Leukaemic Presentation of Small-Cell Alk-Positive Anaplastic Large Cell Lymphoma in a Young Woman—Report of a Case with 9-Year Survival
Abstract
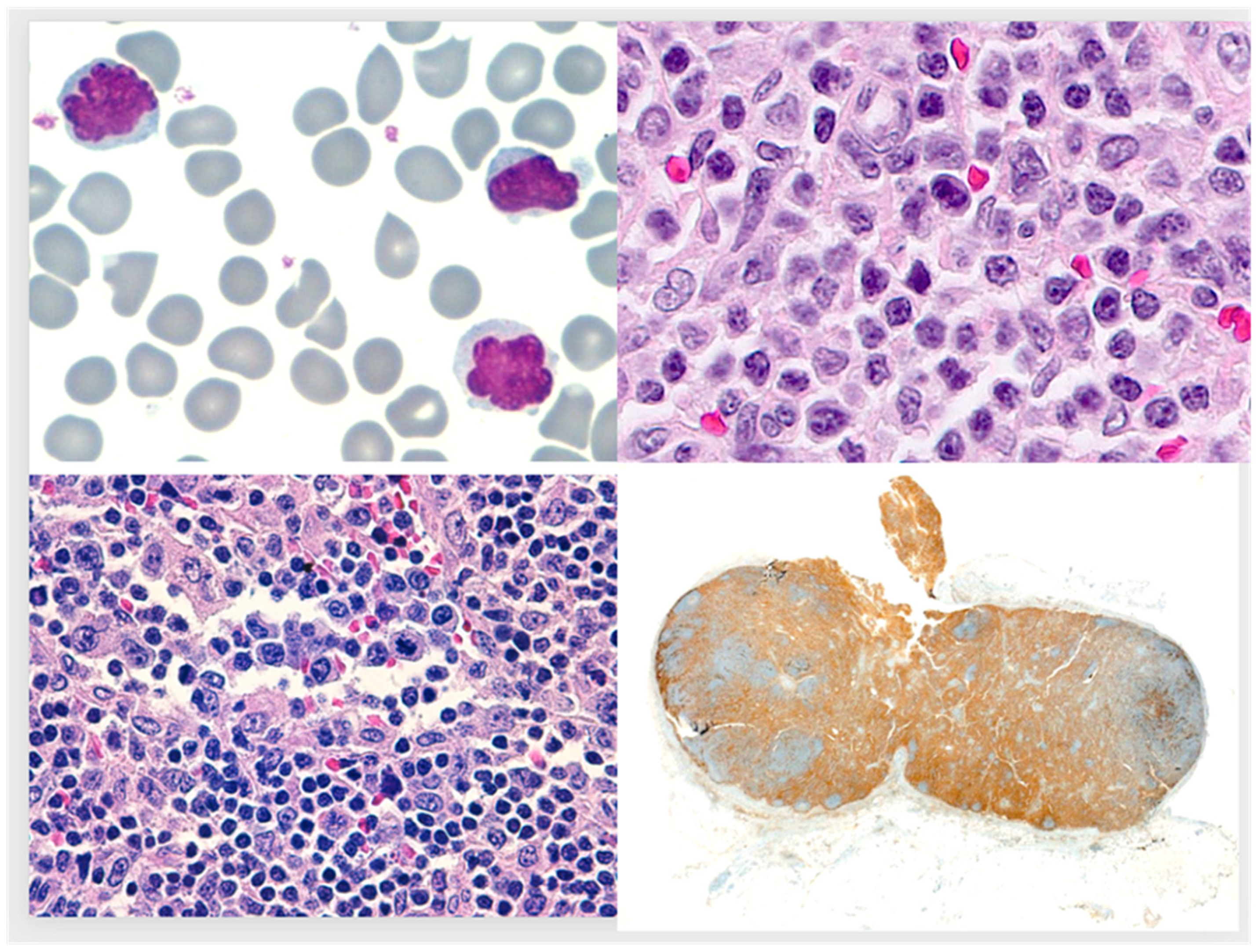
:1. Case Report
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability Statement
Conflicts of Interest
References
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martínez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A Report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Ikawa, Y.; Takenaka, M.; Sakai, Y.; Fujiki, T.; Kuroda, R.; Wada, T. Characterisation of two tumour cell populations in the small cell variant of anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Br. J. Haematol. 2022, 196, 241–243. [Google Scholar] [CrossRef]
- Bayle, C.; Charpentier, A.; Duchayne, E.; Manel, A.M.; Pages, M.P.; Robert, A.; Lamant, N.; Dastugue, N.; Bertrand, Y.; Dijoud, F.; et al. Leukaemic presentation of small cell variant anaplastic large cell lymphoma: Report of four cases. Br. J. Haematol. 1999, 104, 680–688. [Google Scholar] [CrossRef]
- Grewal, J.S.; Smith, L.B.; Winegarden, J.D., 3rd; Krauss, J.C.; Tworek, J.A.; Schnitzer, B. Highly aggressive ALK-positive anaplastic large cell lymphoma with a leukemic phase and multi-organ involvement: A report of three cases and a review of the literature. Ann. Hematol. 2007, 86, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Villamor, N.; Rozman, M.; Esteve, J.; Aymerich, M.; Colomer, D.; Aguilar, J.L.; Campo, E.; Montserrat, E. Anaplastic large-cell lymphoma with rapid evolution to leukemic phase. Ann. Hematol. 1999, 78, 478–482. [Google Scholar] [CrossRef]
- Lesesve, J.F.; Buisine, J.; Gregoire, M.J.; Raby, P.; Lederlin, P.; Béné, M.C.; Froment, N.; Laobuyrie, E. Leukaemic small cell variant anaplastic large cell lymphoma during pregnancy. Clin. Lab. Haematol. 2000, 22, 297–301. [Google Scholar] [CrossRef]
- Kong, S.Y.; Cho, H.J.; Suk, J.H.; Tak, E.Y.; Ko, Y.H.; Kim, K.; Kim, S.H. A novel complex t(2;5;13)(p23;q35;q14) in small cell variant type anaplastic large cell lymphoma with peripheral involvement. Cancer Genet. Cytogenet. 2004, 154, 183–185. [Google Scholar] [CrossRef]
- Spiegel, A.; Paillard, C.; Ducassou, S.; Perel, Y.; Plantaz, D.; Strullu, M.; Eischen, A.; Lutz, P.; Lamant, L.; Le Deley, M.C.; et al. Paediatric anaplastic large cell lymphoma with leukaemic presentation in children: A report of nine French cases. Br. J. Haematol. 2014, 165, 545–551. [Google Scholar] [CrossRef]
- Liu, H.; Hussein, S. Small-cell variant of ALK+ anaplastic large-cell lymphoma with a leukemic phase. Blood 2014, 124, 3175. [Google Scholar] [CrossRef]
- Al-Ahmad, S.; Maertens, V.; Libeer, C.; Schelfhout, V.; Vanhoenacker, F.; Boeckx, N.; Vandevenne, M. The masquerading presentation of a systemic anaplastic large cell lymphoma, ALK positive: A case report and review of the literature. Acta Clin. Belg. 2017, 72, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Zecchini Barrese, T.; Sagramoso, C.; Bacci, F.; Sabattini, E. Small cell variant of anaplastic large cell lymphoma with leukemic presentation: A diagnostic challenge. Rev. Bras. Hematol. Hemoter. 2017, 39, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhou, D. ALK+ small cell variant of anaplastic large cell lymphoma with leukemic presentation. Blood 2018, 131, 1764. [Google Scholar] [CrossRef] [PubMed]
- Graetz, D.; Crews, K.R.; Azzato, E.M.; Singh, R.K.; Raimondi, S.; Mason, J.; Valentine, M.; Mullighan, C.G.; Holland, A.; Inaba, H.; et al. Leukemic presentation of ALK-positive anaplastic large cell lymphoma with a novel partner, poly(A) binding protein cytoplasmic 1 (PABPC1), responding to single-agent crizotinib. Haematologica 2019, 104, e218–e221. [Google Scholar] [CrossRef] [PubMed]
- Kundoo, A.; Sethy, M.; Sable, M.N.; Mishra, P.; Panigrahi, A.; Adhya, A.K. Diagnosis of the leukemic phase of ALK-positive anaplastic large cell lymphoma by immunohistochemistry on cell block prepared from peripheral blood buffy coat. Indian J. Pathol. Microbiol. 2020, 63, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Ramteke, P.; Mathur, S.R.; Saxena, R.; Pati, H.P.; Mallick, S. Small cell variant anaplastic large cell lymphoma presenting as leukemia: A case report and review of Literature. Indian J. Pathol. Microbiol. 2022, 64, 705–708. [Google Scholar]
- Nguyen, J.T.; Condron, M.R.; Nguyen, N.D.; De, J.; Medeiros, L.J.; Padula, A. Anaplastic large cell lymphoma in leukemic phase: Extraordinarily high white blood cell count. Pathol. Int. 2009, 59, 345–353. [Google Scholar] [CrossRef]
- Kinney, M.C.; Collins, R.D.; Greer, J.P.; Whitlock, J.A.; Sioutos, N.; Kadin, M.E. A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am. J. Surg. Pathol. 1993, 17, 859–868. [Google Scholar] [CrossRef]
- Awaya, N.; Mori, S.; Takeuchi, H.; Sugano, Y.; Kamata, T.; Takeuchi, T.; Abe, T. CD30 and the NPM-ALK fusion protein (p80) are differentially expressed between peripheral blood and bone marrow in primary small cell variant of anaplastic large cell lymphoma. Am. J. Hematol. 2002, 69, 200–204. [Google Scholar] [CrossRef]
- Onciu, M.; Behm, F.G.; Raimondi, S.C.; Moore, S.; Harwood, E.L.; Pui, C.H.; Sandlund, J.T. ALK-positive anaplastic large cell lymphoma with leukemic peripheral blood involvement is a clinicopathologic entity with an unfavorable prognosis. Report of three cases and review of the literature. Am. J. Clin. Pathol. 2003, 120, 617–625. [Google Scholar] [CrossRef]
- Sano, F.; Tasaka, T.; Nishimura, H.; Akiyama, T.; Kubo, Y.; Matsuhashi, Y.; Wada, H.; Sugihara, T.; Sadahira, Y. Small cell variant of anaplastic large cell lymphoma diagnosed by a novel chromosomal abnormality t(2;5;3)(p23;q35;p21) of bone marrow cells. Pathol. Int. 2008, 58, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Nagatoshi, Y.; Nagayama, J.; Inagaki, J.; Itonoaga, N.; Takeshita, M.; Okamura, J. Anaplastic large cell lymphoma in leukemic presentation: A case report and a review of the literature. J. Pediatr. Hematol. Oncol. 2008, 30, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Trumper, L.; Ziepert, M.; Nickelsen, M.; Ho, A.D.; Metzner, B.; Peter, N.; Loeffler, M.; Rosenwald, A.; Pfreundschuh, M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: An analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood 2010, 116, 3418–3425. [Google Scholar] [CrossRef] [PubMed]
| Year, Author [Reference] | Sex/Age | Extent at Diagnosis (Other than PB or BM) | Treatment | Folllow-Up |
|---|---|---|---|---|
| 1993, Kinney [18] | M/17 | LNs, liver, skin | MEGA, autologous BM transplantation | Dead of infection at 1 mo |
| 1999, Villamor [6] | M/36 | LNs, liver, spleen | megaCHOP/ESHAP | Relapse at 2 mo |
| 1999, Bayle [4] | F/10 | Mediastinum | COPAD-M BCNU, vinblastine, cytarabine | 1st relapse at 8 w/ A&W at 11 mo |
| 1999, Bayle [4] | F/18 | Mediastinum, cervical LN, skin | CHOP/CT followed by HSCT | 1st relapse at 2 mo/ CR at 18 mo |
| 1999, Bayle [4] | F/20mo | Axillary LN, liver, spleen | COPAD-M/ vinblastine-HSCT | 1st relapse at 1 mo/ PDOD few mo later |
| 1999, Bayle [4] | M/7 | Skin, LNs, mediastinum | vinblastine, adriamycin, dexamethasone, methotrexate | SON |
| 2000, Lesesve [7] | F/28 | LNs, liver, spleen, skin | CHOP | DOD at 3 mo |
| 2002, Awaya [19] | M/63 | LNs, liver | CHOP | Dead of infection at 1 mo |
| 2003, Onciu [20] | F/6 | Lung, kidneys | doxorubicin, vincristine, prednisone; methotrexate, dexamethasone; lomustine, vinblastine, cytarabine; BM transplant | ANED at 17 mo |
| 2003, Onciu [20] | F/9mo | LNs, spleen, liver, lung, skin | corticosteroids, cyclophosphamide, vinblastine; dexamethasone, daunorubicin, asparaginase, methotrexate; cytarabine, etoposide. | DOD at 9 mo |
| 2003, Onciu [20] | M/10 | maxillary sinus, LNs, CNS | methotrexate, ifosfamide, etoposide, dexamethasone; doxorubicin, vincristine, prednisone;methotrexate, cladribine, vinblastine. | DOD at 2 ys |
| 2004, Kong [8] | F/32 | LNs, mediastinum, liver, spleen, CNS | CHOP | DOD at 2 mo |
| 2007, Grewal [5] | M/29 | LNs, mediastinum, liver, spleen | daunorubicin, vincristine, prednisone; Hyper-CVAD; ICE; HSCT | DOD 1 mo after HSCT |
| 2007, Grewal [5] | M/11 | LNs, liver, spleen, colon/CNS | CCG-5941/D-ICE/ /cranial irradiation | DOD 3 mo |
| 2007, Grewal [5] | F/59 | Skin, liver | CHOP | DOD 1 mo |
| 2008, Takahashi [22] | M/10 | LNs, liver, spleen | dexamethasone, cytarabine, vindesine | Dead of infection before treatment end |
| 2008, Sano [21] | F/23 | LNs, liver, spleen, skin | CHOP, autologous BM transplant | Alive at time of report |
| 2009 Nguyen [17] | M/26 | LNs | cyclophosphamide, vincristine, doxorubicin, dexamethasone, intrathecal methotrexate and cytarabine | DOD 2.5 mo |
| 2014, Spiegel [9] | F/10 | LNs, skin, liver, spleen, lung | ALCL 99/CT + HSCT | Alive 23 mo after HSCT |
| 2014, Spiegel [9] | M/13mo | LNs, skin, liver, spleen, lung, CSF | ALCL 99 | Dead PD |
| 2014, Spiegel [9] | F/20mo | LNs, liver, spleen | SFOP-HM 91/vinblastine +HSCT | Dead (toxicity) |
| 2014, Spiegel [9] | M/11 | LNs, skin, iver, spleen, lung | ALCL 99/HSCT | Alive 63mo |
| 2014, Spiegel [9] | F/17 | LNs, liver, spleen, lung | ALCL 99/LMB 96 protocol/vinblastine + HSCT | Dead PD |
| 2014, Spiegel [9] | M/3 | LNs, liver, spleen, lung, CSF | LMB 96 protocol/methotrexate, araC, etoposide/autoSCT/ICI + HSCT | Dead (toxicity) |
| 2014, Spiegel [9] | F/6 | LNs, skin, liver, spleen, lung | ALCL 99 protocol/vinblastine + CT + HSCT | Dead (toxicity) |
| 2014, Spiegel [9] | F/12 | LNs, liver, spleen | ALCL 99 protocol + vinblastine maintenance | Alive 34 mo |
| 2014, Spiegel [9] | F/4 | LNs, skin, liver, spleen, lung, kidney | ALCL 99 protocol/Crizotinib | Alive 15 mo |
| 2014 Liu [10] | M/24 | LNs, retroperitoneal | NS | NS |
| 2017 Al-Ahmad [11] | F/57 | LNs, liver, spleen | CHOEP | CR “Dec. 2016” |
| 2017 Zecchini [12] | F/37 | LNs | MACOP-B | NS |
| 2018 Jiang [13] | M/47 | LN | Brentuximab vedotin + CT | Clinical improvement |
| 2019 Graetz [14] | M/16mo | LNs | ALCL99protocol/crizotinib | In remission |
| 2020 Kundoo [15] | M/25 | NS | None | DOD |
| 2022 Noguchi [3] | M/10 | liver, spleen | ALCL99protocol/alectinib/HSCT | NS |
| 2022 Dutta [16] | M/68 | None | NS | DOD |
| Present case | F/24 | LNs | CHOEP | ANED 114 mo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santonja, C.; Morillo-Giles, D.; Prieto-Pareja, E.; Soto-de Ozaeta, C.; Serrano-del Castillo, C.; Salgado-Sánchez, R.; Yi-Shi, A.-W.-Y.; Manso, R.; Rodríguez-Pinilla, S.M. Leukaemic Presentation of Small-Cell Alk-Positive Anaplastic Large Cell Lymphoma in a Young Woman—Report of a Case with 9-Year Survival. Medicina 2023, 59, 1628. https://doi.org/10.3390/medicina59091628
Santonja C, Morillo-Giles D, Prieto-Pareja E, Soto-de Ozaeta C, Serrano-del Castillo C, Salgado-Sánchez R, Yi-Shi A-W-Y, Manso R, Rodríguez-Pinilla SM. Leukaemic Presentation of Small-Cell Alk-Positive Anaplastic Large Cell Lymphoma in a Young Woman—Report of a Case with 9-Year Survival. Medicina. 2023; 59(9):1628. https://doi.org/10.3390/medicina59091628
Chicago/Turabian StyleSantonja, Carlos, Daniel Morillo-Giles, Elena Prieto-Pareja, Carlos Soto-de Ozaeta, Cristina Serrano-del Castillo, Rocío Salgado-Sánchez, Ana-Wu-Yang Yi-Shi, Rebeca Manso, and Socorro María Rodríguez-Pinilla. 2023. "Leukaemic Presentation of Small-Cell Alk-Positive Anaplastic Large Cell Lymphoma in a Young Woman—Report of a Case with 9-Year Survival" Medicina 59, no. 9: 1628. https://doi.org/10.3390/medicina59091628
APA StyleSantonja, C., Morillo-Giles, D., Prieto-Pareja, E., Soto-de Ozaeta, C., Serrano-del Castillo, C., Salgado-Sánchez, R., Yi-Shi, A.-W.-Y., Manso, R., & Rodríguez-Pinilla, S. M. (2023). Leukaemic Presentation of Small-Cell Alk-Positive Anaplastic Large Cell Lymphoma in a Young Woman—Report of a Case with 9-Year Survival. Medicina, 59(9), 1628. https://doi.org/10.3390/medicina59091628






