Particular Anatomy of the Hyperopic Eye and Potential Clinical Implications
Abstract
:1. Introduction
2. Materials and Methods
- -
- Ocular Response Analyzer (ORA) (Reichert Ophthalmic Instruments Inc, Depew, NY, USA)—in order to determine corneal biomechanical properties: corneal hysteresis (CH), corneal resistance factor (CRF) and the Goldmann-correlated intraocular pressure IOP (IOPg);
- -
- Aladdin biometer (Topcon, Tokyo, Japan)—to determine the axial length (AL), anterior chamber depth (ACD) and central corneal thickness (CCT);
- -
- Specular microscopy (Nidek, Gamagori, Japan) in order to determine corneal endothelial parameters: cell density (CD), coefficient of variation of cell area (CV), percent hexagonality (Hex).
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kandel, H.; Khadka, J.; Goggin, M.; Pesudovs, K. Impact of Refractive Error on Quality of Life: A Qualitative Study. Clin. Exp. Ophthalmol. 2017, 45, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Fotouhi, A.; Yekta, A.; Pakzad, R.; Ostadimoghaddam, H.; Khabazkhoob, M. Global and Regional Estimates of Prevalence of Refractive Errors: Systematic Review and Meta-Analysis. J. Curr. Ophthalmol. 2017, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Horhat, R. Prevalence and Risk Factors of Amblyopia among Refractive Errors in an Eastern European Population. Medicina 2018, 54, 6. [Google Scholar] [CrossRef] [PubMed]
- Bountziouka, V.; Cumberland, P.M.; Rahi, J.S. Impact of Persisting Amblyopia on Socioeconomic, Health, and Well-Being Outcomes in Adult Life: Findings from the UK Biobank. Value Health 2021, 24, 1603–1611. [Google Scholar] [CrossRef]
- Shen, L.; Melles, R.B.; Metlapally, R.; Barcellos, L.; Schaefer, C.; Risch, N.; Herrinton, L.J.; Wildsoet, C.; Jorgenson, E. The Association of Refractive Error with Glaucoma in a Multiethnic Population. Ophthalmology 2016, 123, 92–101. [Google Scholar] [CrossRef]
- Sit, A.J.; Chen, T.C.; Takusagawa, H.L.; Rosdahl, J.A.; Hoguet, A.; Chopra, V.; Richter, G.M.; Ou, Y.; Kim, S.J.; WuDunn, D. Corneal Hysteresis for the Diagnosis of Glaucoma and Assessment of Progression Risk: A Report by the American Academy of Ophthalmology. Ophthalmology 2023, 130, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Gerena Arévalo, V.A.; Ruiz-Moreno, J.M. Choroidal Thickness in a Hyperopic Pediatric Population. Diagnostics 2022, 12, 2330. [Google Scholar] [CrossRef]
- Monika, M.; Durajczyk, M. Evaluation of the Prevalence of Refractive Defects and Ocular Function in a Group of 1518 Children Aged 8 Years in Northwestern Poland-A Retrospective Study. J. Clin. Med. Res. 2023, 12, 2880. [Google Scholar] [CrossRef]
- Hagen, L.A.; Gilson, S.J.; Akram, M.N.; Baraas, R.C. Emmetropia Is Maintained Despite Continued Eye Growth From 16 to 18 Years of Age. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4178–4186. [Google Scholar] [CrossRef]
- Roberts, C.J.; Liu, J. Corneal Biomechanics: From Theory to Practice; Kugler Publications: Amsterdam, The Netherlands, 2017; ISBN 9789062998760. [Google Scholar]
- Chaurasia, S.; Vanathi, M. Specular Microscopy in Clinical Practice. Indian J. Ophthalmol. 2021, 69, 517. [Google Scholar] [CrossRef]
- Mandal, P.; Berrow, E.J.; Naroo, S.A.; Wolffsohn, J.S.; Uthoff, D.; Holland, D.; Shah, S. Validity and Repeatability of the Aladdin Ocular Biometer. Br. J. Ophthalmol. 2014, 98, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Brodie, S.E. 2022–2023 Basic and Clinical Science Course, Section 03: Clinical Optics and Vision... Rehabilitation Print; American Academy of Ophthalmology: San Francisco, CA, USA, 2022; ISBN 9781681045436. [Google Scholar]
- Pniakowska, Z.; Jurowski, P.; Wierzbowska, J. Clinical Evaluation of Corneal Biomechanics Following Laser Refractive Surgery in Myopic Eyes: A Review of the Literature. J. Clin. Med. Res. 2023, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Potop, V.; Coviltir, V.; Schmitzer, S.; Corbu, C.; Ionescu, I.C.; Burcel, M.; Dăscălescu, D. The Relationship Between Corneal Hysteresis and Retinal Ganglion Cells—A Step Forward in Early Glaucoma Diagnosis. Med. Sci. Monit. 2020, 26, e924672. [Google Scholar] [CrossRef] [PubMed]
- Potop, V.; Coviltir, V.; Corbu, C.; Burcel, M.G.; Ionescu, C.I.; Dascalescu, D.M.C. Corneal Hysteresis, A Glaucoma Risk Factor Independent of The Intraocular Pressure. Rev. Roum. Des Sci. Tech. Série Électrotechnique Énergétique 2019, 64, 297–300. [Google Scholar]
- Tur, V.M.; MacGregor, C.; Jayaswal, R.; O’Brart, D.; Maycock, N. A Review of Keratoconus: Diagnosis, Pathophysiology, and Genetics. Surv. Ophthalmol. 2017, 62, 770–783. [Google Scholar]
- Marinescu, M.; Dascalescu, D.; Constantin, M.; Coviltir, V.; Burcel, M.; Darabus, D.; Ciuluvica, R.; Stanila, D.; Voinea, L.; Potop, V. Corneal Biomechanical Properties in Myopic and Emmetropic Children. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3580–3589. [Google Scholar] [CrossRef]
- Zhang, B.; Shweikh, Y.; Khawaja, A.P.; Gallacher, J.; Bauermeister, S.; Foster, P.J. UKBiobank Eye and Vision Consortium Associations with Corneal Hysteresis in a Population Cohort: Results from 96 010 UK Biobank Participants. Ophthalmology 2019, 126, 1500–1510. [Google Scholar] [CrossRef]
- Sharifipour, F.; Panahi-bazaz, M.; Bidar, R.; Idani, A.; Cheraghian, B. Age-Related Variations in Corneal Biomechanical Properties. J. Curr. Ophthalmol. 2016, 28, 117. [Google Scholar] [CrossRef]
- Murphy, M.L.; Pokrovskaya, O.; Galligan, M.; O’Brien, C. Corneal Hysteresis in Patients with Glaucoma-like Optic Discs, Ocular Hypertension and Glaucoma. BMC Ophthalmol. 2017, 17, 1. [Google Scholar] [CrossRef]
- Hashemi, H.; Jafarzadehpur, E.; Mehravaran, S.; Yekta, A.; Ostadimoghaddam, H.; Norouzirad, R.; Khabazkhoob, M. Corneal Resistance Factor and Corneal Hysteresis in a 6- to 18-Year-Old Population. J. Cataract. Refract. Surg. 2014, 40, 1446–1453. [Google Scholar] [CrossRef]
- Refai, T.A. Correlation between Apical Protrusion in the Scheimflug Imaging and Corneal Hysteresis and Corneal Resistance Factor by Ocular Response Analyzer, among Refractive Non-Keratoconic Egyptian Patients. Electron. Physician 2015, 7, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Rosa, N.; Lanza, M.; De Bernardo, M.; Signoriello, G.; Chiodini, P. Relationship Between Corneal Hysteresis and Corneal Resistance Factor with Other Ocular Parameters. Semin. Ophthalmol. 2015, 30, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Park, S.K.; Kim, M.S. The Biomechanical Properties of the Cornea and Anterior Segment Parameters. BMC Ophthalmol. 2013, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Amador, C.; Tormanen, K.; Ghiam, S.; Saghizadeh, M.; Arumugaswami, V.; Kumar, A.; Kramerov, A.A.; Ljubimov, A.V. Systemic Diseases and the Cornea. Exp. Eye Res. 2021, 204, 108455. [Google Scholar] [CrossRef] [PubMed]
- del Buey, M.A.; Casas, P.; Caramello, C.; López, N.; de la Rica, M.; Subirón, A.B.; Lanchares, E.; Huerva, V.; Grzybowski, A.; Ascaso, F.J. An Update on Corneal Biomechanics and Architecture in Diabetes. J. Ophthalmol. 2019, 2019, 7645352. [Google Scholar] [CrossRef]
- Sedaghat, M.-R.; Momeni-Moghaddam, H.; Azimi, A.; Fakhimi, Z.; Ziaei, M.; Danesh, Z.; Roberts, C.J.; Monfared, N.; Jamali, A. Corneal Biomechanical Properties in Varying Severities of Myopia. Front. Bioeng. Biotechnol. 2021, 8, 595330. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901. [Google Scholar] [CrossRef]
- Phu, J.; Tong, J.; Zangerl, B.; Le, J.L.; Kalloniatis, M. Cluster Analysis Reveals Patterns of Age-related Change in Anterior Chamber Depth for Gender and Ethnicity: Clinical Implications. Ophthalmic Physiol. Opt. 2020, 40, 632. [Google Scholar] [CrossRef]
- Trikha, S.; Perera, S.A.; Husain, R.; Aung, T. The Role of Lens Extraction in the Current Management of Primary Angle-Closure Glaucoma. Curr. Opin. Ophthalmol. 2015, 26, 128–134. [Google Scholar] [CrossRef]
- Ong, A.Y.; Ng, S.M.; Swaroop Vedula, S.; Friedman, D.S. Lens Extraction for Chronic Angle-closure Glaucoma. Cochrane Database Syst. Rev. 2021, 2021, CD005555. [Google Scholar] [CrossRef]
- Bueno-Gimeno, I.; Martínez-Albert, N.; Gené-Sampedro, A.; España-Gregori, E. Anterior Segment Biometry and Their Correlation with Corneal Biomechanics in Caucasian Children. Curr. Eye Res. 2019, 44, 118–124. [Google Scholar] [CrossRef]
- Vaiciuliene, R.; Rylskyte, N.; Baguzyte, G.; Jasinskas, V. Risk Factors for Fluctuations in Corneal Endothelial Cell Density (Review). Exp. Ther. Med. 2022, 23, 129. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Kaur, P.; Singh, K.; Kumar, D.; Chopra, R.; Sehgal, G. Evaluation and Correlation of Corneal Endothelium Parameters with the Severity of Primary Glaucoma. Indian J. Ophthalmol. 2022, 70, 3540–3543. [Google Scholar] [CrossRef]
- Reinprayoon, U.; Jermjutitham, M.; Kasetsuwan, N. Rate of Cornea Endothelial Cell Loss and Biomechanical Properties in Fuchs’ Endothelial Corneal Dystrophy. Front. Med. 2021, 8, 757959. [Google Scholar] [CrossRef] [PubMed]
- Ramm, L.; Spoerl, E.; Pillunat, L.E.; Terai, N. Is the Corneal Thickness Profile Altered in Diabetes Mellitus? Curr. Eye Res. 2020, 45, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, A.; Devasena, M.A.; Thomas, M.; Periyathambi, D. A Cross Sectional Study on Evaluating the Corneal Endothelial Cell Density and Central Corneal Thickness in Eyes with Primary Glaucoma. J. Fam. Med. Prim. Care 2022, 11, 4650–4654. [Google Scholar] [CrossRef]
- Akova-Budak, B.; Kıvanç, S.A. Does Corneal Hysteresis Correlate with Endothelial Cell Density? Med. Sci. Monit. 2015, 21, 1460. [Google Scholar] [CrossRef]
- Iancu, R.C.; Bujor, I.A.; Iliuță, C.; Ștefania, T.; Ungureanu, E.; Pașca, I.G.; Istrate, S. Correlations between Corneal Biomechanics and Specular Microscopy in Patient with Cataract. Rom. J. Ophthalmol. 2020, 64, 132. [Google Scholar] [CrossRef]
| Mean (±Standard Deviation) | |||||
|---|---|---|---|---|---|
| Entire Cohort (n = 74) | Hyperopic Study Group (n = 40) | Emmetropic Control Group (n = 34) | Mean Difference (Standard Error) | p Value | |
| Age (years) | 22.93 (±12.07) | 19.83 (±12.37) | 26.59 (±10.77) | −6.76 (±2.72) | 0.015 |
| SE (D) | 1.25 (±1.71) | 2.25 (±1.79) | 0.07 (±0.29) | 2.18 (±0.29) | <0.001 |
| AL (mm) | 23.03 (±0.86) | 22.54 (±0.68) | 23.61 (±0.69) | −1.07 (±0.16) | <0.001 |
| CCT (mm) | 0.568 (±0.034) | 0.566 (±0.030) | 0.569 (±0.038) | −0.003 (±0.008) | 0.738 |
| ACD (mm) | 3.47 (±0.38) | 3.37 (±0.40) | 3.59 (±0.33) | −0.22 (±0.09) | 0.013 |
| CH (mmHg) | 12.39 (±1.56) | 12.80 (±1.61) | 11.90 (±1.35) | 0.91 (±0.35) | 0.012 |
| CRF (mmHg) | 12.31 (±1.76) | 12.71 (±1.91) | 11.85 (±1.47) | 0.86 (±0.40) | 0.036 |
| CD (cells/mm2) | 3000.65 (±361.51) | 3075.70 (±377.27) | 2912.35 (±325.63) | 163.35 (±82.70) | 0.052 |
| CV (%) | 28.72 (±4.89) | 29.30 (±5.17) | 28.03 (±4.52) | 0.013 (±0.011) | 0.268 |
| Hex (%) | 65.68 (±5.26) | 65.68 (±5.32) | 65.68 (±5.27) | −0.00001 (±0.0123) | 0.999 |
| Hyperopic Study Group (n = 40) | Emmetropic Control Group (n = 34) | |||||
|---|---|---|---|---|---|---|
| Adult (n = 18) | Children (n = 22) | p Value | Adult (n = 21) | Children (n = 13) | p Value | |
| Age (years) | 32.22 (±6.60) | 9.68 (±3.06) | <0.001 | 34.14 (±5.55) | 14.38 (±2.47) | <0.001 |
| SE (D) | 2.49 (±2.10) | 2.06 (±1.51) | 0.458 | 0.01 (±0.29) | 0.17 (±0.26) | 0.110 |
| AL (mm) | 22.62 (±0.60) | 22.48 (±0.74) | 0.531 | 23.55 (±0.59) | 23.71 (±0.84) | 0.513 |
| CCT (mm) | 0.569 (±0.029) | 0.564 (±0.032) | 0.567 | 0.561 (±0.032) | 0.582 (±0.045) | 0.117 |
| ACD (mm) | 3.18 (±0.34) | 3.52 (±0.39) | 0.007 | 3.39 (±0.21) | 3.90 (±0.24) | <0.001 |
| CH (mmHg) | 11.94 (±1.69) | 13.51 (±1.17) | 0.001 | 11.35 (±1.04) | 12.79 (±1.35) | 0.001 |
| CRF (mmHg) | 11.88 (±2.00) | 13.39 (±1.56) | 0.011 | 11.18 (±1.17) | 12.93 (±1.28) | 0.001 |
| CD (cells/mm2) | 2833.33 (±321.70) | 3274.00 (±298.35) | <0.001 | 2749.52 (±213.85) | 3175.38 (±306.63) | <0.001 |
| CV (%) | 31.94 (±4.33) | 27.14 (±4.85) | 0.002 | 29.14 (±4.09) | 26.23 (±4.75) | 0.067 |
| Hex (%) | 65.28 (±5.45) | 66.00 (±5.31) | 0.675 | 66.86 (±4.39) | 63.77 (±6.15) | 0.097 |
| Pearson Correlation Coefficients (p Value) | |||
|---|---|---|---|
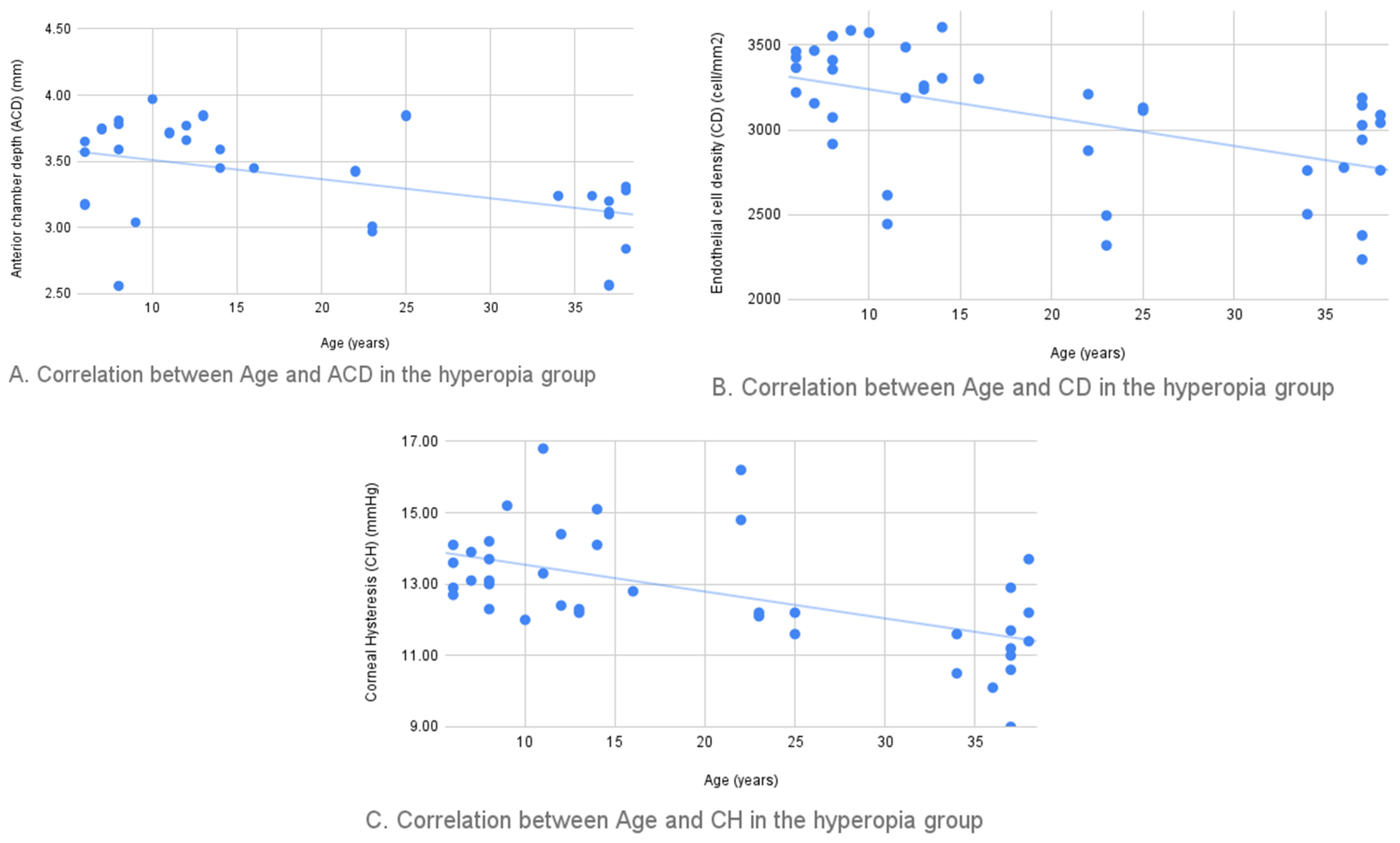
| Age-ACD | −0.447 (0.007) | Age-CV | 0.470 (0.004) |
| Age-CH | −0.544 (0.001) | Age-CD | −0.546 (0.001) |
| Age-CRF | −0.539 (0.001) | ACD-CD | 0.509 (0.002) |
| AL-CH | −0.335 (0.049) | ACD-CV | −0.528 (0.001) |
| AL-CRF | −0.334 (0.041) | CH-CD | 0.384 (0.023) |
| AL-SE | −0.593 (<0.001) | CRF-CV | −0.438 (0.009) |
| CCT-CH | 0.393 (0.019) | CH-CV | −0.379 (0.025) |
| CCT-CRF | 0.435 (0.009) | CD-CV | −0.396 (0.019) |
| Hex-CV | −0.480 (0.004) | ||
| Pearson Correlation Coefficients (p Value) | |||
|---|---|---|---|
| Age-ACD | −0.702 (<0.001) | Age-CV | 0.425 (0.014) |
| Age-CH | −0.455 (0.008) | ACD-CV | −0.390 (0.025) |
| Age-CRF | −0.445 (0.009) | Hex-CV | −0.463 (0.007) |
| CCT-CH | 0.574 (<0.001) | ACD-CD | 0.383 (0.028) |
| CCT-CRF | 0.571 (0.001) | CRF-CD | 0.560 (0.001) |
| ACD-CH | 0.566 (0.001) | CH-CD | 0.562 (0.001) |
| ACD-CRF | 0.561 (0.001) | SE-CD | 0.441 (0.010) |
| AL-CD | −0.452 (0.008) | Age-CD | −0.553 (0.001) |
| Hex-CD | −0.372 (0.033) | Hex-AL | 0.425 (0.014) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinescu, M.-C.; Dascalescu, D.-M.-C.; Constantin, M.-M.; Coviltir, V.; Potop, V.; Stanila, D.; Constantin, F.; Alexandrescu, C.; Ciuluvica, R.-C.; Voinea, L.-M. Particular Anatomy of the Hyperopic Eye and Potential Clinical Implications. Medicina 2023, 59, 1660. https://doi.org/10.3390/medicina59091660
Marinescu M-C, Dascalescu D-M-C, Constantin M-M, Coviltir V, Potop V, Stanila D, Constantin F, Alexandrescu C, Ciuluvica R-C, Voinea L-M. Particular Anatomy of the Hyperopic Eye and Potential Clinical Implications. Medicina. 2023; 59(9):1660. https://doi.org/10.3390/medicina59091660
Chicago/Turabian StyleMarinescu, Maria-Cristina, Dana-Margareta-Cornelia Dascalescu, Mihaela-Monica Constantin, Valeria Coviltir, Vasile Potop, Dan Stanila, Farah Constantin, Cristina Alexandrescu, Radu-Constantin Ciuluvica, and Liliana-Mary Voinea. 2023. "Particular Anatomy of the Hyperopic Eye and Potential Clinical Implications" Medicina 59, no. 9: 1660. https://doi.org/10.3390/medicina59091660
APA StyleMarinescu, M.-C., Dascalescu, D.-M.-C., Constantin, M.-M., Coviltir, V., Potop, V., Stanila, D., Constantin, F., Alexandrescu, C., Ciuluvica, R.-C., & Voinea, L.-M. (2023). Particular Anatomy of the Hyperopic Eye and Potential Clinical Implications. Medicina, 59(9), 1660. https://doi.org/10.3390/medicina59091660






