Lateral Peri-Implantitis: Successful Management via Guided Bone Regeneration at Mandibular First Molar Implant
Abstract
:1. Introduction
2. Case Presentation
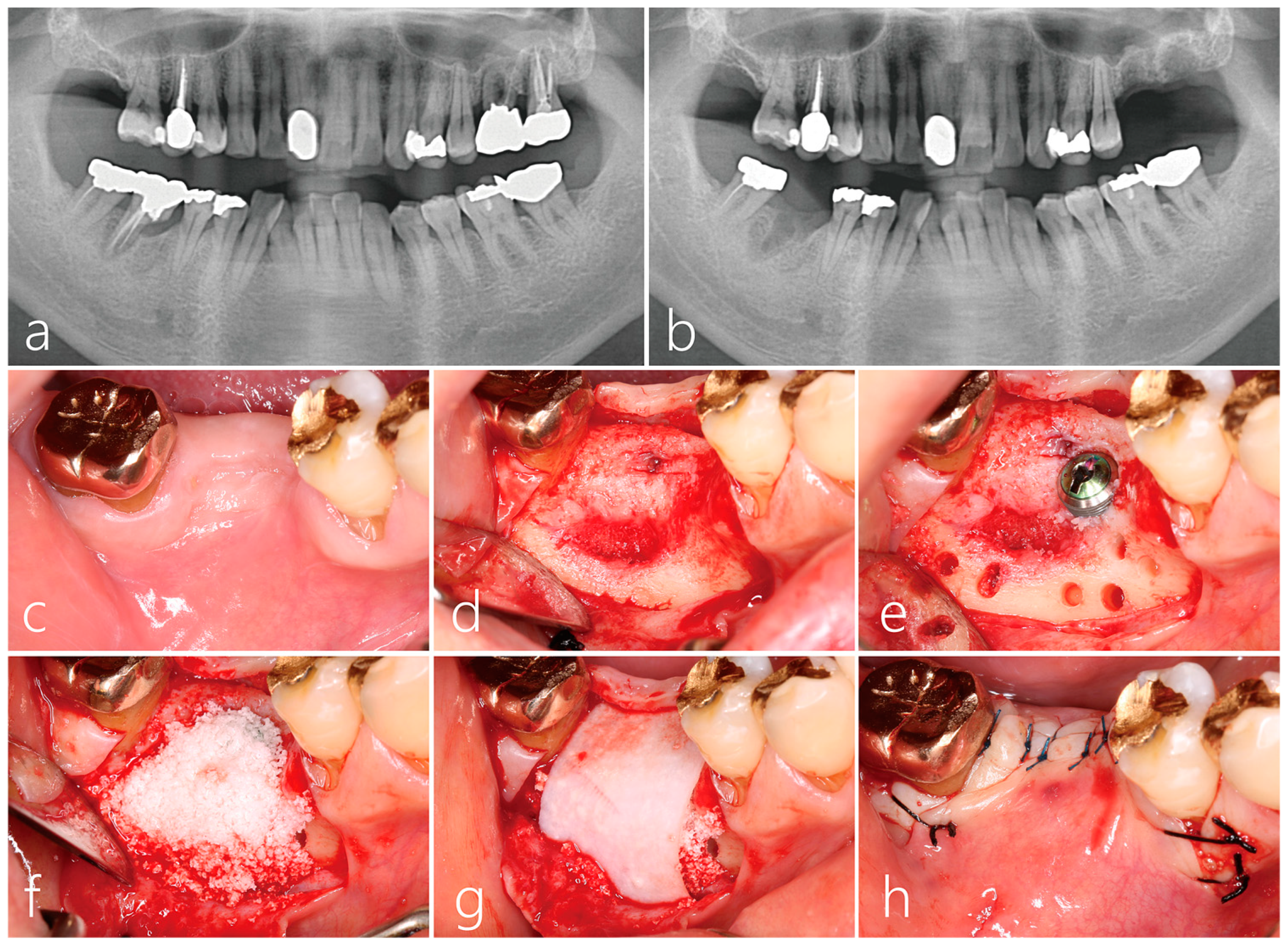
2.1. Implant Placement and Lateral Bone Augmentation
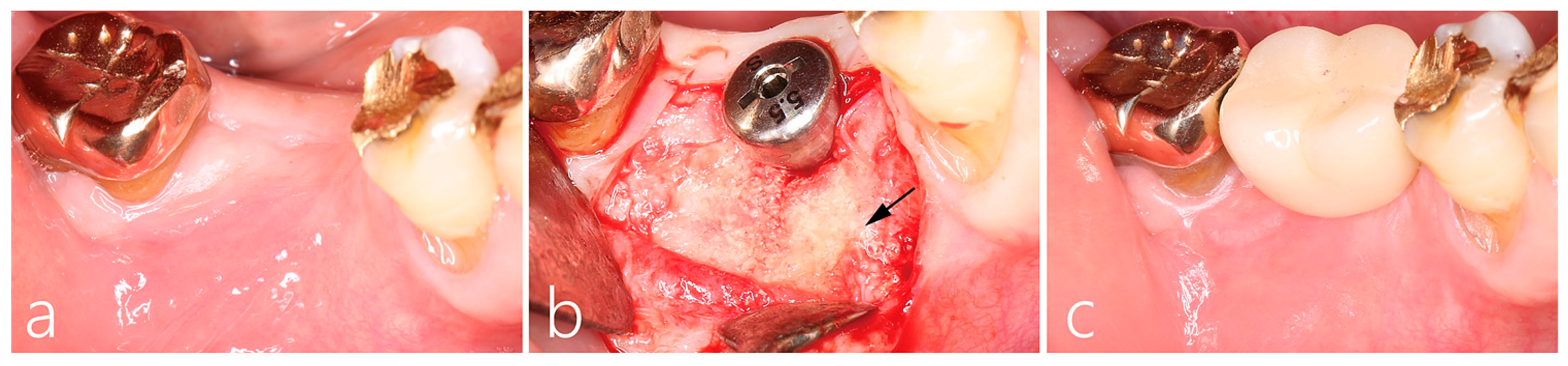
2.2. Uncovering Procedure
2.3. Prosthesis Delivery and Follow-Up
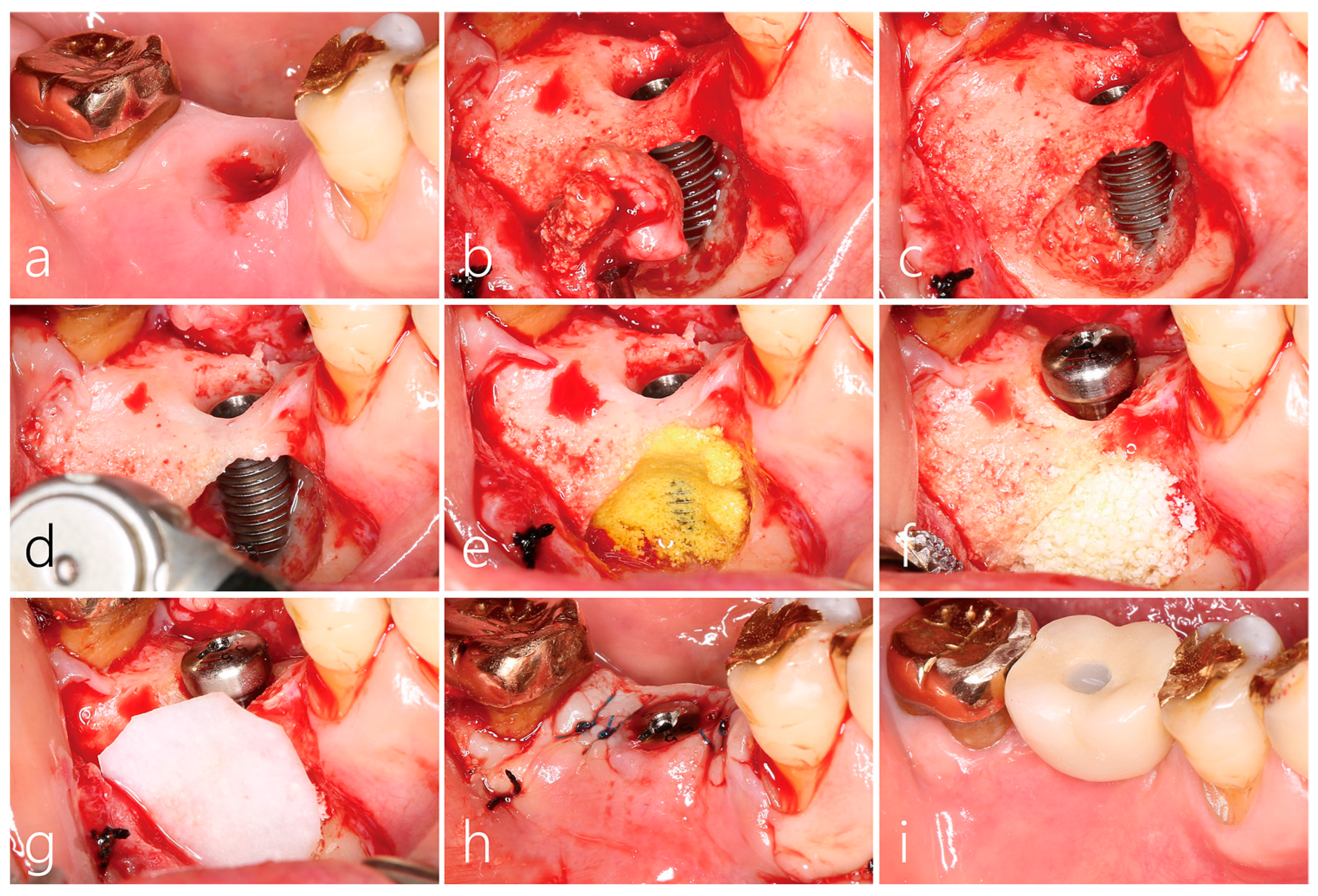
2.4. Surgical Reentry
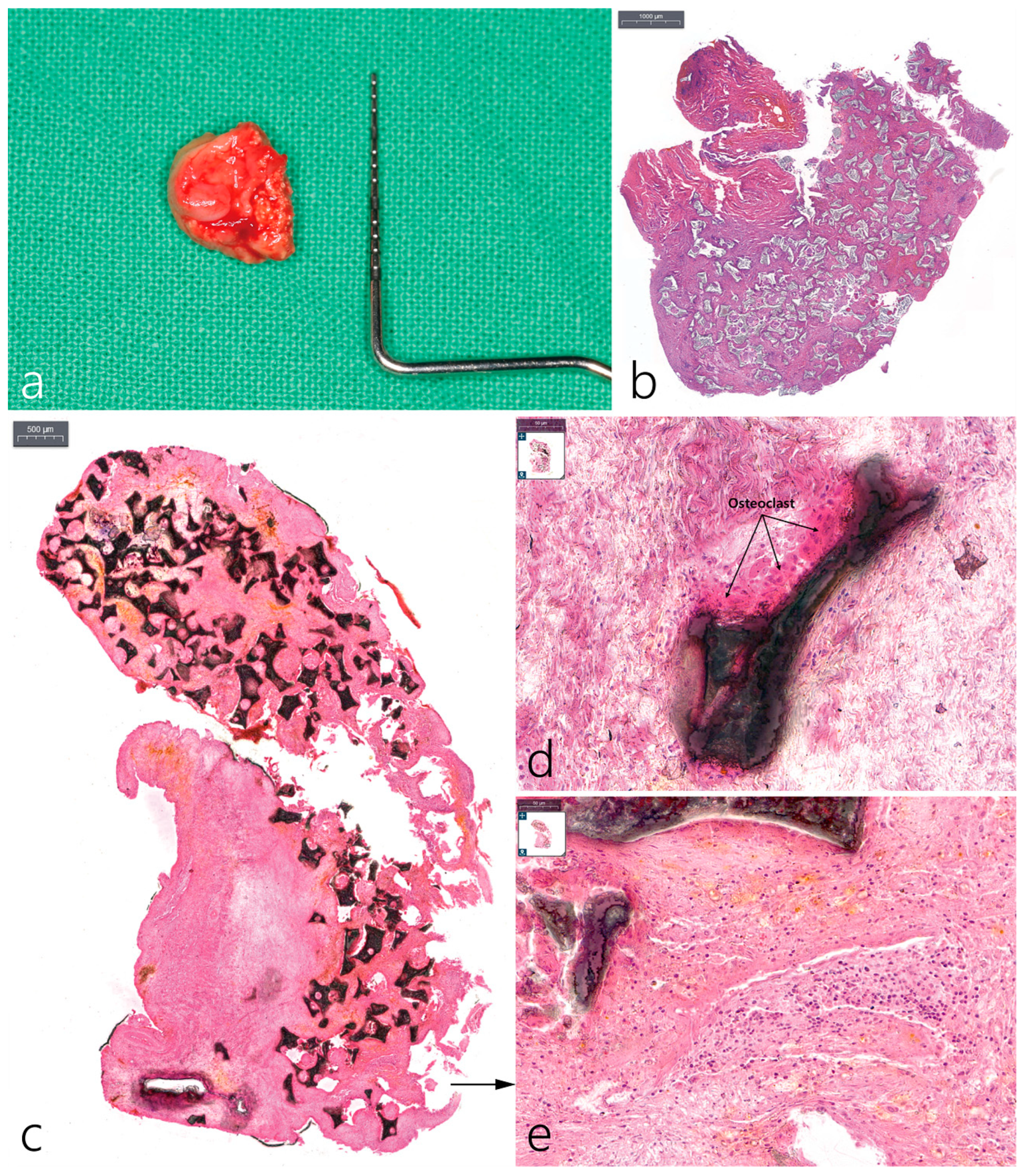
2.5. Histopathological Examination
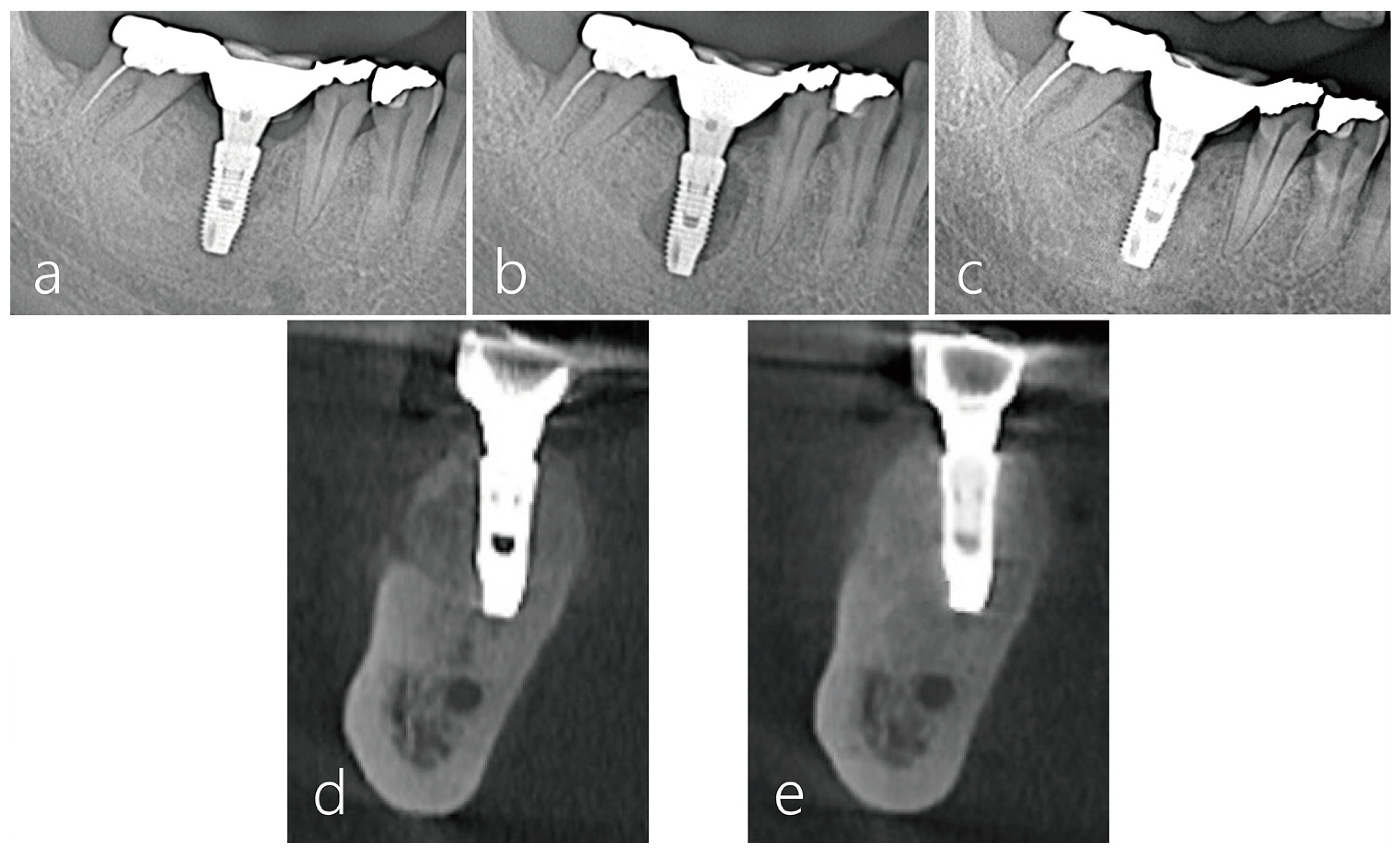
2.6. Radiographic Evaluation
3. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monje, A.; Pons, R.; Insua, A.; Nart, J.; Wang, H.-L.; Schwarz, F. Morphology and severity of peri-implantitis bone defects. Clin. Implant. Dent. Relat. Res. 2019, 21, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Abrahamsson, I.; Berglundh, T.; Lindhe, J. Soft tissue reactions to plaque formation at implant abutments with different surface topography. An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sculean, A.; Engebretson, S.P.; Becker, J.; Sager, M. Animal models for peri-implant mucositis and peri-implantitis. Periodontology 2000 2015, 68, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Becker, B.E.; Newman, M.G.; Nyman, S. Clinical and microbiologic findings that may contribute to dental implant failure. Int. J. Oral Maxillofac. Implant. 1990, 5, 31–38. [Google Scholar]
- Derks, J.; Tomasi, C. Peri-implant health and disease; A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, 158–171. [Google Scholar] [CrossRef]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.L.; Cochran, D.; Sculean, A.; Canullo, L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef]
- Reiser, G.M.; Nevins, M. The implant periapical lesion: Etiology, prevention, and treatment. Compend. Contin. Educ. Dent. 1995, 16, 768–770, 772 passim. [Google Scholar]
- Quirynen, M.; Vogels, R.; Alsaadi, G.; Naert, I.; Jacobs, R.; van Steenberghe, D. Predisposing conditions for retrograde peri-implantitis, and treatment suggestions. Clin. Oral Implant. Res. 2005, 16, 599–608. [Google Scholar] [CrossRef]
- Sarmast, N.D.; Wang, H.H.; Soldatos, N.K.; Angelov, N.; Dorn, S.; Yukna, R.; Iacono, V.J. A Novel Treatment Decision Tree and Literature Review of Retrograde Peri-Implantitis. J. Periodontol. 2016, 87, 1458–1467. [Google Scholar] [CrossRef]
- Flanagan, D. Apical (retrograde) peri-implantitis: A case report of an active lesion. J. Oral Implant. 2002, 2, 92–96. [Google Scholar] [CrossRef]
- Peñarrocha-Oltra, D.; Blaya-Tárraga, J.A.; Menéndez-Nieto, I.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M. Factors associated with early apical peri-implantitis: A retrospective study covering a 20-year period. Int. J. Oral Implant. 2020, 13, 65–73. [Google Scholar]
- Di Murro, B.; Pranno, N.; Raco, A.; Pistilli, R.; Pompa, G.; Papi, P. Knowledge and Attitude towards Retrograde Peri-Implantitis among Italian Implantologists: A Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2020, 17, 8356. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, A.; Lefever, D.; Teughels, W.; Balshi, T.J.; Balshi, S.F.; Quirynen, M. Etiology and treatment of periapical lesions around dental implants. Periodontology 2000 2014, 66, 247–254. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.S.; Masters, D.; Meffert, R.M. Treatment of implants demonstrating periapical radiolucencies. Pr. Periodontics Aesthet Dent. 1992, 4, 37–41. [Google Scholar]
- Lefever, D.; Van Assche, N.; Temmerman, A.; Teughels, W.; Quirynen, M. Aetiology, microbiology and therapy of periapical lesions around oral implants: A retrospective analysis. J. Clin. Periodontol. 2013, 40, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Wang, H.L.; Bashutski, J.D.; Edwards, P.C.; Fu, J.H.; Oh, T.J. Retrograde peri-implantitis: A case report introducing an approach to its management. J. Periodontol. 2011, 82, 1080–1088. [Google Scholar] [CrossRef]
- Ayangco, L.; Sheridan, P.J. Development and treatment of retrograde peri-implantitis involving a site with a history of failed endodontic and apicoectomy procedures: A series of reports. Int. J. Oral Maxillofac. Implant. 2001, 16, 412–417. [Google Scholar]
- Zhou, W.; Han, C.; Li, D.; Li, Y.; Song, Y.; Zhao, Y. Endodontic treatment of teeth induces retrograde periimplantitis. Clin. Oral Implant. Res. 2009, 20, 1326–1332. [Google Scholar] [CrossRef]
- Machtei, E.E. The effect of membrane exposure on the outcome of regenerative procedures in humans: A meta-analysis. J. Periodontol. 2001, 72, 512–516. [Google Scholar] [CrossRef]
- Balshi, S.F.; Wolfinger, G.J.; Balshi, T.J. A retrospective evaluation of a treatment protocol for dental implant periapical lesions: Long-term results of 39 implant apicoectomies. Int. J. Oral Maxillofac. Implant. 2007, 22, 267–272. [Google Scholar]
- Dahlin, C.; Nikfarid, H.; Alse’n, B.; Kashani, H. Apical peri-implantitis: Possible predisposing factors, case reports, and surgical treatment suggestions. Clin. Implant. Dent. Relat. Res. 2009, 11, 222–227. [Google Scholar] [PubMed]
- Meffert, R.M. How to treat ailing and failing implants. Implant. Dent. 1992, 1, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Blaya-Tárraga, J.A.; Cervera-Ballester, J.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M. Periapical implant lesion: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e737–e749. [Google Scholar] [CrossRef]
- Park, W.B.; Kim, Y.J.; Han, J.Y.; Kang, P. Successful Management of Dental Implants in Postoperative Maxillary Cyst: A Case Report With a 13-Year Follow-Up. J. Oral Implant. 2020, 46, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Park, W.B.; Park, W.; Han, J.Y.; Kang, P. Successful Management of Apically Exposed Implants in the Maxillary Sinus and Associated Sinus Pathologies. J. Oral Implant. 2022, 48, 491–499. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.-B.; Villa, M.; Han, J.-Y.; Lim, H.-C.; Kang, P. Lateral Peri-Implantitis: Successful Management via Guided Bone Regeneration at Mandibular First Molar Implant. Medicina 2023, 59, 1691. https://doi.org/10.3390/medicina59091691
Park W-B, Villa M, Han J-Y, Lim H-C, Kang P. Lateral Peri-Implantitis: Successful Management via Guided Bone Regeneration at Mandibular First Molar Implant. Medicina. 2023; 59(9):1691. https://doi.org/10.3390/medicina59091691
Chicago/Turabian StylePark, Won-Bae, Michael Villa, Ji-Young Han, Hyun-Chang Lim, and Philip Kang. 2023. "Lateral Peri-Implantitis: Successful Management via Guided Bone Regeneration at Mandibular First Molar Implant" Medicina 59, no. 9: 1691. https://doi.org/10.3390/medicina59091691
APA StylePark, W.-B., Villa, M., Han, J.-Y., Lim, H.-C., & Kang, P. (2023). Lateral Peri-Implantitis: Successful Management via Guided Bone Regeneration at Mandibular First Molar Implant. Medicina, 59(9), 1691. https://doi.org/10.3390/medicina59091691






