PNU-74654 Induces Cell Cycle Arrest and Inhibits EMT Progression in Pancreatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assay
2.3. Colony Formation Assay
2.4. Cell Cycle Analysis
2.5. Wound-Healing Assay
2.6. Transwell Invasion Assay
2.7. Western Blotting
2.8. Isolation of Nuclear and Cytoplasmic Proteins
2.9. Proteome Profiling with a Human XL Oncology Array
2.10. Statistical Analysis
3. Results
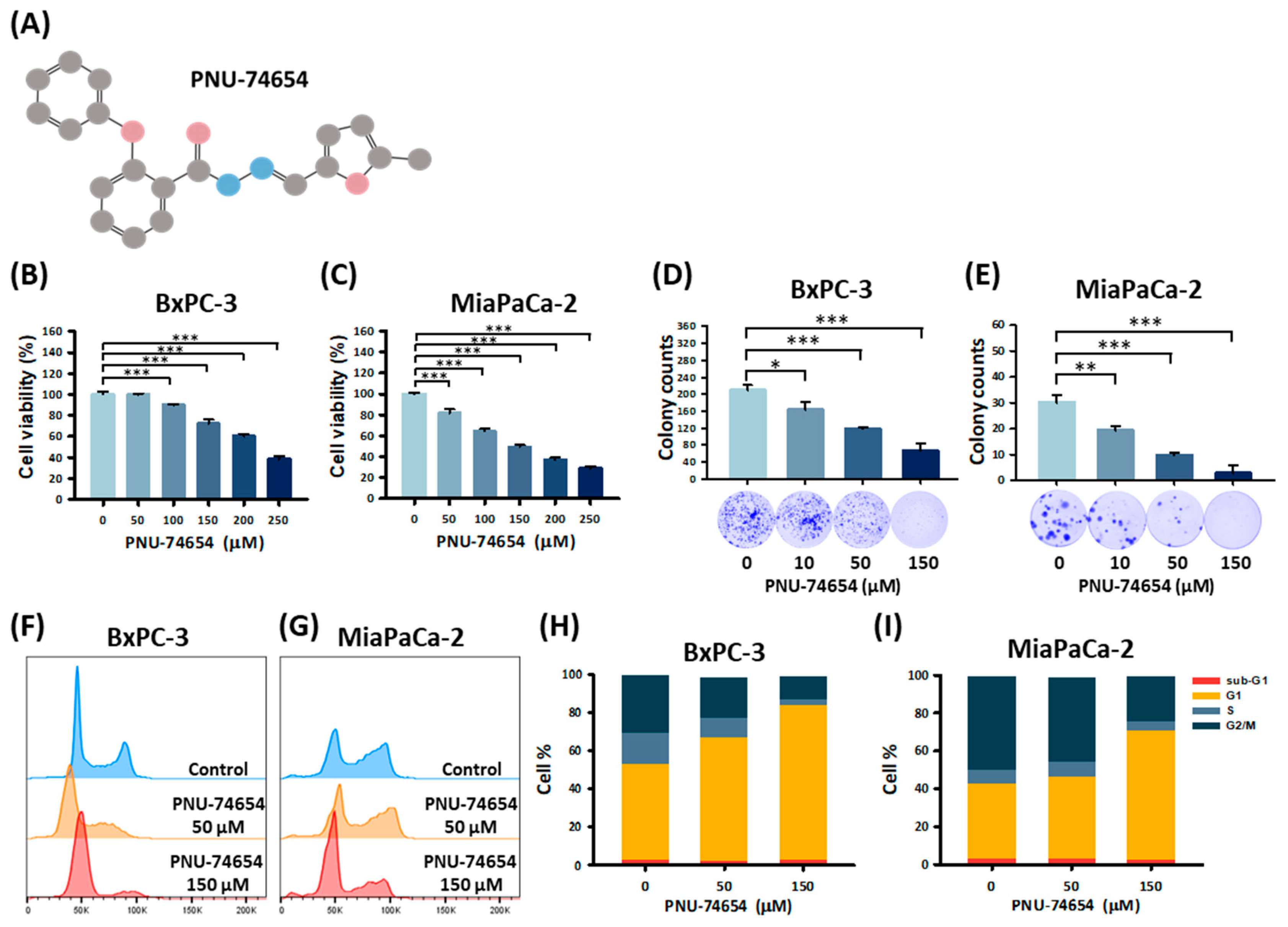
3.1. PNU-74654 Suppresses PC Cell Proliferation by Promoting Cell Cycle Arrest
3.2. PNU-74654 Inhibits the Migration of PC Cells
3.3. PC Cell Invasion Is Suppressed by PNU-74654
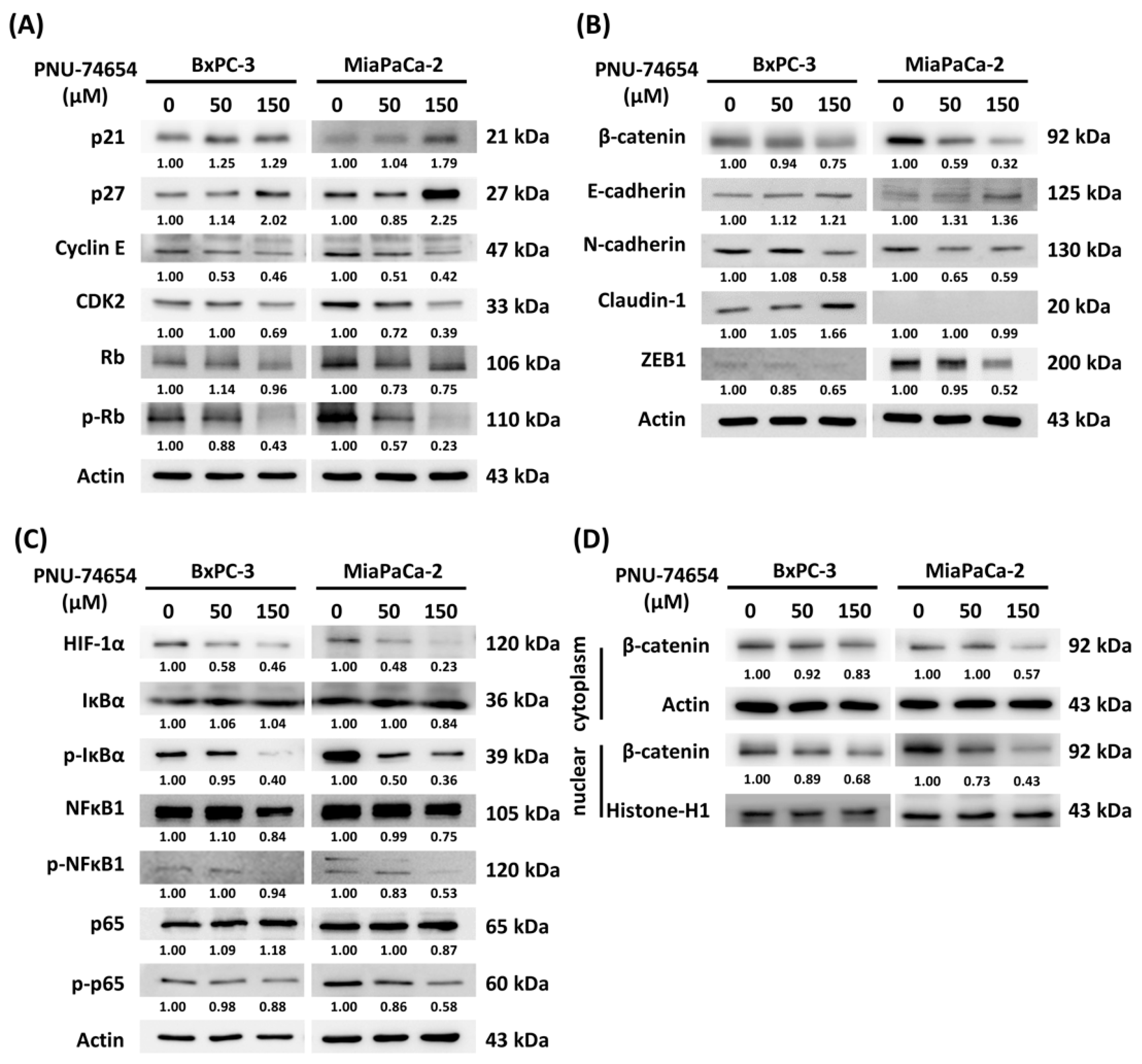
3.4. Molecular Mechanism of PNU-74654-Induced Cell Cycle Arrest and Inhibition of the Epithelial–Mesenchymal Transition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Peng, C.M.; Huang, C.Y.; Wu, S.Y.; Tsai, M.C.; Wang, C.C.; Chen, S.L.; Lin, C.C.; Huang, C.N.; Sung, W.W. Is mortality-to-incidence ratio associated with health disparity in pancreatic cancer? A cross-sectional database analysis of 57 countries. BMJ Open 2018, 8, e020618. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef]
- Gupta, R.; Amanam, I.; Chung, V. Current and future therapies for advanced pancreatic cancer. J. Surg. Oncol. 2017, 116, 25–34. [Google Scholar] [CrossRef]
- Bramhall, S.R.; Rosemurgy, A.; Brown, P.D.; Bowry, C.; Buckels, J.A. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: A randomized trial. J. Clin. Oncol. 2001, 19, 3447–3455. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Nusse, R. Wnt signaling in disease and in development. Cell Res. 2005, 15, 28–32. [Google Scholar] [CrossRef]
- Giles, R.H.; van Es, J.H.; Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta BBA—Rev. Cancer 2003, 1653, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jiang, W.; Wang, S.; Wang, L.; Xie, K. Role of Wnt/β-catenin signaling in drug resistance of pancreatic cancer. Curr. Pharm. Des. 2012, 18, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kurzrock, R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 2018, 62, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Itakura, J.; Ishiwata, T.; Friess, H.; Fujii, H.; Matsumoto, Y.; Büchler, M.W.; Korc, M. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin. Cancer Res. 1997, 3, 1309–1316. [Google Scholar]
- Qiao, Q.; Ramadani, M.; Gansauge, S.; Gansauge, F.; Leder, G.; Beger, H.G. Reduced membranous and ectopic cytoplasmic expression of beta-catenin correlate with cyclin D1 overexpression and poor prognosis in pancreatic cancer. Int. J. Cancer 2001, 95, 194–197. [Google Scholar] [CrossRef]
- Gansauge, S.; Gansauge, F.; Ramadani, M.; Stobbe, H.; Rau, B.; Harada, N.; Beger, H.G. Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res. 1997, 57, 1634–1637. [Google Scholar]
- Ryan, B.M.; O’Donovan, N.; Duffy, M.J. Survivin: A new target for anti-cancer therapy. Cancer Treat. Rev. 2009, 35, 553–562. [Google Scholar] [CrossRef]
- Satoh, K.; Kaneko, K.; Hirota, M.; Masamune, A.; Satoh, A.; Shimosegawa, T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer 2001, 92, 271–278. [Google Scholar] [CrossRef]
- Biswal, B.; Tripathi, S. Wnt/b-Catenin: The Main Culprit behind Cancer Chemoresistance. J. Appl. Microbiol. Biochem. 2017, 1, 16. [Google Scholar] [CrossRef]
- Leal, L.F.; Bueno, A.C.; Gomes, D.C.; Abduch, R.; de Castro, M.; Antonini, S.R. Inhibition of the Tcf/beta-catenin complex increases apoptosis and impairs adrenocortical tumor cell proliferation and adrenal steroidogenesis. Oncotarget 2015, 6, 43016–43032. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Amerizadeh, F.; Hassanian, S.M.; Hashemzehi, M.; Nasiri, S.N.; Fiuji, H.; Ferns, G.A.; Khazaei, M.; Avan, A. PNU-74654 enhances the antiproliferative effects of 5-FU in breast cancer and antagonizes thrombin-induced cell growth via the Wnt pathway. J. Cell. Physiol. 2019, 234, 14123–14132. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Zhou, X.; Zhang, W.; Qu, Y.; Ke, X. Is β-Catenin a Druggable Target for Cancer Therapy? Trends Biochem. Sci. 2018, 43, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Amerizadeh, F.; Rahmani, F.; Maftooh, M.; Nasiri, S.-N.; Hassanian, S.M.; Giovannetti, E.; Moradi-Marjaneh, R.; Sabbaghzadeh, R.; Shahidsales, S.; Joudi-Mashhad, M.; et al. Inhibition of the Wnt/b-catenin pathway using PNU-74654 reduces tumor growth in in vitro and in vivo models of colorectal cancer. Tissue Cell 2022, 77, 101853. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Wang, C.-C.; Chang, Y.-C.; Yu, C.-Y.; Sung, W.-W.; Chen, C.-J.; Tsai, M.-C. The Therapeutic Role of PNU-74654 in Hepatocellular Carcinoma May Involve Suppression of NF-κB Signaling. Medicina 2022, 58, 798. [Google Scholar]
- Sung, W.W.; Wang, Y.C.; Lin, P.L.; Cheng, Y.W.; Chen, C.Y.; Wu, T.C.; Lee, H. IL-10 promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 4092–4103. [Google Scholar] [CrossRef]
- Sung, W.W.; Wang, Y.C.; Cheng, Y.W.; Lee, M.C.; Yeh, K.T.; Wang, L.; Wang, J.; Chen, C.Y.; Lee, H. A polymorphic -844T/C in FasL promoter predicts survival and relapse in non-small cell lung cancer. Clin. Cancer Res. 2011, 17, 5991–5999. [Google Scholar] [CrossRef]
- Wang, S.C.; Chang, Y.C.; Wu, M.Y.; Yu, C.Y.; Chen, S.L.; Sung, W.W. Intravesical Instillation of Azacitidine Suppresses Tumor Formation through TNF-R1 and TRAIL-R2 Signaling in Genotoxic Carcinogen-Induced Bladder Cancer. Cancers 2021, 13, 3933. [Google Scholar]
- Wang, S.C.; Yu, C.Y.; Wu, Y.C.; Chang, Y.C.; Chen, S.L.; Sung, W.W. Chidamide and mitomycin C exert synergistic cytotoxic effects against bladder cancer cells in vitro and suppress tumor growth in a rat bladder cancer model. Cancer Lett. 2022, 530, 8–15. [Google Scholar] [CrossRef]
- Voronkov, A.; Krauss, S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr. Pharm. Des. 2013, 19, 634–664. [Google Scholar] [CrossRef]
- Liu, C.; Tu, Y.; Sun, X.; Jiang, J.; Jin, X.; Bo, X.; Li, Z.; Bian, A.; Wang, X.; Liu, D.; et al. Wnt/beta-Catenin pathway in human glioma: Expression pattern and clinical/prognostic correlations. Clin. Exp. Med. 2011, 11, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Brown, A.M.C. Wnt Signaling and Breast Cancer. Cancer Biol. Ther. 2004, 3, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kolligs, F.T.; Bommer, G.; Göke, B. Wnt/beta-catenin/tcf signaling: A critical pathway in gastrointestinal tumorigenesis. Digestion 2002, 66, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef]
- Hu, C.-J.; Wang, L.-Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential Roles of Hypoxia-Inducible Factor 1α (HIF-1α) and HIF-2α in Hypoxic Gene Regulation. Mol. Cell. Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down regulation of HIF-1α in cancer progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef]
- Jin, X.; Dai, L.; Ma, Y.; Wang, J.; Liu, Z. Implications of HIF-1α in the tumorigenesis and progression of pancreatic cancer. Cancer Cell Int. 2020, 20, 273. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Chien, W.; Lee, D.H.; Zheng, Y.; Wuensche, P.; Alvarez, R.; Wen, D.L.; Aribi, A.M.; Thean, S.M.; Doan, N.B.; Said, J.W.; et al. Growth inhibition of pancreatic cancer cells by histone deacetylase inhibitor belinostat through suppression of multiple pathways including HIF, NFkB, and mTOR signaling in vitro and in vivo. Mol. Carcinog. 2014, 53, 722–735. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.-P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Sasaki, C.Y.; Barberi, T.J.; Ghosh, P.; Longo, D.L. Phosphorylation of RelA/p65 on serine 536 defines an IκBα-independent NF-κB pathway. J. Biol. Chem. 2005, 280, 34538–34547. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, G.; Xu, Y.; Li, J.; Ruan, N.; Chen, Y.; Zhang, Q.; Xia, Q. Porcupine Inhibitor LGK974 Downregulates the Wnt Signaling Pathway and Inhibits Clear Cell Renal Cell Carcinoma. BioMed Res. Int. 2020, 2020, 2527643. [Google Scholar] [CrossRef] [PubMed]
- Giefing, M.; Wierzbicka, M.; Szyfter, K.; Brenner, J.C.; Braakhuis, B.J.; Brakenhoff, R.H.; Bradford, C.R.; Sorensen, J.A.; Rinaldo, A.; Rodrigo, J.P.; et al. Moving towards personalised therapy in head and neck squamous cell carcinoma through analysis of next generation sequencing data. Eur. J. Cancer 2016, 55, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Kahn, M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014, 105, 1087–1092. [Google Scholar] [CrossRef]
- Gabata, R.; Harada, K.; Mizutani, Y.; Ouchi, H.; Yoshimura, K.; Sato, Y.; Kitao, A.; Kimura, K.; Kouji, H.; Miyashita, T.; et al. Anti-tumor Activity of the Small Molecule Inhibitor PRI-724 Against β-Catenin-activated Hepatocellular Carcinoma. Anticancer Res. 2020, 40, 5211. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, O.-H.; Lee, S.K.; Kim, S.-J. IWR-1 inhibits epithelial-mesenchymal transition of colorectal cancer cells through suppressing Wnt/β-catenin signaling as well as survivin expression. Oncotarget 2015, 6, 27146–27159. [Google Scholar] [CrossRef]
- Shetti, D.; Zhang, B.; Fan, C.; Mo, C.; Lee, B.H.; Wei, K. Low Dose of Paclitaxel Combined with XAV939 Attenuates Metastasis, Angiogenesis and Growth in Breast Cancer by Suppressing Wnt Signaling. Cells 2019, 8, 892. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, T.-L.; Wu, Y.-C.; Lee, H.-L.; Sung, W.-W.; Yu, C.-Y.; Chang, Y.-C.; Lin, C.-C.; Wang, C.-C.; Tsai, M.-C. PNU-74654 Induces Cell Cycle Arrest and Inhibits EMT Progression in Pancreatic Cancer. Medicina 2023, 59, 1531. https://doi.org/10.3390/medicina59091531
Chien T-L, Wu Y-C, Lee H-L, Sung W-W, Yu C-Y, Chang Y-C, Lin C-C, Wang C-C, Tsai M-C. PNU-74654 Induces Cell Cycle Arrest and Inhibits EMT Progression in Pancreatic Cancer. Medicina. 2023; 59(9):1531. https://doi.org/10.3390/medicina59091531
Chicago/Turabian StyleChien, Tai-Long, Yao-Cheng Wu, Hsiang-Lin Lee, Wen-Wei Sung, Chia-Ying Yu, Ya-Chuan Chang, Chun-Che Lin, Chi-Chih Wang, and Ming-Chang Tsai. 2023. "PNU-74654 Induces Cell Cycle Arrest and Inhibits EMT Progression in Pancreatic Cancer" Medicina 59, no. 9: 1531. https://doi.org/10.3390/medicina59091531
APA StyleChien, T.-L., Wu, Y.-C., Lee, H.-L., Sung, W.-W., Yu, C.-Y., Chang, Y.-C., Lin, C.-C., Wang, C.-C., & Tsai, M.-C. (2023). PNU-74654 Induces Cell Cycle Arrest and Inhibits EMT Progression in Pancreatic Cancer. Medicina, 59(9), 1531. https://doi.org/10.3390/medicina59091531








