.36–3.20) | 0.001 |
| Ischemic heart disease | −0.823 | 0.176 | 0.44 (0.31–0.62) | 0.000 |
| Chronic obstructive pulmonary disease | −1.004 | 0.256 | 0.37 (0.22–0.61) | 0.000 |
| Obesity | 1.164 | 0.533 | 3.20 (1.13–9.10) | 0.029 |
| Heavy smoking (>20 cigarettes/day) | −2.540 | 0.549 | 0.08 (0.03–0.23) | 0.000 |
| Subacute onset | −0.630 | 0.306 | 0.53 (0.29–0.97) | 0.039 |
| Internal capsule | 0.502 | 0.229 | 1.65 (1.06–2.59) | 0.028 |
| Centrum semiovale | −1.226 | 0.610 | 0.29 (0.09–0.97) | 0.044 |
| Vertebral artery | −1.104 | 0.543 | 0.33 (0.11–0.96) | 0.042 |
| Variables | Alive (n = 463) | Dead (n = 139) | p |
|---|---|---|---|
| Demographics | |||
| Age—years, mean (SD) | 80.22 (9.21) | 84.71 (6.27) | 0.000 |
| ≥85 years old—no. (%) | 158 (34.1%) | 69 (49.6%) | 0.001 |
| Cerebrovascular risk factors | |||
| Hypertension | 270 (58.3) | 70 (50.4) | 0.097 |
| Diabetes mellitus | 84 (18.1) | 22 (15.8) | 0.530 |
| Valvular heart disease | 95 (20.5) | 22 (15.8) | 0.220 |
| Ischemic heart disease | 71 (15.3) | 20 (14.4) | 0.785 |
| Atrial fibrillation | 350 (75.6) | 112 (80.6) | 0.223 |
| Heart failure | 52 (11.2) | 21 (15.1) | 0.219 |
| Previous ischemic stroke | 68 (14.7) | 32 (23.0) | 0.021 |
| Chronic obstructive pulmonary disease | 22 (4.8) | 12 (8.6) | 0.082 |
| Peripheral vascular disease | 26 (5.6) | 11 (7.9) | 0.322 |
| Obesity | 18 (3.9) | 4 (2.9) | 0.578 |
| Heavy smoking (>20 cigarettes/day) | 3 (0.6) | 1 (0.7) | 1.000 |
| Hyperlipidemia | 60 (13.0) | 11 (7.9) | 0.106 |
| Clinical features | |||
| Sudden onset | 296 (63.9) | 94 (67.6) | 0.424 |
| Acute onset (hours) | 107 (23.1) | 26 (18.7) | 0.272 |
| Headache | 34 (7.3) | 7 (5.0) | 0.344 |
| Vertigo | 14 (3.0) | 1 (0.7) | 0.223 |
| Seizures | 5 (1.1) | 7 (5.0) | 0.010 |
| Nausea, vomiting | 23 (5.0) | 10 (7.2) | 0.312 |
| Altered consciousness | 83 (17.9) | 89 (64.0) | 0.000 |
| Limb weakness | 366 (79.0) | 129 (92.8) | 0.000 |
| Sensory deficits | 163 (35.2) | 60 (43.2) | 0.088 |
| Hemianopia | 95 (20.5) | 33 (23.7) | 0.415 |
| Speech disorders (aphasia, dysarthria) | 305 (65.9) | 78 (56.1) | 0.036 |
| Extrapyramidal symptoms | 10 (2.2) | 0 (0.0) | 0.171 |
| Brain topography | |||
| Frontal lobe | 101 (21.8) | 38 (27.3) | 0.175 |
| Parietal lobe | 161 (34.8) | 74 (53.2) | 0.000 |
| Temporal lobe | 174 (37.6) | 72 (51.8) | 0.003 |
| Occipital lobe | 50 (10.8) | 20 (14.4) | 0.247 |
| Internal capsule | 69 (14.9) | 26 (18.7) | 0.281 |
| Thalamus | 20 (4.3) | 3 (2.2) | 0.244 |
| Basal ganglia | 60 (13.0) | 36 (25.9) | 0.000 |
| Vascular topography | |||
| Anterior cerebral artery | 18 (3.9) | 7 (5.0) | 0.552 |
| Middle cerebral artery | 298 (64.4) | 103 (74.1) | 0.033 |
| Posterior cerebral artery | 41 (8.9) | 6 (4.3) | 0.080 |
| Basilar artery | 8 (1.7) | 5 (3.6) | 0.319 |
| Vertebral artery | 2 (0.4) | 4 (2.9) | 0.040 |
| Early outcome | |||
| Neurological complications | 28 (6.0) | 54 (38.8) | 0.000 |
| Respiratory complications | 35 (7.6) | 52 (37.4) | 0.000 |
| Gastrointestinal complications | 6 (1.3) | 11 (7.9) | 0.000 |
| Renal complications | 5 (1.1) | 6 (4.3) | 0.033 |
| Urinary tract complications | 42 (9.1) | 11 (7.9) | 0.673 |
| Cardiac complications | 23 (5.0) | 30 (21.6) | 0.000 |
| Vascular complications | 9 (1.9) | 7 (5.0) | 0.092 |
| Hemorrhagic complications | 9 (1.9) | 7 (5.0) | 0.092 |
| Infectious complications * | 66 (14.3) | 49 (35.3) | 0.000 |
| Regression Model | Coefficient (β) | Standard Error | Odds Ratio (95% Confidence Interval) | p Value |
|---|---|---|---|---|
| Model based on demographics and risk factors | ||||
| Age | 0.073 | 0.014 | 1.08 (1.05–1.08) | 0.000 |
| Previous ischemic stroke | 0.560 | 0.248 | 1.75 (1.08–2.85) | 0.024 |
| Model based on demographics, risk factors, and clinical features | ||||
| Age | 0.064 | 0.016 | 1.07 (1.03–1.10) | 0.000 |
| Early seizures | 1.818 | 0.704 | 6.16 (1.55–24.47) | 0.010 |
| Altered consciousness | 1.952 | 0.222 | 7.05 (4.56–10.90) | 0.000 |
| Limb weakness | 1.098 | 0.376 | 3.00 (1.44–6.26) | 0.003 |
| Model based on demographics, risk factors, clinical features, location, vascular topography, and complications | ||||
| Age | 0.060 | 0.019 | 1.06 (1.02–1.10) | 0.001 |
| Altered consciousness | 1.946 | 0.272 | 7.00 (4.11–11.92) | 0.000 |
| Limb weakness | 0.931 | 0.428 | 2.54 (1.10–5.87) | 0.030 |
| Neurological complications | 2.266 | 0.327 | 9.64 (5.08–18.30) | 0.000 |
| Respiratory complications | 1.879 | 0.316 | 6.55 (3.53–12.16) | 0.000 |
| Gastrointestinal complications | 1.415 | 0.673 | 4.12 (1.10–15.39) | 0.035 |
| Renal dysfunction | 2.020 | 0.907 | 7.54 (1.27–44.62) | 0.026 |
| Cardiac complications | 1.785 | 0.403 | 5.96 (2.71–13.12) | 0.000 |
| Vascular complications | 1.478 | 0.652 | 4.39 (1.22–15.73) | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inogés, M.; Arboix, A.; García-Eroles, L.; Sánchez-López, M.J. Gender Predicts Differences in Acute Ischemic Cardioembolic Stroke Profile: Emphasis on Woman-Specific Clinical Data and Early Outcome—The Experience of Sagrat Cor Hospital of Barcelona Stroke Registry. Medicina 2024, 60, 101. https://doi.org/10.3390/medicina60010101
Inogés M, Arboix A, García-Eroles L, Sánchez-López MJ. Gender Predicts Differences in Acute Ischemic Cardioembolic Stroke Profile: Emphasis on Woman-Specific Clinical Data and Early Outcome—The Experience of Sagrat Cor Hospital of Barcelona Stroke Registry. Medicina. 2024; 60(1):101. https://doi.org/10.3390/medicina60010101
Chicago/Turabian StyleInogés, Marc, Adrià Arboix, Luís García-Eroles, and María José Sánchez-López. 2024. "Gender Predicts Differences in Acute Ischemic Cardioembolic Stroke Profile: Emphasis on Woman-Specific Clinical Data and Early Outcome—The Experience of Sagrat Cor Hospital of Barcelona Stroke Registry" Medicina 60, no. 1: 101. https://doi.org/10.3390/medicina60010101
APA StyleInogés, M., Arboix, A., García-Eroles, L., & Sánchez-López, M. J. (2024). Gender Predicts Differences in Acute Ischemic Cardioembolic Stroke Profile: Emphasis on Woman-Specific Clinical Data and Early Outcome—The Experience of Sagrat Cor Hospital of Barcelona Stroke Registry. Medicina, 60(1), 101. https://doi.org/10.3390/medicina60010101







