1. Introduction
Guided bone regeneration (GBR) is a well-established technique used to increase the amount of bone and provide optimal bone support for osseointegrated dental implants. The application of GBR for vertical augmentation is well documented, with high implant success rates [
1,
2]. As proposed by Dahlin et al., the GBR therapeutic protocol involves the surgical placement of a space-creating barrier device that protects the site of regeneration, excluding cells that may impede bone formation (epithelial cells and connective tissue) [
3]. The use of PTFE non-resorbable barriers, compared to resorbable barriers, can guarantee an adequate space-making effect up to the second surgical phase, which usually takes place nine months after the first operation [
2]. However, in the case of accidental exposure during the healing period, in most cases, the semipermeable PTFE barriers have to be removed within a couple of weeks, due to their contamination by oral fluids [
4]. The use of occlusive titanium membranes (OTBs) has been proposed to create and maintain an isolated space [
5]. An OTB is a thin, preformed titanium barrier, which, being without openings and non-permeable, can ensure better biological protection of the graft in the case of accidental exposure.
Perret et al. [
6] demonstrated that the use of an intentionally exposed occlusive barrier in extraction sockets can lead to bone regeneration, despite exposure. In order to reduce the intra-surgical time of OTB adaptation, the pre-shaping of the barrier on a printed stereolithographic model (STLM) has been proposed [
7,
8,
9]. The osteoconductive and mechanical properties, and the possibility of autoclaving the titanium barriers, can guarantee an optimal biocompatible response for this device. Furthermore, the authors of the present article observed an increase in flap thickness above the OTB during the healing period [
9].
Although this phenomenon is not yet completely explained, it can be assumed that it is a consequence of the occlusivity of the barrier, which hinders the penetration of the soft tissues from the flap to the regenerative space, maintaining not only the initial thickness of the flap, but even favoring its thickening. As described by Dahlin et al. [
10], a white layer of connective tissue, called the pseudo-periosteum, can be observed above the newly formed bone when a non-resorbable barrier is removed. This is a dense, connective soft tissue layer with low cellularity and no mineralization (
Figure 1a,b).
A classification of this layer of the pseudo-periosteum has been proposed by Cucchi et al. [
11] into three types based on morphological and histological characteristics. Some authors believe that this tissue should be maintained to protect newly formed bone and avoid bone exposure in cases of secondary healing after membrane removal [
12,
13]. However, further studies will be needed to fully understand the role and clinical potential of the pseudo-periosteum.
The aim of the present study is to introduce a new graftless protocol for the augmentation of adherent gingiva in posterior areas treated with vertical GBR, to be applied in the OTB removal phase.
The proposed procedure provides for the possibility of leaving a vestibular portion of the pseudo-periosteum intentionally exposed, promoting its healing by secondary intention. This procedure is performed in order to restore the depth of the vestibule in the regenerated areas and increase the amount of adherent keratinized tissue without a free gingival graft.
2. Materials and Methods
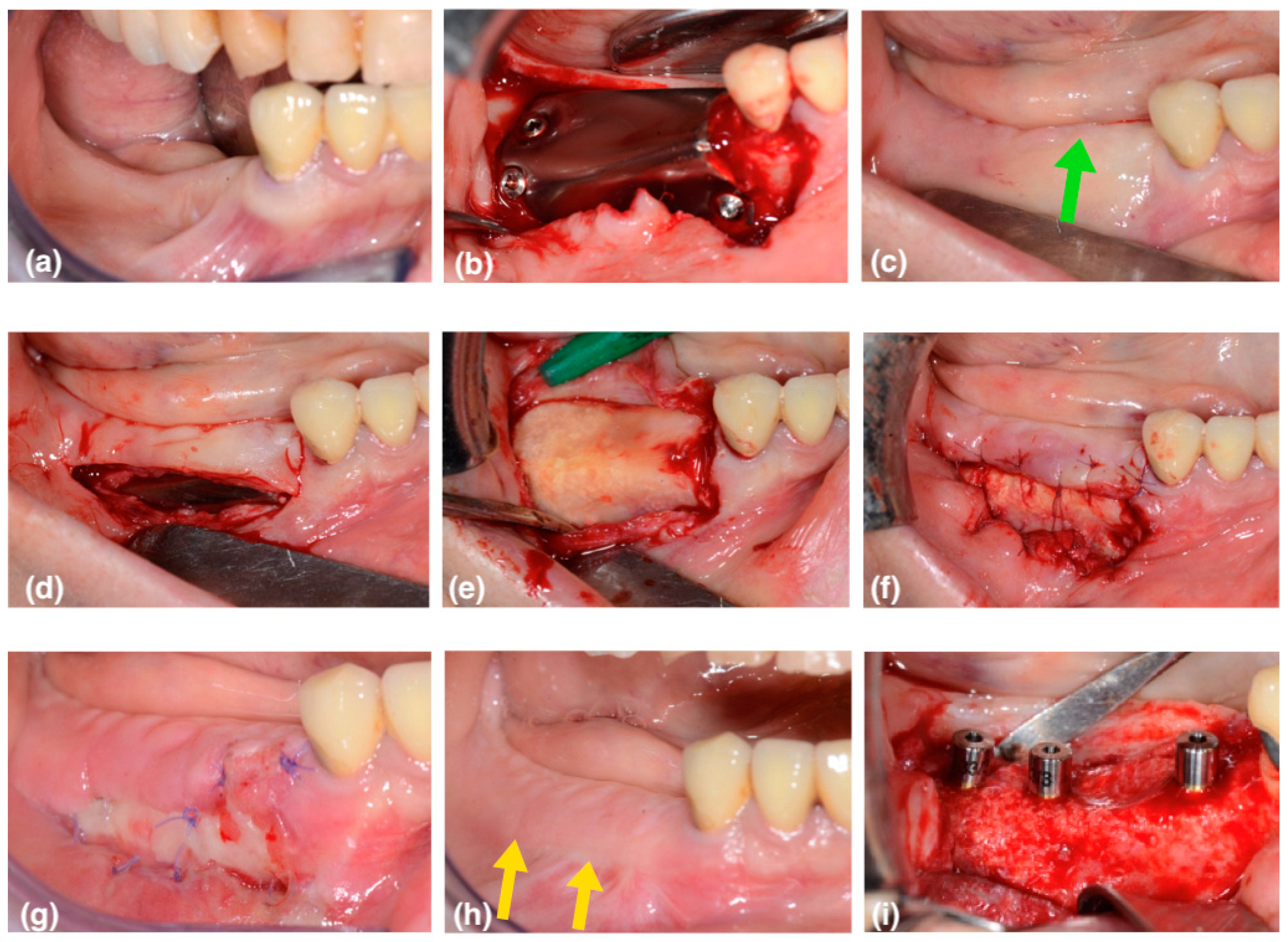
This case series includes 6 patients (5 female, 1 male; average 59 years old) in need of vertical bone augmentation for implant placement (
Figure 2a and
Figure 3a). The patients underwent 5 mandibular surgeries and 1 in the upper jaw. All the patients were systemically healthy, had never smoked, and were not taking any regular medications associated with a compromised bone healing response. Prior to treatment, informed consent was obtained from patients regarding the treatment goal and protocols. In the present article, the authors describe a new approach in order to reach the proper vestibule depth and keratinized attached tissue width by taking advantage of the secondary healing of the white layer of pseudo-periosteum.
2.1. Occlusive Titanium Barrier Preparation
For the selected patients, cone beam computed tomography (CBCT) (Cranex 3D, Soredex—Kavo Dental, Brea, CA, USA) was performed, and showed the reduced thickness and height of the residual bone. A lack of keratinized tissue (KT) was also evident. For each CBCT, a three-dimensional STLM (stereolithographic model) of the recipient site was printed to evaluate the case and pre-shape the titanium barrier. The GBRs were planned directly on each printed STLM. A 0.12 mm thickness OTB (Regenplate
® Shape 2 and Shape 4, Bio-Micron, Sweden & Martina, Due Carrare, Padova, Italy) was trimmed and shaped on each STLM to maintain a distance of 1.5 mm from the adjacent teeth, from the mental nerve, and from the mylohyoid line. The smooth side of the barrier was placed externally, while the treated rough surface was placed on the inner side of the dome, in order to obtain better blood clot adhesion [
14]. Every sharp edge was bent inward with pliers to avoid damage to the flap. Various of holes were drilled with a burr (2 mm diameter) in the mesial–buccal and distal–occlusal areas of the barrier to stabilize it using fixation screws. The device was then sterilized by means of an autoclave before surgery.
2.2. First-Phase Surgery
After local anesthesia was performed with articaine hydrochloride 4% plus epinephrine 1:100,000 (Citocartin, Molteni Dental, Milan, Italy), a flap design was made to ensure primary tension-free closure. In detail, a crestal full-thickness incision was made with a No. 15 surgical blade, to equally divide the KT. Posteriorly, the incision ended with a vestibular oblique incision (45°) at the level of the occlusal plane. In the presence of a molar, the posterior incision was marginal and distally released. Lingually, to obtain an adequate length of the flap, a marginal horizontal incision was extended mesially to at least the two adjacent teeth, avoiding vertical incisions. To obtain passivation of the lingual flap in the middle area, the superficial fibers of the mylohyoid muscle were carefully detached by a periodontal probe. In the distal area, the full thickness of the adherent tissues of the retro-molar pad was lifted using a Prichard elevator. On the buccal side, the mesial extension of the flap involved one or two adjacent teeth and ended with a “hockey-stick” vertical incision with preservation of the papillae [
15]. The full thickness of the flap was then carefully raised to locate the mental foramen area and the neurovascular bundle. A shallow periosteum incision was made from the distal to the medial vertical incision, by using a new No. 15 blade, in order to obtain flap passivation and elongation of about 8 mm. The buccal flap was further carefully “brushed” on its internal surface until the desired elongation was achieved [
16]. Then, the collection of autologous bone was performed from the recipient site by using a scraper (Safescraper
® TWIST, META, Reggio Emilia, Italy). The bone scraping procedure was considered sufficient to activate the regional acceleratory phenomenon at the recipient site [
17]. A recent systematic review shows that there is no evidence supporting creating bone perforations to promote new angiogenesis in GBR [
18]. A 1:1 mixture of particulated autogenous bone and porcine-derived xenograft (Regeneross
®, Zimmer, Columbus, OH, USA) was placed in the inner surface of the membrane.
The device was, at this point, placed on the recipient site and fixed, first on the buccal side, then on the distal–occlusal area, and finally on the lingual side using self-tapping fixation screws (0220Q-4-10; 0220Q-6-10, Cizeta, Sweden & Martina, Due Carrare, Padova, Italy) (
Figure 2b). Contact between the barrier and neighboring teeth was carefully avoided, keeping a minimum distance of 1.5 mm. Flaps were then closed using two layers of sutures: horizontal mattress sutures were used in the first layer, and then single interrupted sutures were placed to ensure an adequate closure, especially on the edges of the flaps. Vertical incisions were then closed with single sutures.
2.3. Second-Phase Surgery
The barrier was removed 6 months after vertical augmentation, to allow a sufficient period of healing in order to obtain hard tissue regeneration. In all cases, a lack of keratinized tissue and vestibule depth was evident at the second surgical phase (
Figure 2c and
Figure 3b—the green arrows show the coronal shifting of the mucogingival junction). A buccal horizontal full-thickness incision was placed in the alveolar mucosa, extending to the titanium barrier surface, at the same level of the mucogingival junction of the adjacent teeth. The flap was completed with two small mesial and distal vertical releasing incisions that occlusally reached the crestal residual keratinized gingiva. This access flap allows the easy removal of the fixation screws and the titanium barrier (
Figure 2d).
The absence of holes in the barrier surface, contrary to the perforated titanium meshes, hinders the penetration of connective tissue, permitting very fast and easy removal of the titanium barrier.
A thin white layer of dense connective tissue, called the pseudo-periosteum, was always observed underneath the titanium occlusive barrier. It was a type 1 pseudo-periosteum with a thickness < 1 mm according to the classification proposed by Cucchi et al. [
11]. This layer was left in place to protect the new regenerated hard tissue, promoting its maturation and corticalization (
Figure 2e and
Figure 3c).
The alveolar mucosa apical to the incision line was sutured to the bottom of the vestibule, with 6/0 resorbable sutures anchored to the apical periosteum. The lingual flap was placed crestally in the same original position. Compression sutures were applied with the purpose of stabilizing the primary flap against the pseudo-periosteum and the newly regenerated bone.
In this way, a buccal portion of the pseudo periosteum was left intentionally exposed, with second-intention healing in order to obtain an increase in vestibulum depth and in the keratinized tissue (
Figure 2f and
Figure 3d).
Rinses with 0.20% chlorhexidine mouthwash were prescribed, suspending the use of the toothbrush until the sutures were removed.
At the check-up after one week, the first healing tissue was visible (
Figure 2g and
Figure 3e). After two weeks, the area was partially epithelized, with a good increase in thickness.
After six weeks, a band of adherent and keratinized gingiva and the mucogingival line were created, aligned with the mucogingival line of the adjacent remaining teeth.
After three months, corticalization and bone maturation were observed radiographically, as well as the restoration of the original gingival anatomy (
Figure 2h and
Figure 3f—the yellow arrows show the new position of the mucogingival line).
A small flap was raised, and the implant sites were prepared in a prosthetically driven manner, using dedicated drills according to the manufacturer’s instructions. The implants were then placed with a minimum torque of 35 ncm, placing a healing abutment (
Figure 2i). In one case, immediate loading with a splinted temporary crown was performed.
2.4. Prosthetic Loading
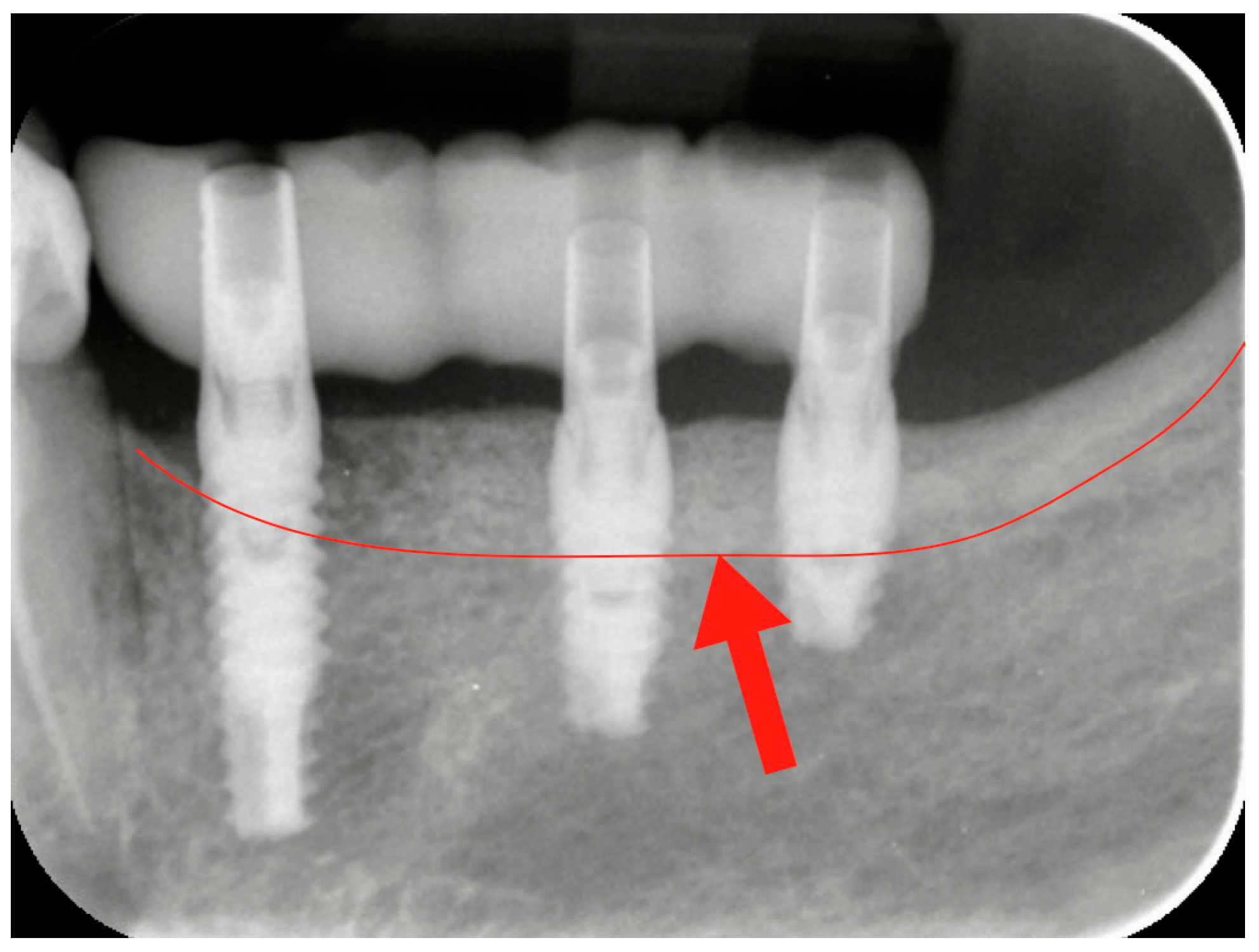
After three months, the healing abutment was replaced with a permanent abutment, and acrylic resin provisional restorations were placed. They were left in situ for two months, before being replaced by definitive zirconia–ceramic restorations. A radiographic follow-up was performed before prosthetic finalization for all cases to verify the conditions of hard peri-implant tissues (
Figure 4).
2.5. Data Collection
The following variables were recorded by an independent operator and are presented in
Table 1.
- -
Vertical bone gain (VBG): For each case, this was represented by the mean value of maximum vertical regeneration achieved, on both vestibular/buccal and lingual/palatal sides, by means of CT scans, measured at baseline and before the implant placement (9 months later). The measurements were carried out by means of OsiriX software (v. 3.5.1—32 bit, Pixmeo, Geneva, Switzerland).
- -
Adherent Soft Tissue Gain (ADSG): The procedure was performed with a periodontal probe (CPC 15, Hu-friedy) measuring the buccal adherent tissue height from the crestal point to the most apical point. The gain was evaluated by recording the difference between the two values before the barrier removal and 3 months after the white layer approach (WLA).
3. Results
Six patients (five females and one male) with a mean age of 59 years were enrolled in the present study. All patients followed the entire protocol, without no withdrawals.
In one case (patient 1), the healing time between the GBR procedure and the WLA was 4 months. Despite the reduced healing time, the clinical consistency was good, with no penetration of the probe.
The average Vertical Bone Gain was 4.08 mm, ranging from a minimum of 3 mm to a maximum of 6 mm.
The average Adherent Soft Tissue Gain was 6.75 mm, ranging from a minimum of 5 mm to a maximum of 8 mm.
4. Discussion
Guided bone regeneration surgery always leads to a deformation of the soft tissues consequent to passivation of the flap. As a result, there is often a loss in the vestibule depth and a coronal migration of the mucogingival line, resulting in the loss of keratinized tissue. In order to maintain the stability of the peri-implant crestal bone over the medium-long term, the need for adequate gingival thickness around the neck of the implant has been highlighted. Furthermore, an adequate depth of the vestibule guarantees the health of the peri-implant marginal mucosa, which should remain stable during phonation and chewing movements.
In order to restore the soft tissue anatomy following vertical augmentation in the posterior areas, some authors proposed a surgical protocol that involves the uncovering of the implants with a partial thickness flap and the simultaneous positioning of a free gingival graft on the buccal side of the regenerated site, in order to restore the correct depth of the vestibule [
19]. This surgical step is generally performed after two previous surgeries, which are the first phase of GBR and the second phase of membrane removal and implant insertion. The restoration of the keratinized tissue and the vestibule depth is then reserved for a third surgical phase. However, at this point, the preparation of the receiving periosteal bed in the posterior mandibular area must be performed with a deep partial thickness flap and consequently requires an operator with great expertise, as the risk of damaging the mental nerve bundles is quite high.
For these reasons, the approach proposed in this paper suggests the possibility of exploiting the only moment in which there is the spontaneous presence of the pseudo-periosteum, during the removal of the titanium barrier, without raising a partial-thickness flap.
Histologic studies demonstrated that the pseudo-periosteum underneath the non-resorbable barriers is composed of dense connective tissue having multi-directional fiber organization, a variable degree of cellularity, little or no vascularization, and an absence of inflammatory reactions [
11]. In particular, in human and animal studies, Simion et al. [
20,
21] showed that most coronal portions of implants placed with a one-stage GBR using non-resorbable Ti-reinforced membranes were immersed in a dense fibrous tissue over the crestal level of the regenerated bone. Histologic analysis at 9 months showed that this tissue consisted of densely packed collagen fibers with few cells and a scarcity of blood vessels. No inflammatory reactions or epithelial tissue were present [
21]. Although numerous studies about the pseudo-periosteum formation consider dense PTFE membranes, a similar type of tissue has been found underneath the non-resorbable occlusive titanium barriers (
Figure 1e and
Figure 2c).
These histological features of dense connective tissue seem to be compatible with secondary-intention healing; Lim et al. [
12] suggested that the pseudo-periosteum formation facilitates secondary-intention healing in cases of barrier exposure.
In the present article, a surgical approach to restore the depth of the vestibule and increase the attached gingiva with secondary healing of the pseudo-periosteum has been proposed. The incision technique, as described in the materials and methods section, involves a horizontal incision in the alveolar mucosa, placed buccally at the level of the mucogingival line of the adjacent teeth. The buccal–apical portion of the flap is sutured apically, leaving exposed the layer of the pseudo-periosteum newly formed underneath the OTB. This white layer undergoes secondary healing with the aim of generating new adherent tissue. It has been observed that the new mucogingival junction tends to be aligned with the MGJ of the adjacent teeth. This is probably linked to the fact that the presence of adjacent keratinized tissue favors the re-creation of thick, keratinized tissue. The thickness of the new adherent tissue seems to be influenced by the thickening of the flap following the use of an OTB, an aspect already observed by the same authors in a previous article [
9].
As regards the quality of the regenerated bone with GBR with non-resorbable barriers, it has been observed that the regenerated bone underneath the barrier is quite immature and requires a maturation period of 3–4 months in which the primary flap remains in contact with the regenerated tissue, promoting its crestal vascularization, maturation, and corticalization. It is possible to deduce that the placement of implants in more mature and corticalized regenerated bone could be favorable for the maintenance of the crestal bone.
Although many authors usually remove the newly formed pseudo-periosteum, other authors think that this tissue should be maintained to protect newly formed bone and avoid bone exposure in cases of secondary healing after membrane removal [
13,
22].
It is not yet clear how well the pseudo-periosteum is able to preserve bone resorption if left exposed. In any case, the portion of the buccal pseudo-periosteum that remains exposed is far from the critical crestal area of the implant neck; therefore, even a potential slight reabsorption of the regenerated volume could be well tolerated and irrelevant to the final esthetic and functional outcome.
5. Conclusions
In cases of vertical bone regeneration in the posterior areas, this article has demonstrated the possibility of recreating the depth of the fornix and new adherent gingiva by the secondary healing of an area of pseudo-periosteum left exposed.
The advantages compared to the FGG techniques are related to the lower invasiveness, operative simplicity, lower risk of mental nerve damage, reduction in healing time and finally the better mimesis and final esthetic outcome.
This approach can also be applied in cases of accidental exposure and the consequent anticipated removal of the titanium barrier. In this case, the implant placement must also be delayed by at least 3–4 months from the barrier removal.
Due to the limitations of the present study, including the small number of patients recruited, further randomized controlled cases will be needed to confirm the predictability of the proposed approach.
Author Contributions
Provided substantial contributions to the study’s conception and performed the clinical cases and data acquisition: F.P.; performed data collection and interpretation: E.D.; provided for the bibliographic review: L.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was performed in compliance with good clinical practice and the WMA Declaration of Helsinki for ethical principles in medical research involving human subjects (amended by the 64th WMA General Assembly, Fortaleza, Brazil, 16–19 October 2013). Since simple data collection was performed before and during the surgeries, the presence of an ethics committee was not required.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study regarding the treatment protocols and the publication of related clinical pictures.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buser, D.; Brägger, U.; Lang, N.P.; Nyman, S. Regenerationand enlargement of jaw bone using guided tissue regeneration. Clin. Oral. Implant. Res. 1990, 1, 22–32. [Google Scholar] [CrossRef]
- Buser, D.; Ingimarsson, S.; Dula, K.; Lussi, A.; Hirt, H.P.; Belser, U.C. Long-term stability of osseointegrated implants in aug- mented bone: A 5-year prospective study in partially edentu- lous patients. Int. J. Periodontics Restor. Dent. 2002, 22, 109–117. [Google Scholar]
- Dahlin, C.; Sennerby, L.; Lekholm, U.; Linde, A.; Nyman, S. Generation of new bone around titanium implants using a mem- brane technique: An experimental study in rabbits. Int. J. Oral. Maxillofac. Implant. 1989, 4, 19–25. [Google Scholar]
- Carbonell, J.M.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.D.; Nart, J. High-density polytetrafluoroethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral. Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef]
- Van Steenberghe, D.; Johansson, C.; Quirynen, M.; Molly, L.; Albrektsson, T.; Naert, I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin. Oral. Implant. Res. 2003, 14, 63–71. [Google Scholar] [CrossRef]
- Perret, F.; Romano, F.; Ferrarotti, F.; Aimetti, M. Occlusive Titanium Barrier for Immediate Bone Augmentation of Severely Resorbed Alveolar Sockets with Secondary Soft Tissue Healing: A 2-Year Case Series. Int. J. Periodontics Restor. Dent. 2019, 39, 97–105. [Google Scholar] [CrossRef]
- Molly, L.; Quirynen, M.; Michiels, K.; van Steenberghe, D. Comparison between jaw bone augmentation by means of a stiff occlusive titanium membrane or an autologous hip graft: A retrospective clinical assessment. Clin. Oral. Implant. Res. 2006, 17, 481–487. [Google Scholar] [CrossRef]
- Andreasi Bassi, M.; Andrisani, C.; Lopez, M.A.; Gaudio, R.M.; Lombardo, L.; Lauritano, D. Guided bone regeneration in distal mandibular atrophy by means of a pre-formed titanium foil: A case series. J. Biol. Regul. Homeost. Agents 2016, 30, 61–68. [Google Scholar]
- Perret, F.; Aimetti, M.; Andreasi Bassi, M. Hard and soft tissue augmentation with occlusive titanium barriers in jaw vertical defects: A novel approach. Plast. Aesthet. Res. 2022, 9, 7. [Google Scholar] [CrossRef]
- Dahlin, C.; Simion, M.; Nanmark, U.; Sennerby, L. Histological morphology of the e-PTFE/tissue interface in hu- mans subjected to guided bone regen- eration in conjunction with oral implant treatment. Clin. Oral. Implant. Res. 1998, 9, 100–106. [Google Scholar] [CrossRef]
- Cucchi, A.; Sartori, M.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. A Proposal of Pseudo-periosteum Classification After GBR by Means of Titanium-Reinforced d-PTFE Membranes or Titanium Meshes Plus Cross-Linked Collagen Membranes. Int. J. Periodontics Restor. Dent. 2019, 39, e157–e165. [Google Scholar] [CrossRef]
- Lim, H.C.; Lee, J.S.; Choi, S.H.; Jung, U.W. The effect of overlaying titanium mesh with collagen membrane for ridge preser- vation. J. Periodontal Implant. Sci. 2015, 45, 128–135. [Google Scholar] [CrossRef]
- Lizio, G.; Corinaldesi, G.; Marchetti, C. Alveolar ridge reconstruction with ti- tanium mesh: A three-dimensional evaluation of factors affecting bone augmentation. Int. J. Oral. Maxillofac. Implant. 2014, 29, 1354–1363. [Google Scholar] [CrossRef]
- Lundgren, A.K.; Lundgren, D.; Wennerberg, A.; Hämmerle, C.H.; Nyman, S. Influence of surface roughness of barrier walls on guided bone augmentation: Experimental study in rabbits. Clin. Implant. Dent. Relat. Res. 1999, 1, 41–48. [Google Scholar] [CrossRef]
- Tinti, C.; Parma-Benfenati, S. Vertical ridge augmentation: Surgical protocol and retrospective evaluation of 48 consecutively inserted implants. Int. J. Periodontics Restor. Dent. 1998, 18, 434–443. [Google Scholar]
- Ronda, M.; Stacchi, C. A novel approach for the coronal advancement of the buccal flap. Int. J. Periodontics Restor. Dent. 2015, 35, 795–801. [Google Scholar] [CrossRef]
- Andreasi Bassi, M.; Lopez, M.A.; Confalone, L.; Carinci, F. Hydraulic sinus lift technique in future site development: Clinical and histomorphometric analysis of human biopsies. Implant. Dent. 2015, 24, 117–124. [Google Scholar] [CrossRef]
- Alvira-González, J.; De Stavola, L. The role of cortical perforations in bone regeneration: A systematic review. Int. J. Oral. Maxillofac. Surg. 2020, 49, 945–951. [Google Scholar] [CrossRef]
- Bassetti, R.G.; Stähli, A.; Bassetti, M.A.; Sculean, A. Soft tissue augmentation procedures at second-stage surgery: A systematic review. Clin. Oral. Investig. 2016, 20, 1369–1387. [Google Scholar] [CrossRef]
- Simion, M.; Trisi, P.; Piattelli, A. Vertical ridge augmentation using a membrane technique associated with osseointe- grated implants. Int. J. Periodontics Restor. Dent. 1994, 14, 496–511. [Google Scholar]
- Simion, M.; Dahlin, C.; Rocchietta, I.; Stavropoulos, A.; Sanchez, R.; Karring, T. Vertical ridge augmentation with guided bone regeneration in association with dental implants: An experimental study in dogs. Clin. Oral. Implant. Res. 2007, 18, 86–94. [Google Scholar] [CrossRef]
- Gutta, R.; Baker, R.A.; Bartolucci, A.A.; Louis, P.J. Barrier membranes used for ridge augmentation: Is there an opti- mal pore size? J. Oral. Maxillofac. Surg. 2009, 67, 1218–1225. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).