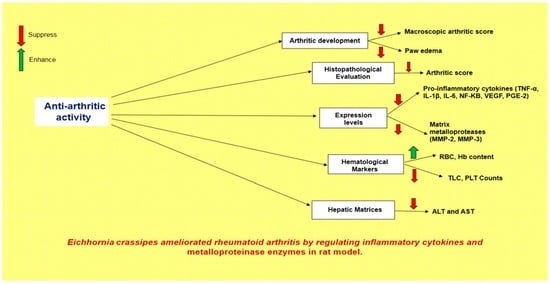
Eichhornia crassipes Ameliorated Rheumatoid Arthritis by Modulating Inflammatory Cytokines and Metalloproteinase Enzymes in a Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Identification
2.2. Preparation of Methanol Extract and Fractions
2.3. Test Animals
2.4. Assessment of Anti-Arthritic Effect
2.5. Measurement of Arthritic Score and Paw Volume
2.6. Histopathological Evaluation
2.7. Analysis of mRNA Expression Values of the Pro-Inflammatory Cytokines TNF-α, IL-1β, IL-6, NF-κB, MMP-2, MMP-3, and VEGF
2.8. Determination of PGE2 Levels
2.9. Assessment of Hematological Parameters
2.10. Biochemical Parameters
2.11. GC-MS Investigation
2.12. Statistical Analysis
3. Results
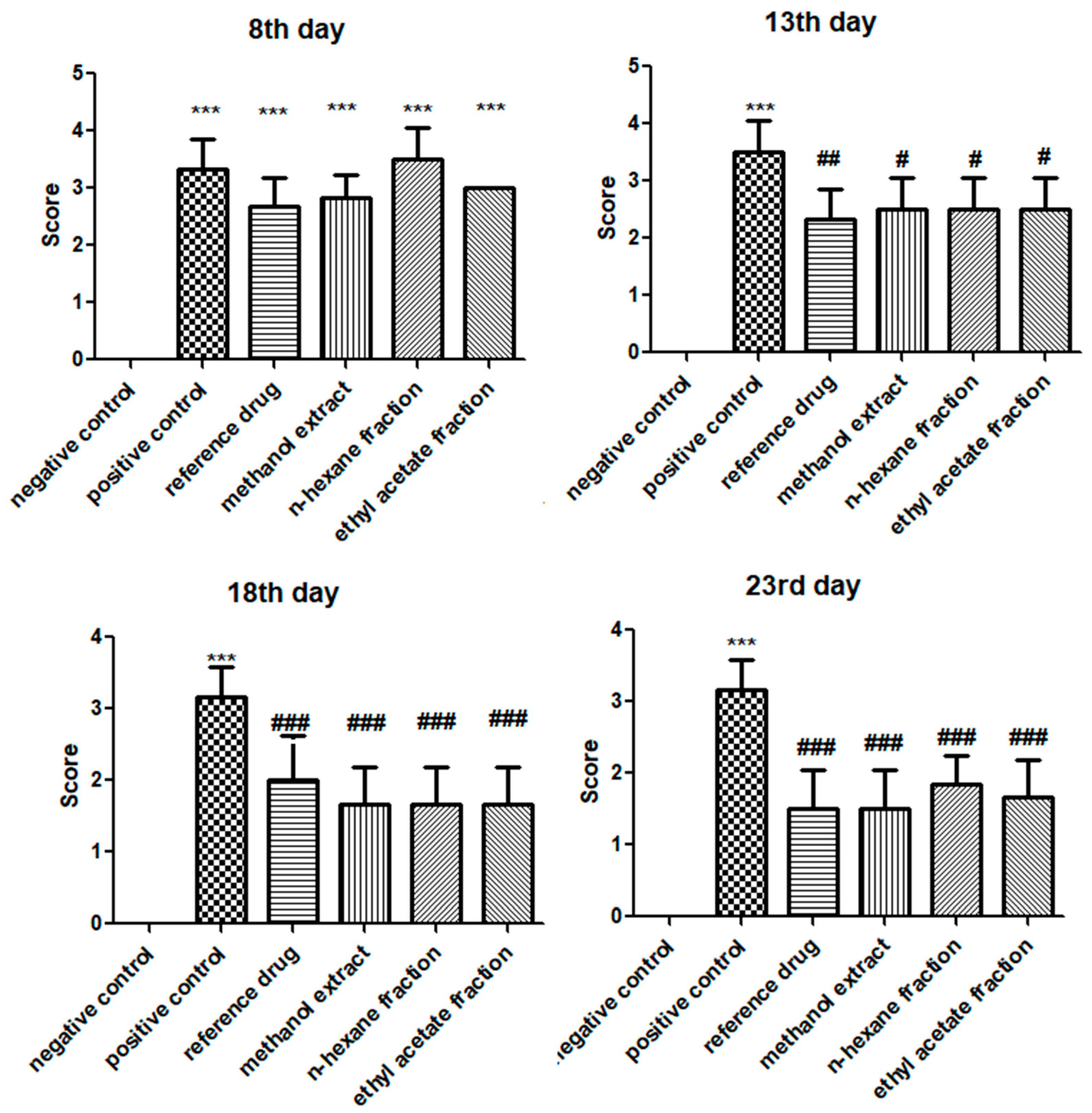
3.1. E. crassipes Repressed Arthritic Development
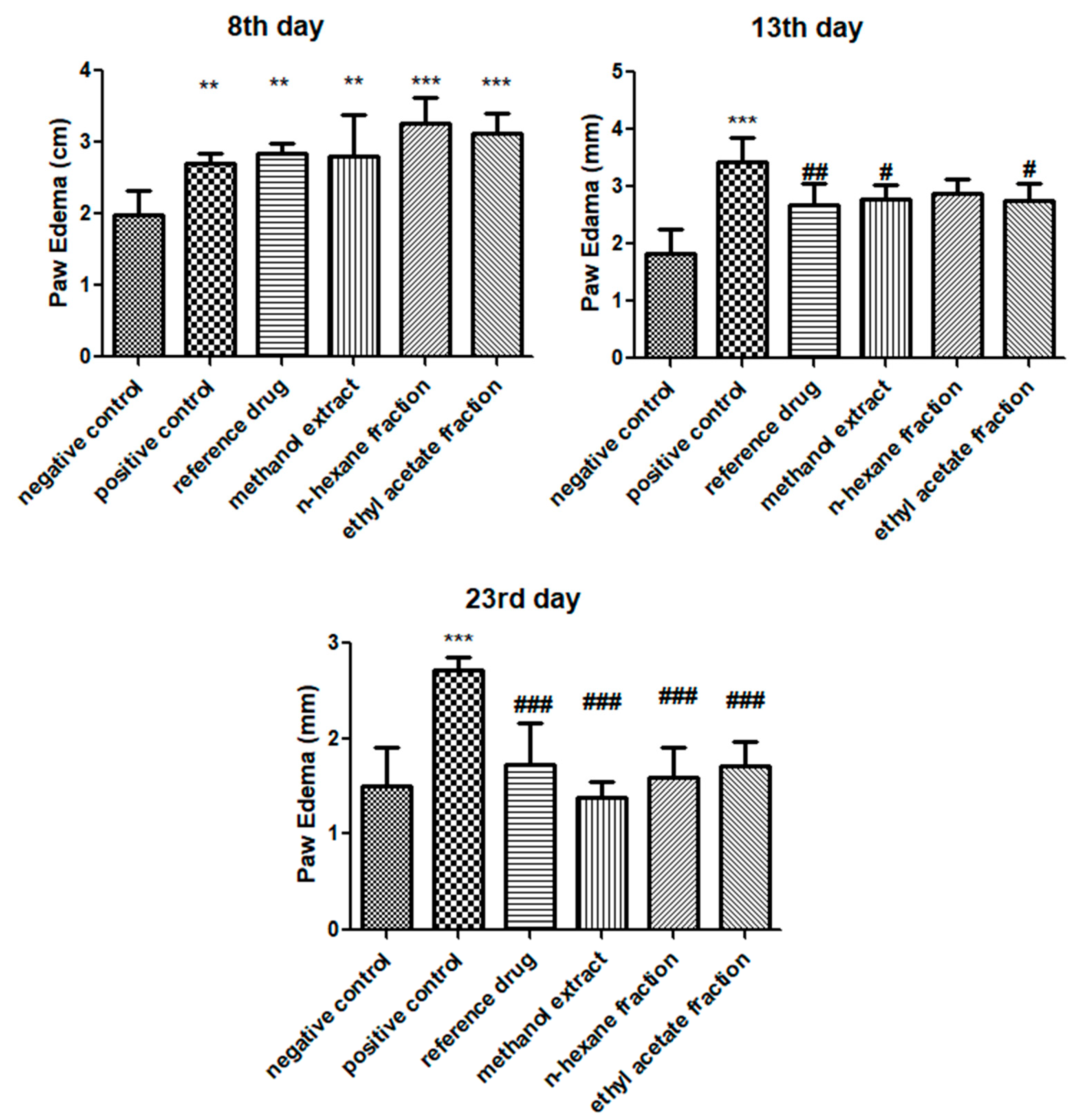
3.2. E. crassipes Decreased Paw Edema
3.3. E. crassipes Attenuated Histopathological Scoring
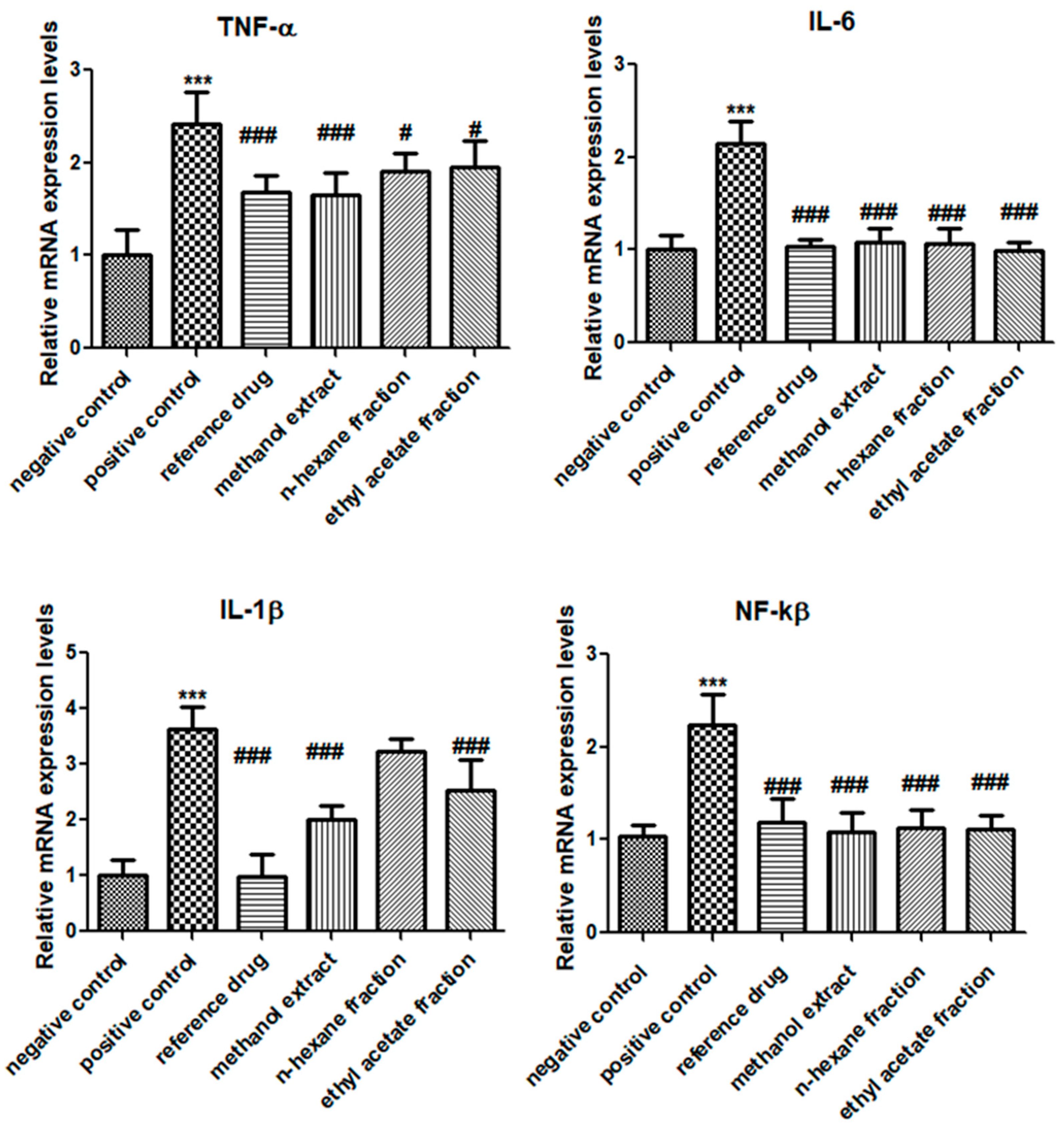
3.4. E. crassipes Down-Regulated TNF-α, IL-1β, IL-6, and NF-κB
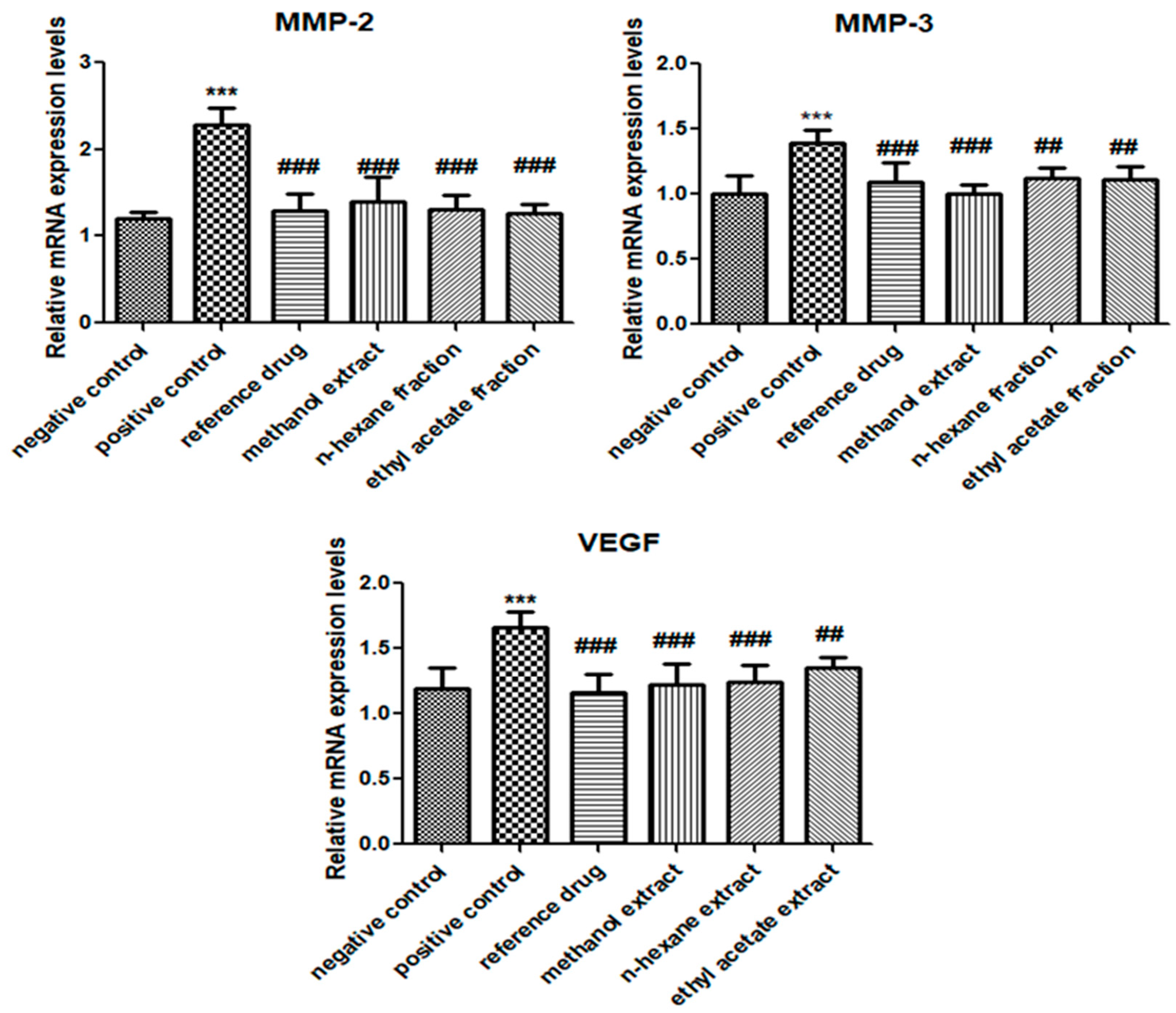
3.5. E. crassipes Attenuated MMP-2, MMP-3, and VEGF Expression Levels
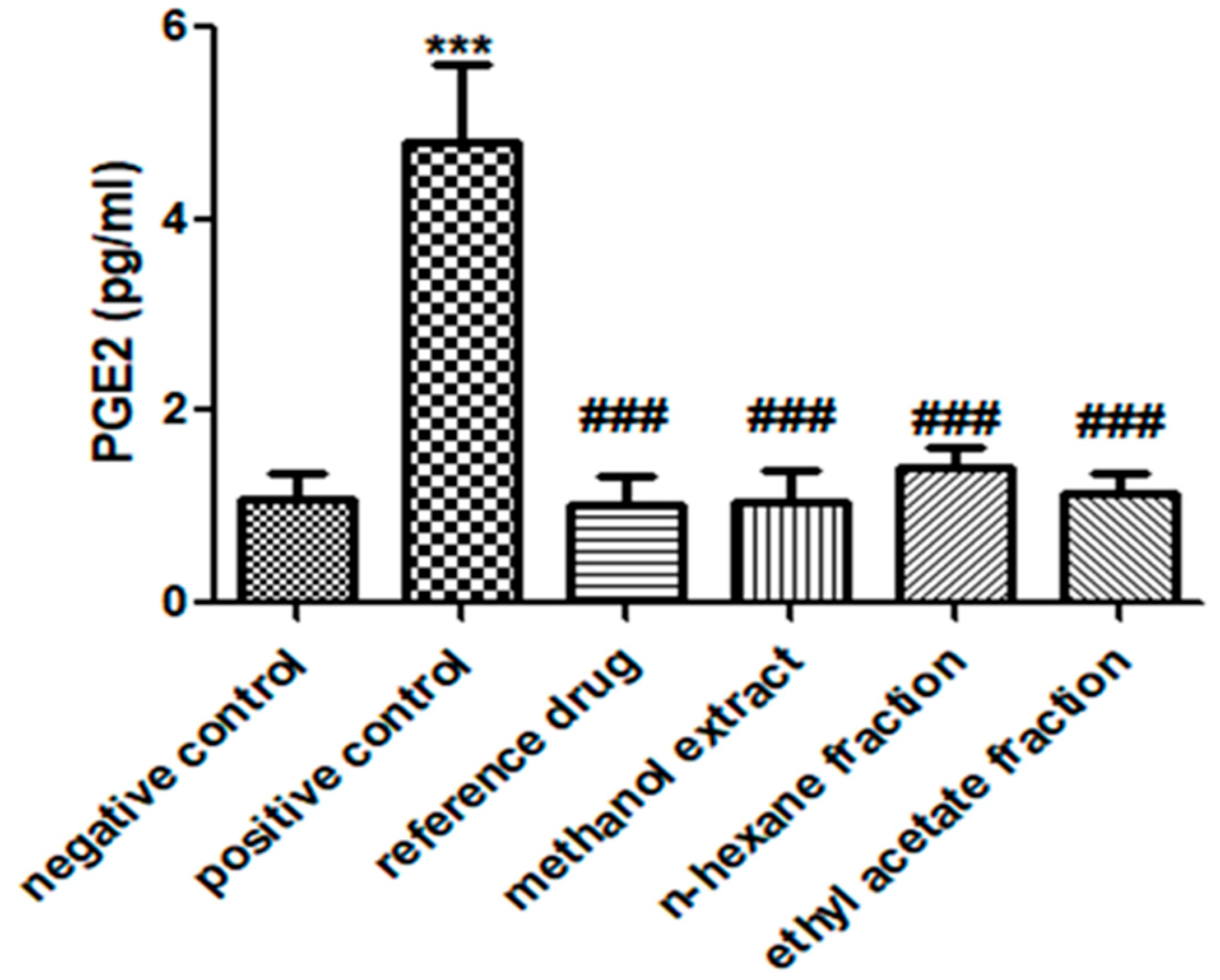
3.6. E. crassipes Significantly Reduced PGE2 Levels
3.7. E. Crassipes Modulated Hematological Markers
3.8. E. crassipes Improved Markers of Liver Function Tests
3.9. No Nephrotoxic Effect of E. crassipes Was Observed
3.10. GC-MS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.-B.; Liu, Y.-P.; Yang, Y.-Y.; Liu, X.-Y.; Xiang, D.-X. Anti-arthritic effect of total saponins from Clematis henryi Oliv. on collagen-induced arthritis rats. Eur. J. Inflamm. 2016, 14, 71–77. [Google Scholar] [CrossRef]
- Monti, S.; Montecucco, C.; Bugatti, S.; Caporali, R. Rheumatoid arthritis treatment: The earlier the better to prevent joint damage. RMD Open 2015, 1, e000057. [Google Scholar] [CrossRef] [PubMed]
- Romao, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef]
- Sudoł-Szopińska, I.; Kontny, E.; Maśliński, W.; Prochorec-Sobieszek, M.; Kwiatkowska, B.; Zaniewicz-Kaniewska, K.; Warczyńska, A. The pathogenesis of rheumatoid arthritis in radiological studies. Part I: Formation of inflammatory infiltrates within the synovial membrane. J. Ultrason. 2012, 12, 202. [Google Scholar] [CrossRef]
- Gautam, R.K.; Singh, D.; Nainwani, R. Medicinal plants having anti-arthritic potential: A review. Int. J. Pharm. Sci. Rev. Res 2013, 19, 96–102. [Google Scholar]
- Radu, A.F.; Bungau, S.G.; Negru, P.A.; Marcu, M.F.; Andronie-Cioara, F.L. In-depth bibliometric analysis and current scientific mapping research in the context of rheumatoid arthritis pharmacotherapy. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 154, 113614. [Google Scholar] [CrossRef] [PubMed]
- Avau, A.; Mitera, T.; Put, S.; Put, K.; Brisse, E.; Filtjens, J.; Uyttenhove, C.; Van Snick, J.; Liston, A.; Leclercq, G. Systemic juvenile idiopathic arthritis–like syndrome in mice following stimulation of the immune system with Freund’s complete adjuvant: Regulation by interferon-γ. Arthritis Rheumatol. 2014, 66, 1340–1351. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N.; et al. The global burden of rheumatoid arthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef]
- Machado-Duque, M.E.; Ramirez-Valencia, D.M.; Murillo-Munoz, M.M.; Machado-Alba, J.E. Trends in Opioid Use in a Cohort of Patients with Rheumatoid Arthritis. Pain Res. Manag. 2020, 2020, 3891436. [Google Scholar] [CrossRef]
- Yap, H.Y.; Tee, S.Z.; Wong, M.M.; Chow, S.K.; Peh, S.C.; Teow, S.Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef]
- Zago, B.A.; Priyadharshini, A.; Vijayakumar, T.M. Safety and efficacy of newer biologics DMARDs in the management of rheumatoid arthritis: A systematic review. Osteoarthr. Cartil. Open 2020, 2, 100116. [Google Scholar] [CrossRef]
- Dixon, W.G.; Hyrich, K.L.; Watson, K.D.; Lunt, M.; Galloway, J.; Ustianowski, A.; Consortium, B.S.R.B.R.C.C.; Symmons, D.P.; Register, B.S.R.B. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register (BSRBR). Ann. Rheum. Dis. 2010, 69, 522–528. [Google Scholar] [CrossRef]
- Park, D.W.; Kim, Y.J.; Sung, Y.K.; Chung, S.J.; Yeo, Y.; Park, T.S.; Lee, H.; Moon, J.Y.; Kim, S.H.; Kim, T.H.; et al. TNF inhibitors increase the risk of nontuberculous mycobacteria in patients with seropositive rheumatoid arthritis in a mycobacterium tuberculosis endemic area. Sci. Rep. 2022, 12, 4003. [Google Scholar] [CrossRef]
- Edwards, C.J. Immunological therapies for rheumatoid arthritis. Br. Med. Bull. 2005, 73, 71–82. [Google Scholar] [CrossRef]
- Gichuki, J.; Omondi, R.; Boera, P.; Okorut, T.; Matano, A.S.; Jembe, T.; Ofulla, A. Water hyacinth Eichhornia crassipes (Mart.) Solms-Laubach dynamics and succession in the Nyanza Gulf of Lake Victoria (east Africa): Implications for water quality and biodiversity conservation. Sci. World J. 2012, 2012, 106429. [Google Scholar] [CrossRef]
- Jayanthi, P.; Lalitha, P.; Sujitha, R.; Thamaraiselvi, A. Anti-inflammatory activity of the various solvent extracts of Eichhornia crassipes (Mart.) Solms. Int. J. Pharm. Tech. Res. 2013, 5, 641–644. [Google Scholar]
- Fileto-Pérez, H.A.; Rutiaga-Quiñones, O.M.; Sytsma, M.D.; Lorne, I.M.; Luo, W.; Pankow, J.F.; Rutiaga-Quiñones, J.G. GC/MS analysis of some extractives from Eichhornia crassipes. BioResources 2015, 10, 7353–7360. [Google Scholar] [CrossRef]
- Ijaz, B.; Shabbir, A.; Shahzad, M.; Mobashar, A.; Sharif, M.; Basheer, M.I.; Tareen, R.B.; Syed, N.I. Amelioration of airway inflammation and pulmonary edema by Teucrium stocksianum via attenuation of pro-inflammatory cytokines and up-regulation of AQP1 and AQP5. Respir. Physiol. Neurobiol. 2021, 284, 103569. [Google Scholar] [CrossRef]
- Shabbir, A.; Batool, S.A.; Basheer, M.I.; Shahzad, M.; Sultana, K.; Tareen, R.B.; Iqbal, J. Ziziphora clinopodioides ameliorated rheumatoid arthritis and inflammatory paw edema in different models of acute and chronic inflammation. Biomed. Pharmacother. 2018, 97, 1710–1721. [Google Scholar] [CrossRef]
- Kyei, S.; Koffuor, G.A.; Boampong, J.N. Antiarthritic effect of aqueous and ethanolic leaf extracts of Pistia stratiotes in adjuvant-induced arthritis in Sprague-Dawley rats. J. Exp. Pharmacol. 2012, 4, 41. [Google Scholar]
- Akhtar, G.; Shabbir, A. Urginea indica attenuated rheumatoid arthritis and inflammatory paw edema in diverse animal models of acute and chronic inflammation. J. Ethnopharmacol. 2019, 238, 111864. [Google Scholar] [CrossRef] [PubMed]
- Mobashar, A.; Shabbir, A.; Shahzad, M.; Gobe, G. Preclinical rodent models of arthritis and acute inflammation indicate Immunomodulatory and Anti-Inflammatory Properties of Juglans regia Extracts. Evid. Based Complement. Altern. Med. 2022, 2022, 1695701. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhou, H.; Wong, Y.F.; Xie, Y.; Liu, Z.Q.; Jiang, Z.H.; Bian, Z.X.; Xu, H.X.; Liu, L. Suppression of the onset and progression of collagen-induced arthritis in rats by QFGJS, a preparation from an anti-arthritic Chinese herbal formula. J. Ethnopharmacol. 2007, 110, 39–48. [Google Scholar] [CrossRef]
- Uttra, A.M.; Alamgeer; Shahzad, M.; Shabbir, A.; Jahan, S. Ephedra gerardiana aqueous ethanolic extract and fractions attenuate Freund Complete Adjuvant induced arthritis in Sprague Dawley rats by downregulating PGE2, COX2, IL-1β, IL-6, TNF-α, NF-kB and upregulating IL-4 and IL-10. J. Ethnopharmacol. 2018, 224, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Shahzad, M.; Ali, A.; Zia-Ur-Rehman, M. Discovery of New Benzothiazine Derivative as Modulator of Pro- and Anti-inflammatory Cytokines in Rheumatoid Arthritis. Inflammation 2016, 39, 1918–1929. [Google Scholar] [CrossRef]
- Zhu, J.; Su, C.; Chen, Y.; Hao, X.; Jiang, J. Electroacupuncture on ST36 and GB39 Acupoints Inhibits Synovial Angiogenesis via Downregulating HIF-1α/VEGF Expression in a Rat Model of Adjuvant Arthritis. Evid. Based Complement. Altern. Med. 2019, 2019, 5741931. [Google Scholar] [CrossRef]
- Shabbir, A.; Shahzad, M.; Ali, A.; Zia-ur-Rehman, M. Anti-arthritic activity of N′-[(2,4-dihydroxyphenyl)methylidene]-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-1(4H)-yl)acetohydrazide. Eur. J. Pharmacol. 2014, 738, 263–272. [Google Scholar] [CrossRef]
- Nasuti, C.; Fedeli, D.; Bordoni, L.; Piangerelli, M.; Servili, M.; Selvaggini, R.; Gabbianelli, R. Anti-Inflammatory, Anti-Arthritic and Anti-Nociceptive Activities of Nigella sativa Oil in a Rat Model of Arthritis. Antioxidants 2019, 8, 342. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.P.; Kumar, B.; Singh, S.B.; Ganju, L. Andrographolide attenuates complete freund’s adjuvant induced arthritis via suppression of inflammatory mediators and pro-inflammatory cytokines. J. Ethnopharmacol. 2020, 261, 113022. [Google Scholar] [CrossRef]
- Aboul-Enein, A.M.; Al-Abd, A.M.; Shalaby, E.; Abul-Ela, F.; Nasr-Allah, A.A.; Mahmoud, A.M.; El-Shemy, H.A. Eichhornia crassipes (Mart) solms: From water parasite to potential medicinal remedy. Plant Signal Behav. 2011, 6, 834–836. [Google Scholar] [CrossRef]
- Haq, M.M.; Chowdhury, M.A.R.; Tayara, H.; Abdelbaky, I.; Islam, M.S.; Chong, K.T.; Jeong, S. A Report on Multi-Target Anti-Inflammatory Properties of Phytoconstituents from Monochoria hastata (Family: Pontederiaceae). Molecules 2021, 26, 7397. [Google Scholar] [CrossRef]
- Khuntia, A.; Martorell, M.; Ilango, K.; Bungau, S.G.; Radu, A.F.; Behl, T.; Sharifi-Rad, J. Theoretical evaluation of Cleome species’ bioactive compounds and therapeutic potential: A literature review. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 151, 113161. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Gautam, D.N.S.; Radu, A.F.; Behl, T.; Bungau, S.G.; Vesa, C.M. Reviewing the Traditional/Modern Uses, Phytochemistry, Essential Oils/Extracts and Pharmacology of Embelia ribes Burm. Antioxidants 2022, 11, 1359. [Google Scholar] [CrossRef] [PubMed]
- Yogisha, S.; Samiulla, D.S.; Prashanth, D.; Padmaja, R.; Amit, A. Trypsin inhibitory activity of Lawsonia inermis. Fitoterapia 2002, 73, 690–691. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. Matrix Metalloproteinase Gene Activation Resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int. J. Mol. Sci. 2017, 18, 905. [Google Scholar] [CrossRef]
- Moelants, E.A.; Mortier, A.; Van Damme, J.; Proost, P. Regulation of TNF-alpha with a focus on rheumatoid arthritis. Immunol. Cell Biol. 2013, 91, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, W.; Zhang, M.; Chen, C. Taraxasterol suppresses inflammation in IL-1beta-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Int. Immunopharmacol. 2019, 70, 274–283. [Google Scholar] [CrossRef]
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling pathways in rheumatoid arthritis: Implications for targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.A.; Kwok, S.K.; Kim, W.U. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: Prospects for therapeutic intervention. Mediat. Inflamm. 2008, 2008, 129873. [Google Scholar] [CrossRef]
- Seibl, R.; Birchler, T.; Loeliger, S.; Hossle, J.P.; Gay, R.E.; Saurenmann, T.; Michel, B.A.; Seger, R.A.; Gay, S.; Lauener, R.P. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am. J. Pathol. 2003, 162, 1221–1227. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, Y.; Adebayo, A.; Pope, R.M. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2007, 56, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Cho, M.-L.; Kim, K.-W.; Juhn, J.-Y.; Hwang, S.-Y.; Yoon, C.-H.; Park, S.-H.; Lee, S.-H.; Kim, H.-Y. Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-, NF-κB-and p38 MAPK-dependent signalling pathways. Rheumatology 2007, 46, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, K.R.; Korotkova, M.; Catrina, A.I.; Backman, L.; Af Klint, E.; Claesson, H.-E.; Rådmark, O.; Jakobsson, P.-J. Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res. Ther. 2009, 11, R83. [Google Scholar] [CrossRef] [PubMed]
- Noufal, K.P.; Rajesh, B.; Nair, S.S. Antiproliferative Effects of the Methanolic Petiole Extract of Eichhornia crassipes Against Sloan Kettering Melanoma 5 Cell Line: An In Vitro Study. Cureus 2022, 14, e30554. [Google Scholar] [CrossRef]
- Jia, X.Y.; Chang, Y.; Sun, X.J.; Dai, X.; Wei, W. The role of prostaglandin E2 receptor signaling of dendritic cells in rheumatoid arthritis. Int. Immunopharmacol. 2014, 23, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.J.; Mirshafiey, A. Prostaglandins and rheumatoid arthritis. Arthritis 2012, 2012, 239310. [Google Scholar] [CrossRef]
- Hashimoto, M.; Fujii, T.; Hamaguchi, M.; Furu, M.; Ito, H.; Terao, C.; Yamamoto, K.; Yamamoto, W.; Matsuo, T.; Mori, M.; et al. Increase of hemoglobin levels by anti-IL-6 receptor antibody (tocilizumab) in rheumatoid arthritis. PLoS ONE 2014, 9, e98202. [Google Scholar] [CrossRef]
- Ekambaram, S.; Perumal, S.S.; Subramanian, V. Evaluation of antiarthritic activity of Strychnos potatorum Linn seeds in Freund’s adjuvant induced arthritic rat model. BMC Complement. Altern. Med. 2010, 10, 56. [Google Scholar] [CrossRef]
- Shanab, S.M.; Shalaby, E.A.; Lightfoot, D.A.; El-Shemy, H.A. Allelopathic effects of water hyacinth [Eichhornia crassipes]. PLoS ONE 2010, 5, e13200. [Google Scholar] [CrossRef]
- Vanco, J.; Travnicek, Z.; Hosek, J.; Suchy, P., Jr. In vitro and in vivo anti-inflammatory active copper(II)-lawsone complexes. PLoS ONE 2017, 12, e0181822. [Google Scholar] [CrossRef]
- Sandeep Birdar, B.V. Protective effects of Lawsone in L-Arginine induced pancreatitis in rats. Indian J. Exp. Biol. 2012, 51, 256–261. [Google Scholar]
- Manivannan, P.; Muralitharan, G.; Balaji, N.P. Prediction aided in vitro analysis of octa-decanoic acid from Cyanobacterium Lyngbya sp. as a proapoptotic factor in eliciting anti-inflammatory properties. Bioinformation 2017, 13, 301–306. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Park, C.; Park, J.; Kim, W.J.; Kim, W.; Cheong, H.; Kim, S.J. Malonic Acid Isolated from Pinus densiflora Inhibits UVB-Induced Oxidative Stress and Inflammation in HaCaT Keratinocytes. Polymers 2021, 13, 816. [Google Scholar] [CrossRef]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Xiao, Z.; Xu, C.; Fan, Y.; Liu, W.; Qiu, J.; Zhang, Q.; et al. Anti-Inflammatory and Antioxidant Properties of Squalene in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2023, 24, 8518. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G. Nanomedical approaches in the realm of rheumatoid arthritis. Ageing Res. Rev. 2023, 87, 101927. [Google Scholar] [CrossRef]

| Genes | Forward Primer | Reverse Primer | Annealing Temperature | Product Size | |
|---|---|---|---|---|---|
| IL-1β | 5′-CCTGCTAGTGTGTGATGTTC-3′ | 5′-GAGGTGCTGATGTACCAGTT-3′ | 58 °C | 390 | ENSRNOG0000004649 |
| IL-6 | 5′-AGACTTCCAGCCAGTTGCCT-3′ | 5′-CTGACAGTGCATCATCGCTG-3′ | 60 °C | 233 | [24] |
| TNF-α | 5′-CCTCTTCTCATTCCTGCTCGT-3″ | 5′-TGAGATCCATGCCATTGGCC-3′ | 60 °C | 266 | [25] |
| NF-κB | 5′-CAAGGAAGAGGATGTGGGGTT-3 | 5′-AGCTGAGCATGAAGGTGGATG-3′ | 60 °C | 207 | [25] |
| VEGF | 5′-GTTCAGAGCGGAGAAAGCATT-3 | 5′-CTTGCAACGCGAGTCTGTGT-3′ | 60 °C | 80 | [26] |
| MMP-2 | 5′-CGAACAAGTATGAGAGCTGC-3′ | 5′-CGGTCATCATCGTAGTTGGT-3′ | 57 °C | 85 | ENSRNOG00000016695 |
| MMP-3 | 5′-CCTTTTGATGGGCCTGGAAT-3′ | 5′-GTGACATCATCTGTCCATCG-3′ | 58 °C | 107 | ENSRNOG00000032626 |
| GAPDH | 5′-GTCATCAACGGGAAACCCAT-3′ | 5′-CTTGCCGTGGGTAGAGTCAT-3 | 60 °C | 229 | [27] |
| Hematological Parameters | Vehicle Control Group | Arthritic Control Group | Reference Drug Group | Methanol Extract | n-hexane Fraction | Ethyl Acetate Fraction |
|---|---|---|---|---|---|---|
| RBC | 8.29 ± 0.666 | 6.9 ± 0.6 *** | 7.9 ± 0.3 ## | 7.66 ± 0.275 # | 7.81 ± 0.32 # | 7.64 ± 0.46 # |
| WBC | 9.60 ± 1.607 | 10.75 ± 0.736 | 9.7 ± 1.288 | 10.15 ± 0.717 | 9.47 ± 1.3 | 9.68 ± 1.422 |
| PLT | 660.5 ± 37.05 | 776.7 ± 48.12 ** | 684.2 ± 62.86 # | 691.5 ± 42.93 # | 685.3 ± 25.46 # | 685.2 ± 42.44 # |
| Hb | 11.73 ± 0.326 | 10.35 ± 0.796 *** | 11.80 ± 0.334 ### | 11.88 ± 0.354 ### | 11.75 ± 0.301 ### | 11.78 ± 0.23 ### |
| Biochemical Parameters | Vehicle Control Group | Arthritic Control Group | Reference Drug Group | Methanol Extract | n-hexane Fraction | Ethyl Acetate Fraction |
|---|---|---|---|---|---|---|
| ALT | 41.33 ± 9.180 | 59.50 ± 3.391 *** | 47.83 ± 4.792 # | 48.83 ± 6.21 # | 45.67 ± 5.046 ## | 44.67 ± 4.590 ## |
| AST | 278.3 ± 58.29 | 386.7 ± 44.57 ** | 291.0 ± 27.21 ## | 298.5 ± 41.23 ## | 307.2 ± 13.0 # | 289.7 ± 46.74 ## |
| Urea | 19.83 ± 5.981 | 19.5 ± 2.665 | 17.17 ± 2.48 | 16.33 ± 3.83 | 15.83 ± 3.371 | 15.83 ± 5.345 |
| Creatinine | 0.666 ± 0.081 | 0.65 ± 0.054 | 0.616 ± 0.04 | 0.65 ± 0.083 | 0.633 ± 0.081 | 0.6 ± 0.089 |
| Sr. No. | Retention Time (Min) | Area % | Name of Detected Compound | Class of Phytochemical Compound | Molecular Formula | Molecular Weight (g/mol) | Structure |
|---|---|---|---|---|---|---|---|
| 1 | 8.691 | 14.62 | 2-Hydroxy-1,4-naphthoquinone (Lawsone) | Naphthoquinone | C10H6O3 | 174.15 | 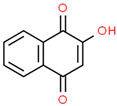 |
| 2 | 17.200 | 12.39 | Octadecanoic acid | Fatty Acid | C18H36O2 | 284.48 | 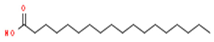 |
| 3 | 11.375 | 11.375 | Pentadecanoic acid | Fatty Acid | C15H30O2 | 242.39 | 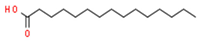 |
| 4 | 17.201 | 3.22 | Hexadecanoic acid | Fatty Acid | C16H32O2 | 256.4 | 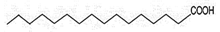 |
| 5 | 13.328 | 0.90 | Linoleic acid | Omega-6 Fatty Acid | C18H32O2 | 280.44 | 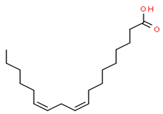 |
| 6 | 23.4 | 0.85 | Vaccenic acid | Fatty Acid | C18H34O2 | 282.461 | 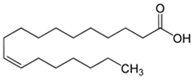 |
| 7 | 15.46 | 0.80 | Malonic acid | Dicarboxylic acid | C3H4O4 | 104.0615 |  |
| 8 | 31.51 | 0.78 | β-stigmasterol | Stigmastane | C29H50O | 414.71 | 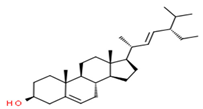 |
| 9 | 14.83 | 0.75 | Caprylic acid | Fatty Acid | C8H16O2 | 144.21 | 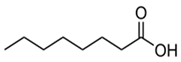 |
| 10 | 16.12 | 0.70 | Nonanoic acid | Fatty Acid | C9H18O2 | 158.23 | 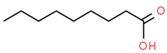 |
| 11 | 18.92 | 0.65 | Myristic acid | Fatty Acid | C14H28O2 | 228.37 | 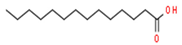 |
| 12 | 28.5 | 0.50 | Squalene | Triterpenoid | C30H50 | 410.73 | 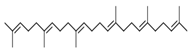 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattar, S.; Shabbir, A.; Shahzad, M.; Akhtar, T.; Ahmad, A.; Alnasser, S.M.; Riaz, B.; Karimullah, S.; Ahmad, A. Eichhornia crassipes Ameliorated Rheumatoid Arthritis by Modulating Inflammatory Cytokines and Metalloproteinase Enzymes in a Rat Model. Medicina 2023, 59, 1594. https://doi.org/10.3390/medicina59091594
Sattar S, Shabbir A, Shahzad M, Akhtar T, Ahmad A, Alnasser SM, Riaz B, Karimullah S, Ahmad A. Eichhornia crassipes Ameliorated Rheumatoid Arthritis by Modulating Inflammatory Cytokines and Metalloproteinase Enzymes in a Rat Model. Medicina. 2023; 59(9):1594. https://doi.org/10.3390/medicina59091594
Chicago/Turabian StyleSattar, Sara, Arham Shabbir, Muhammad Shahzad, Tasleem Akhtar, Arfan Ahmad, Sulaiman Mohammed Alnasser, Bushra Riaz, Shaik Karimullah, and Ashfaq Ahmad. 2023. "Eichhornia crassipes Ameliorated Rheumatoid Arthritis by Modulating Inflammatory Cytokines and Metalloproteinase Enzymes in a Rat Model" Medicina 59, no. 9: 1594. https://doi.org/10.3390/medicina59091594
APA StyleSattar, S., Shabbir, A., Shahzad, M., Akhtar, T., Ahmad, A., Alnasser, S. M., Riaz, B., Karimullah, S., & Ahmad, A. (2023). Eichhornia crassipes Ameliorated Rheumatoid Arthritis by Modulating Inflammatory Cytokines and Metalloproteinase Enzymes in a Rat Model. Medicina, 59(9), 1594. https://doi.org/10.3390/medicina59091594