The Carotid–Hyoid Topography Is Variable
Abstract
:1. Introduction
2. Materials and Methods
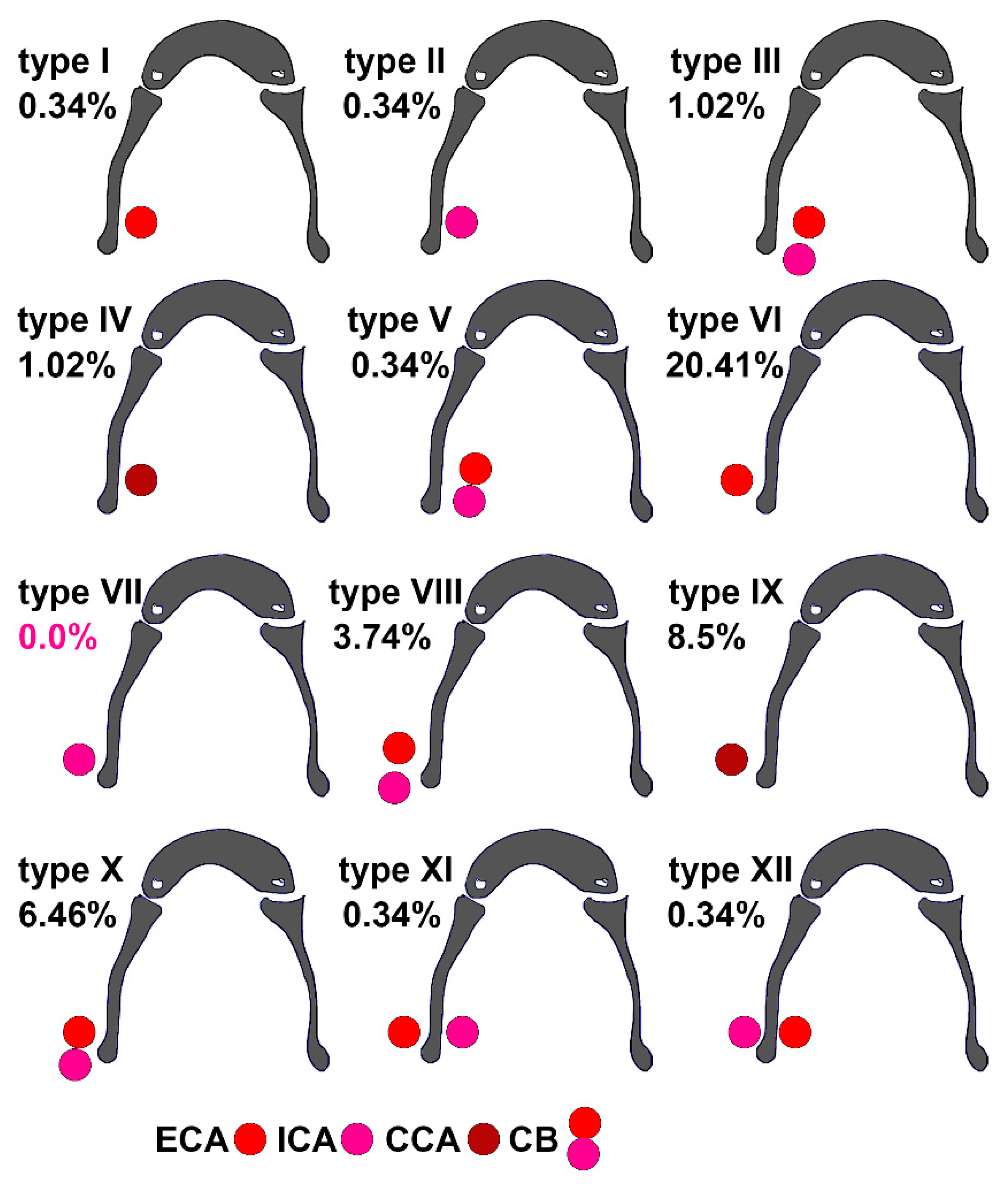
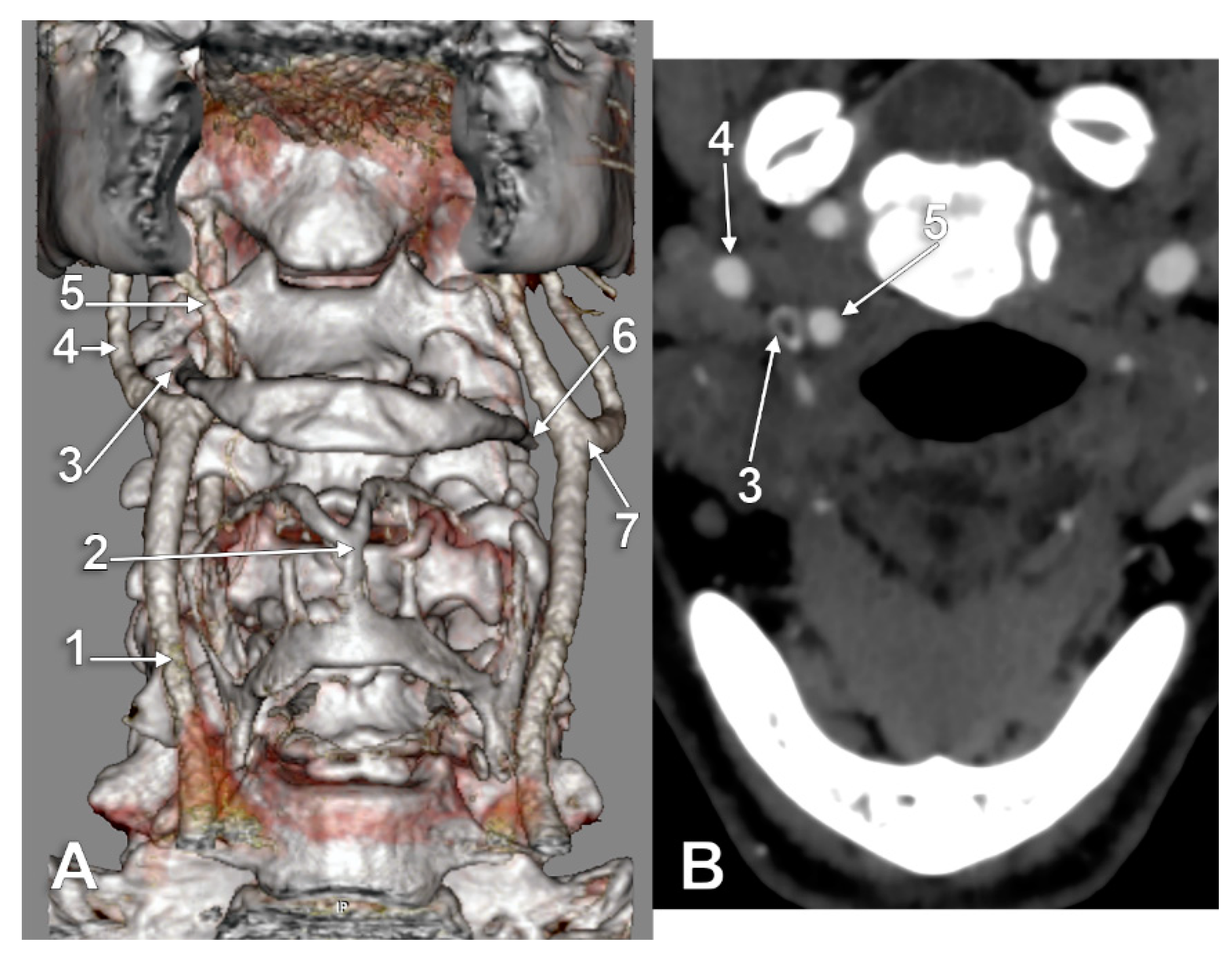
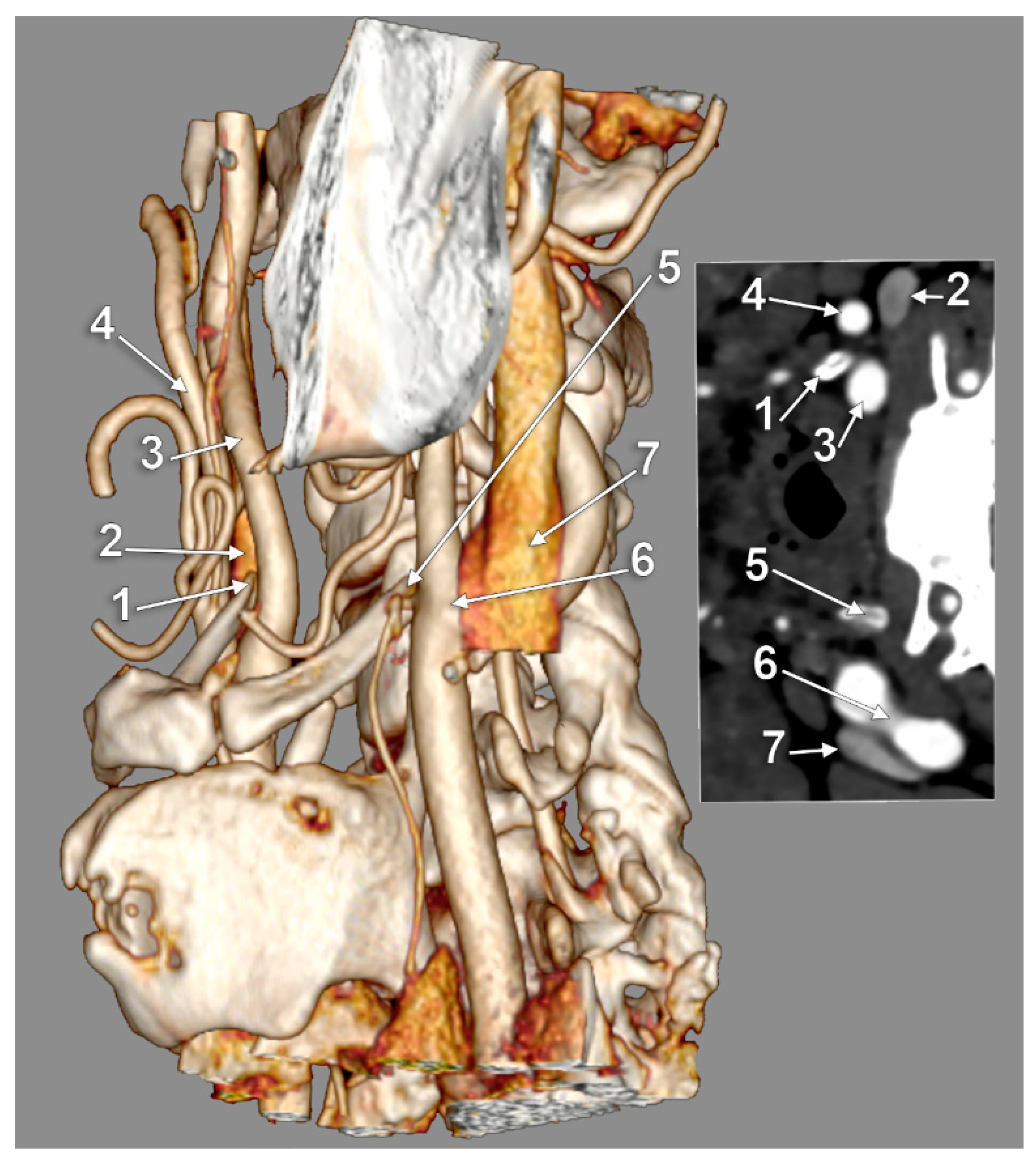
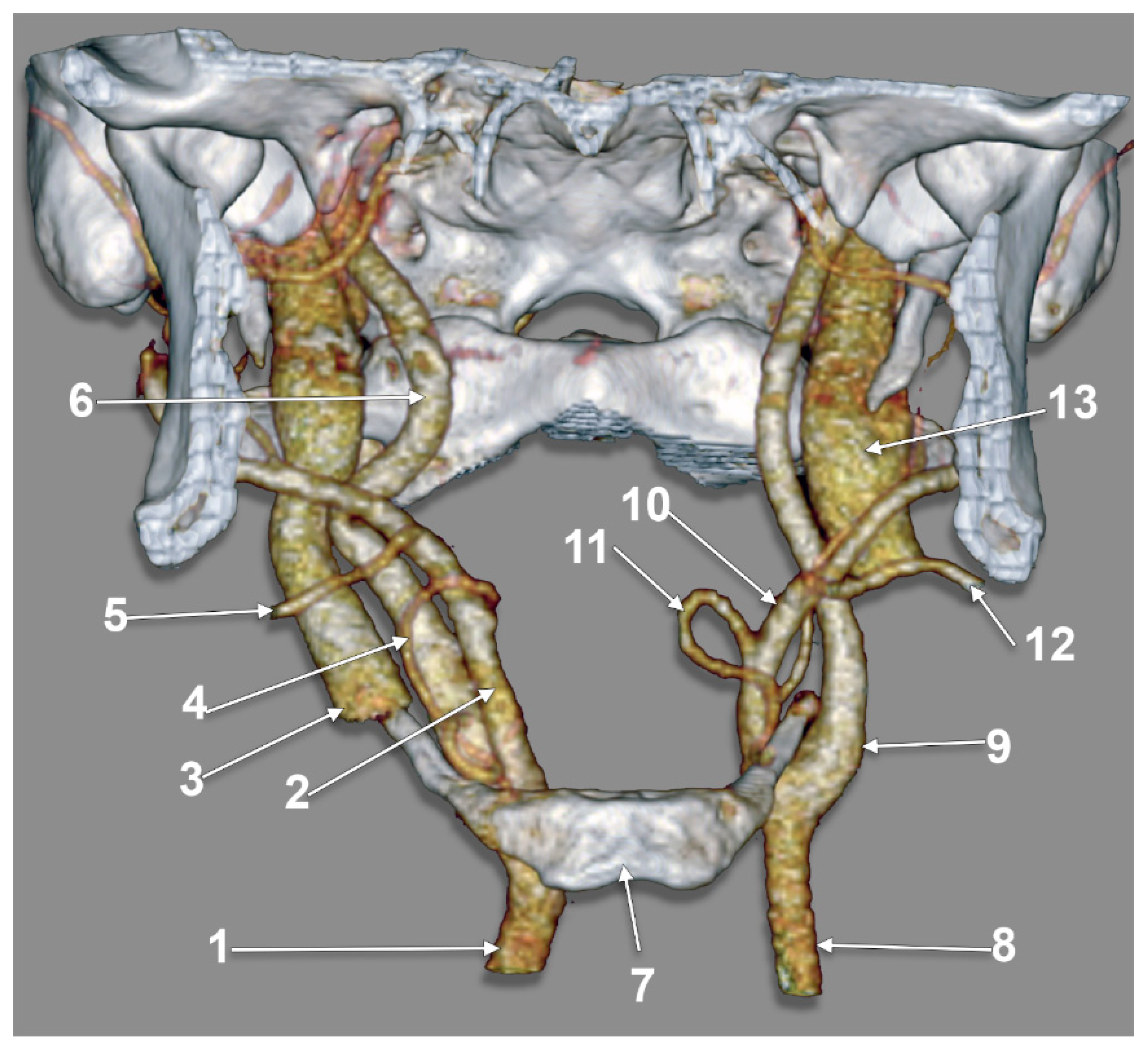
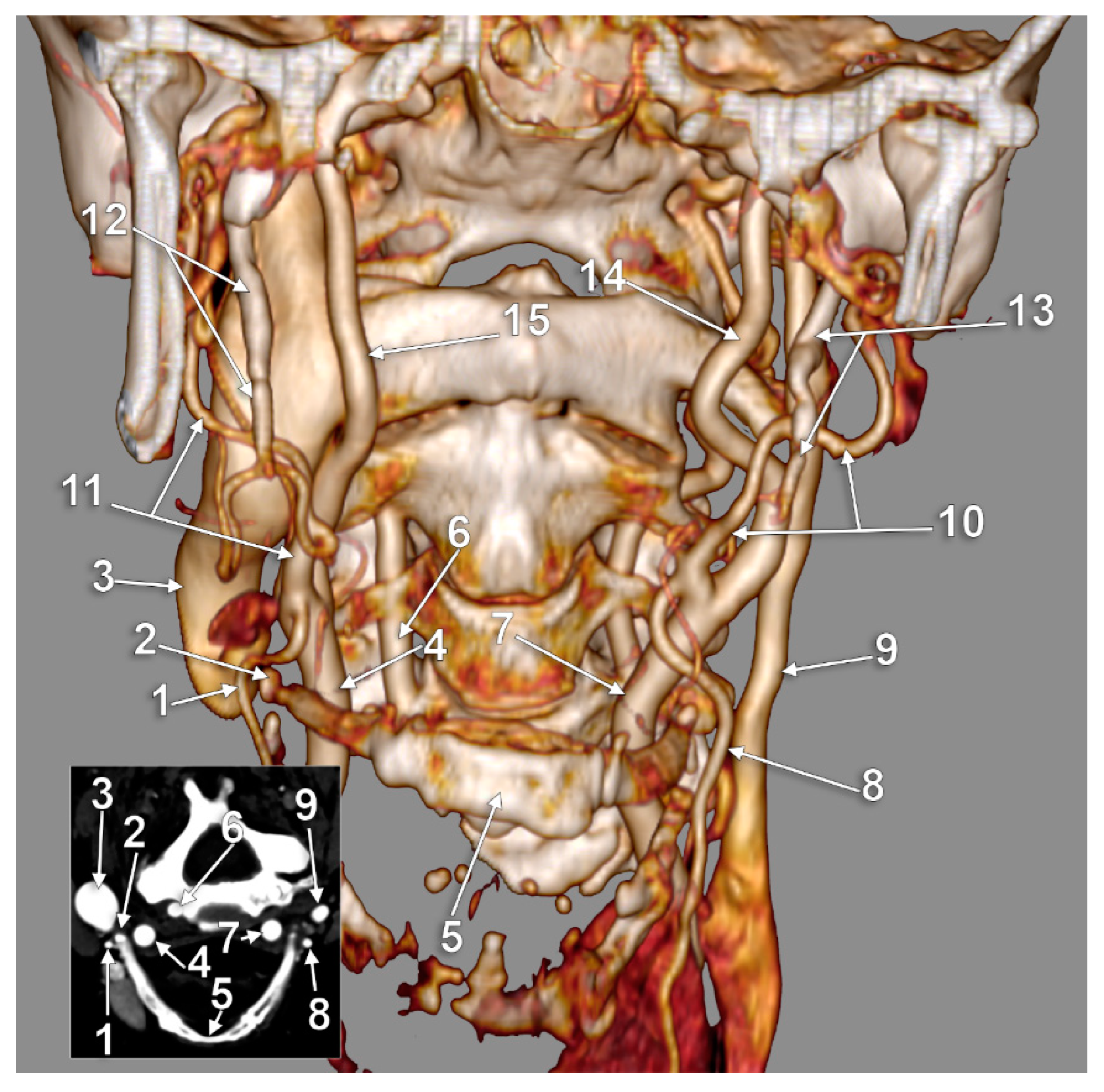
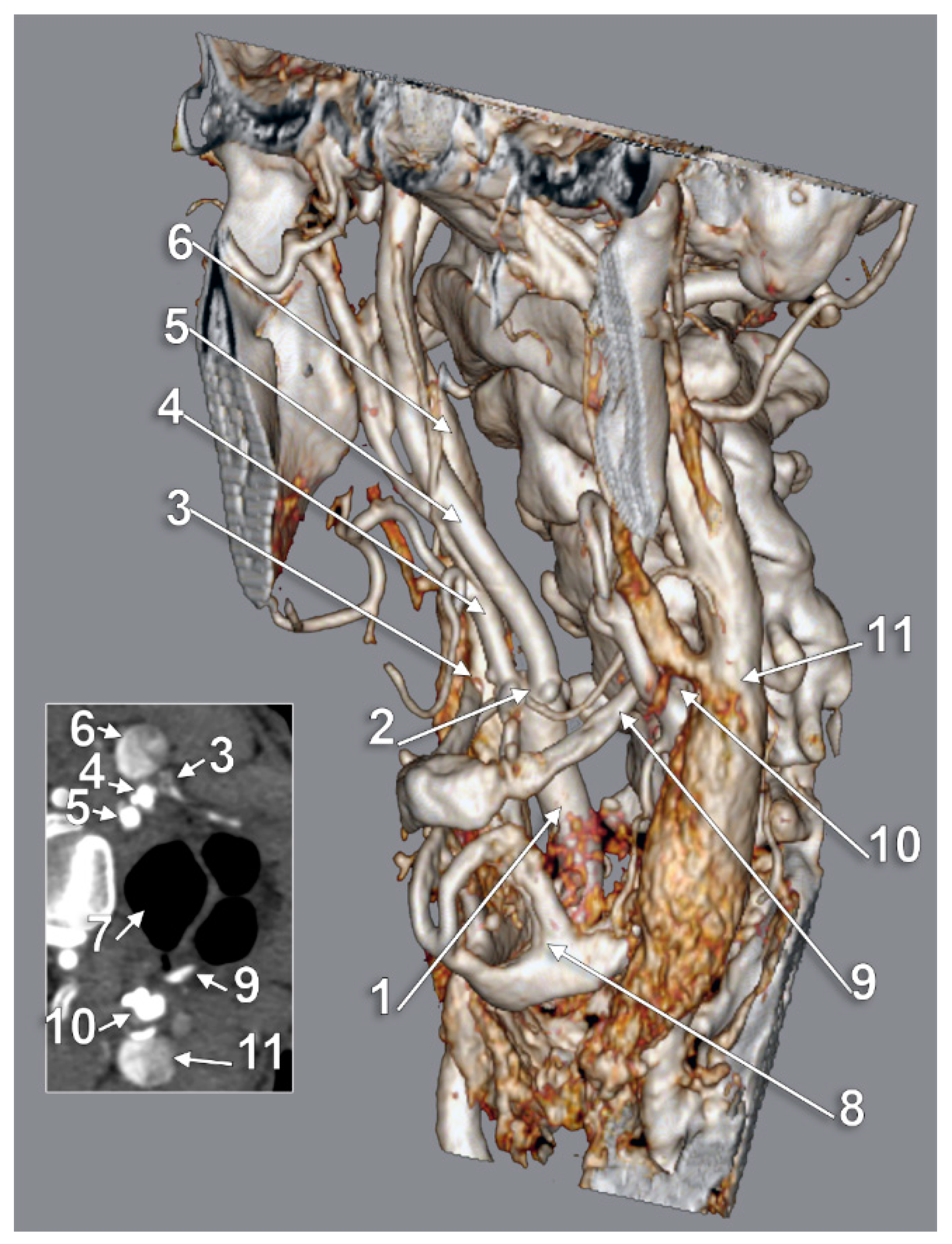
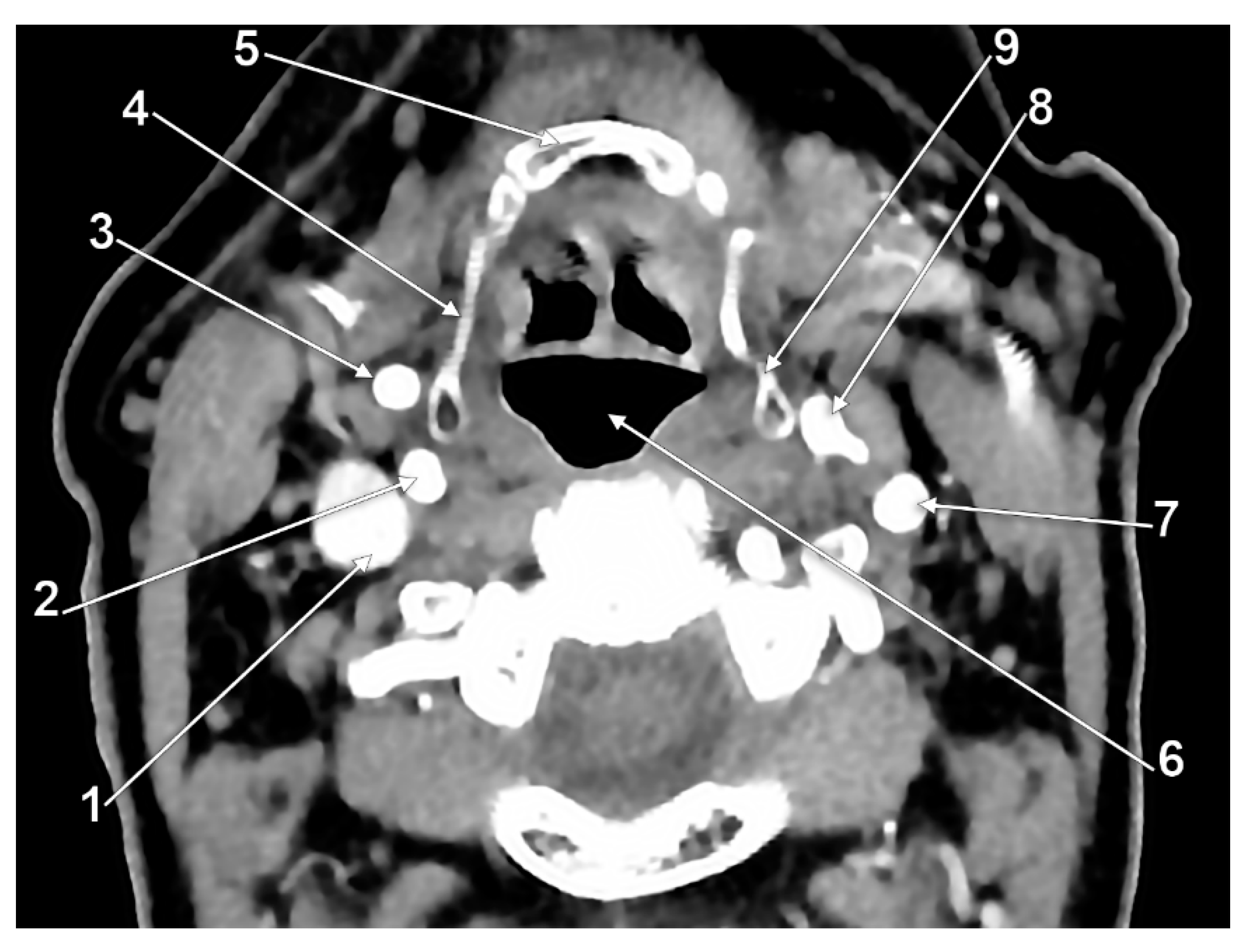
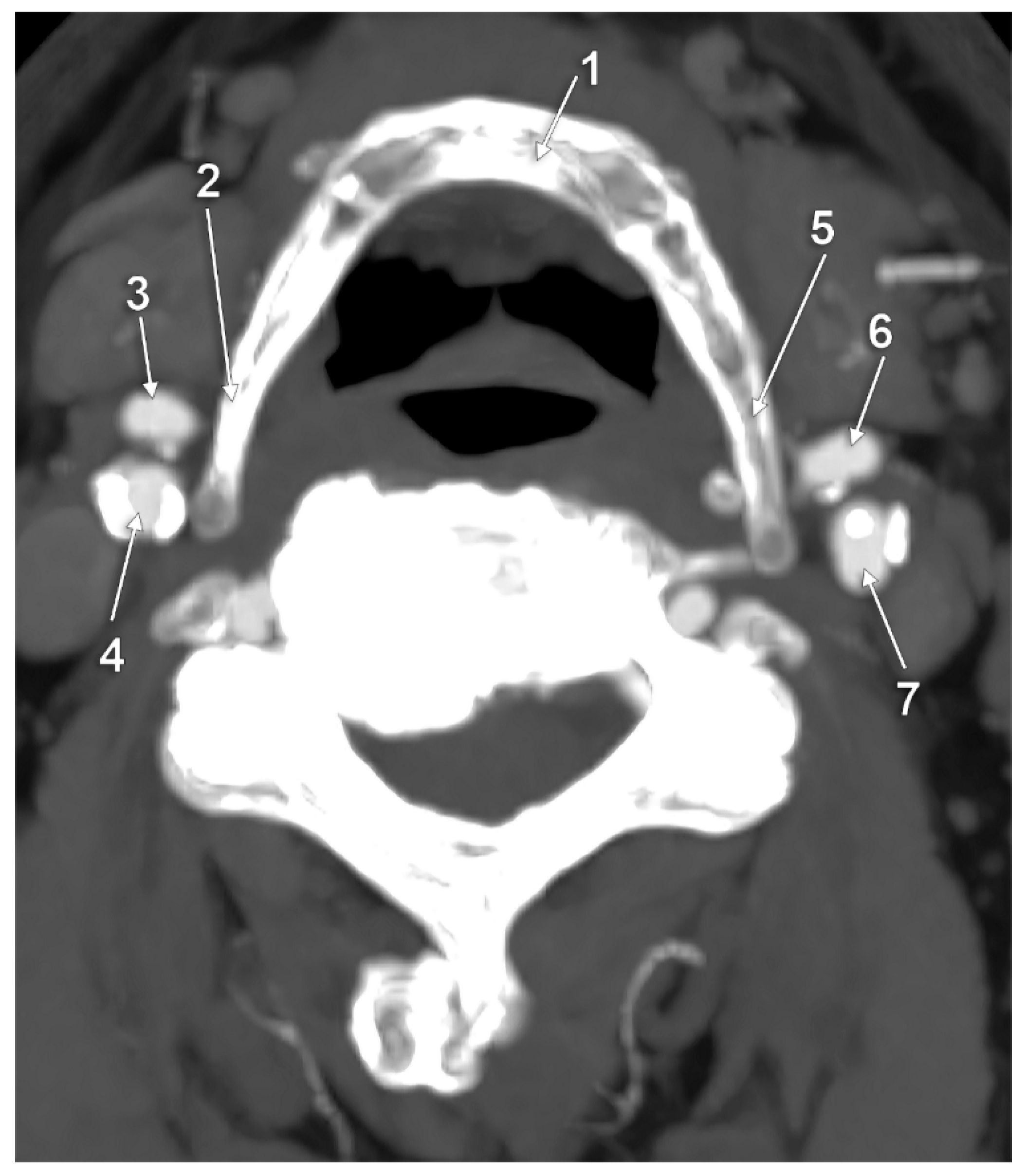
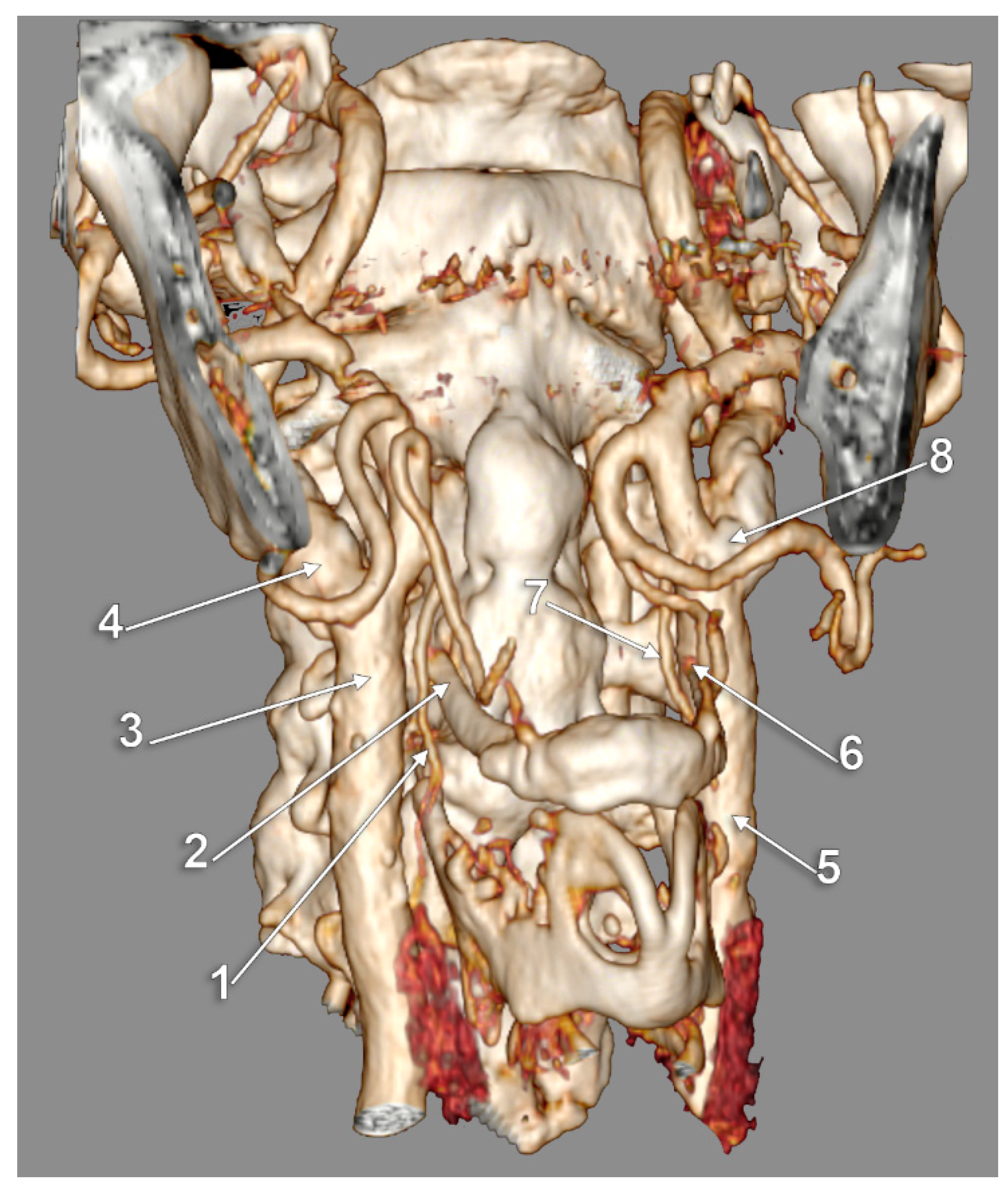
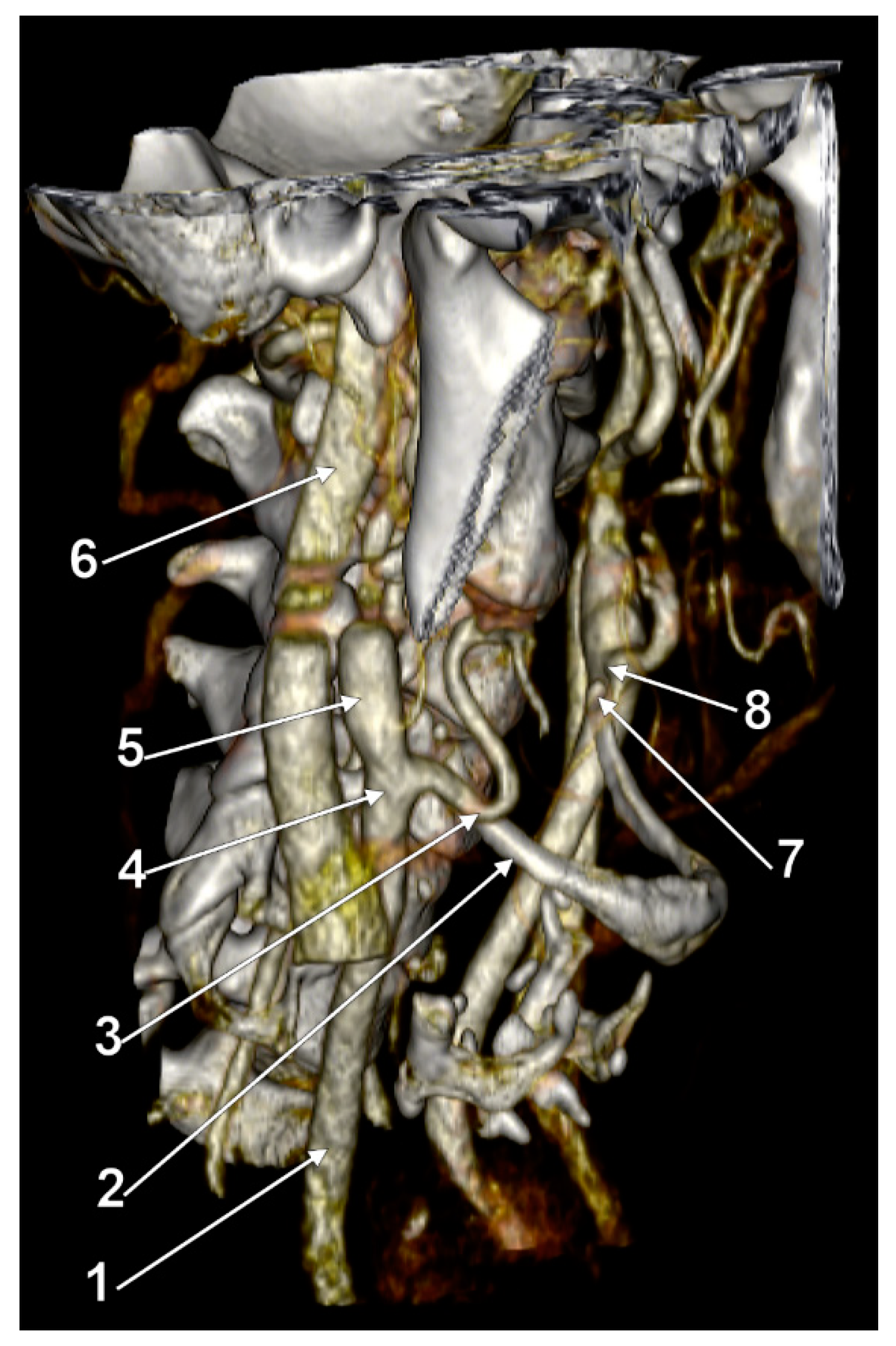
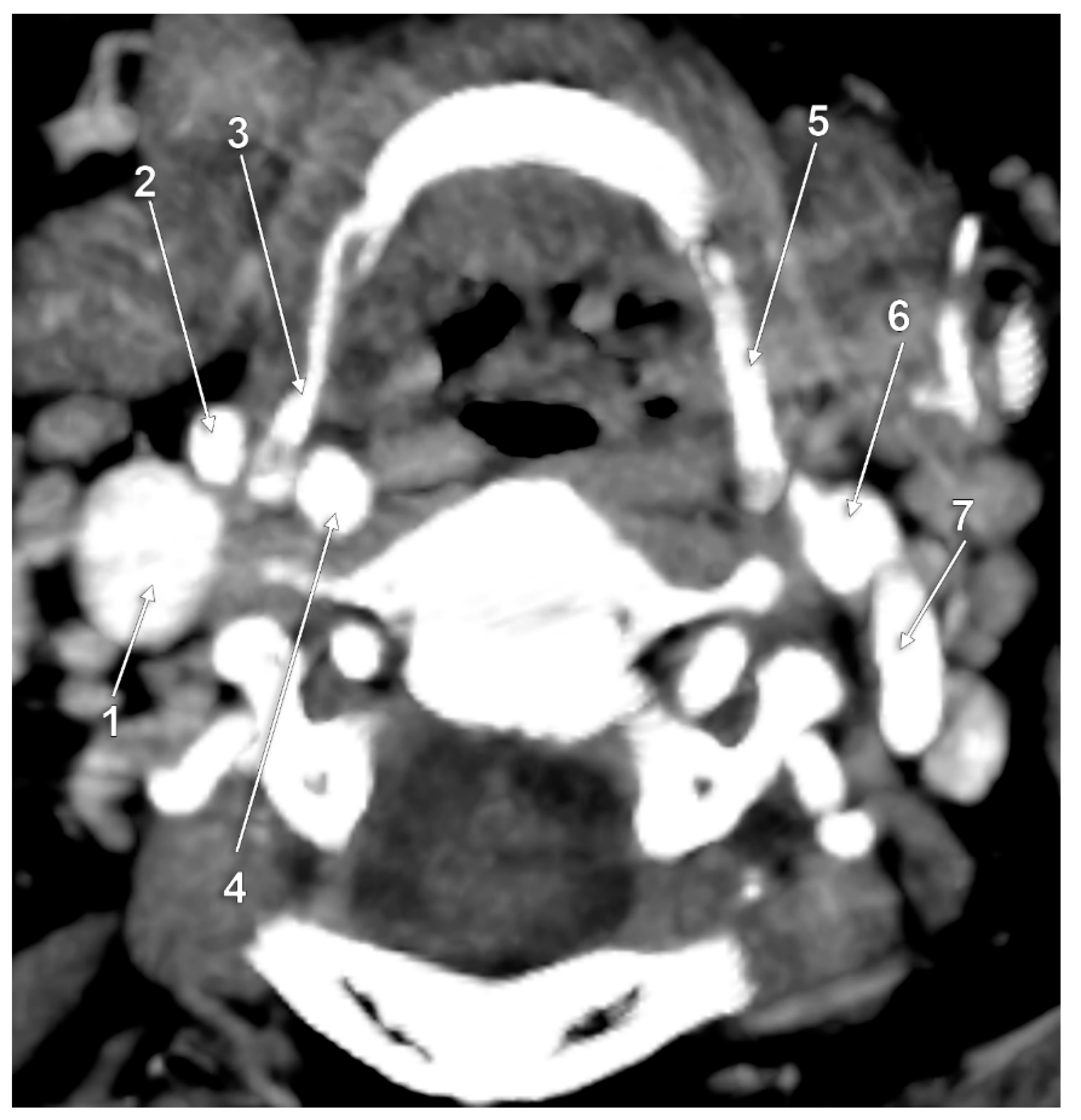
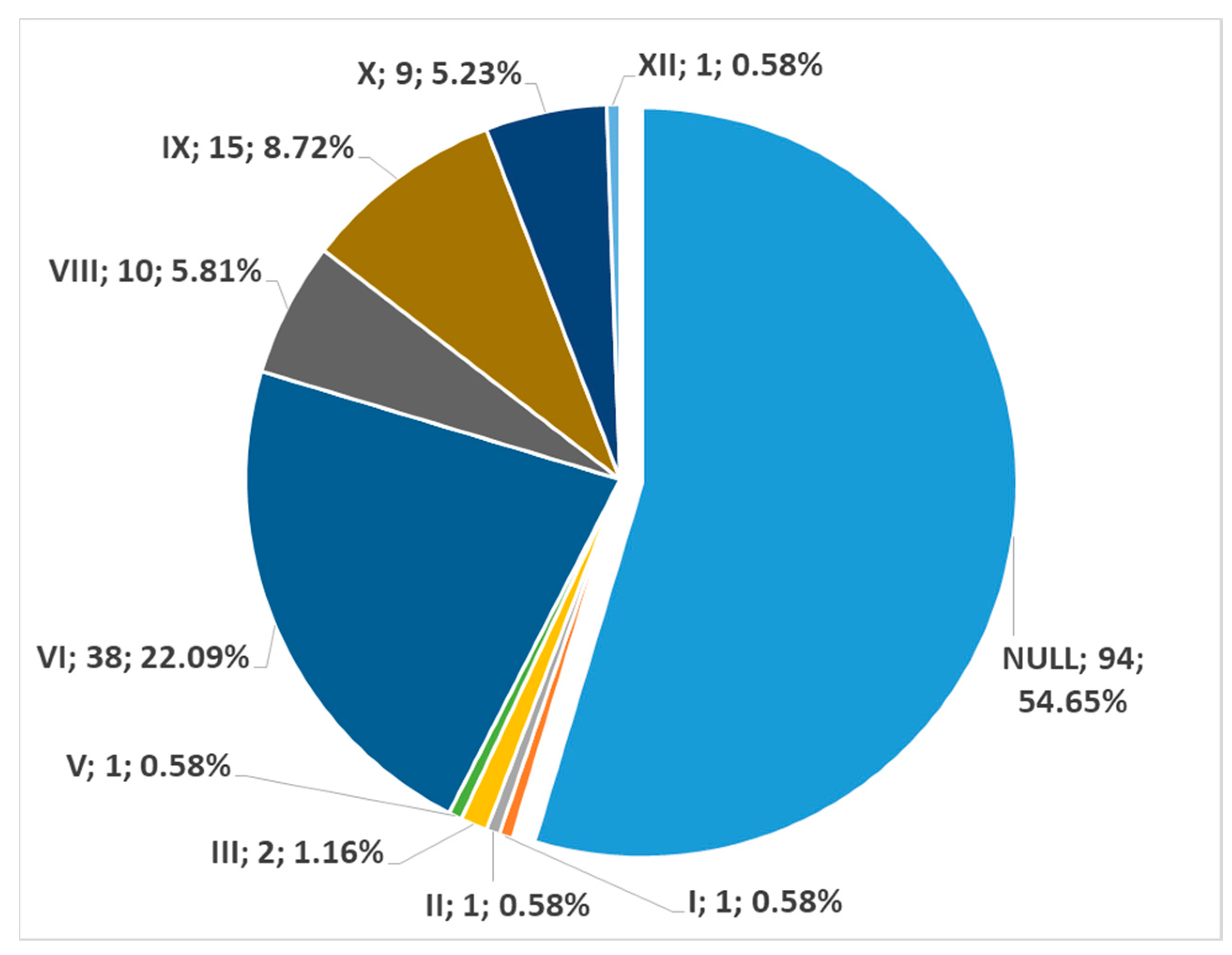
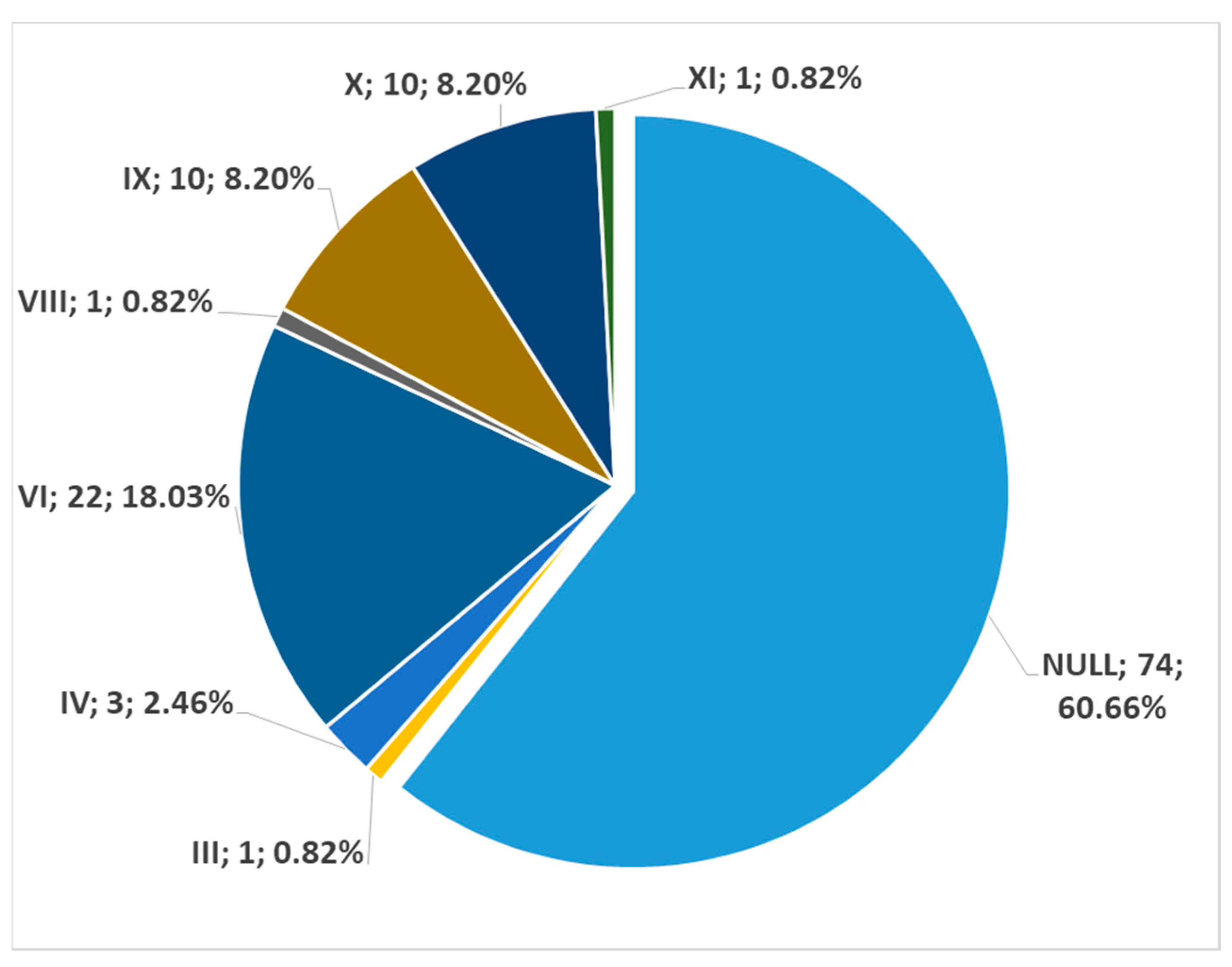
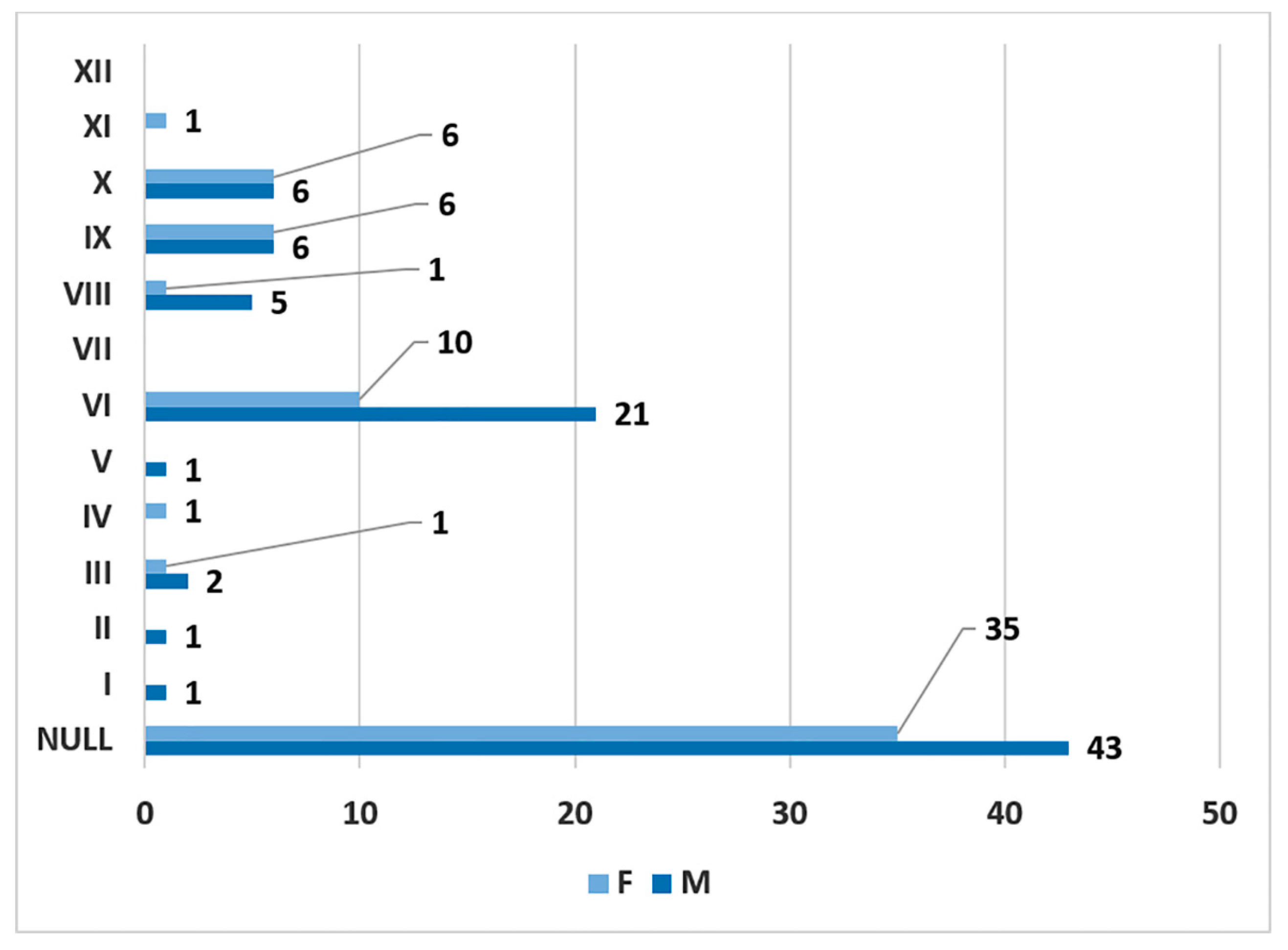
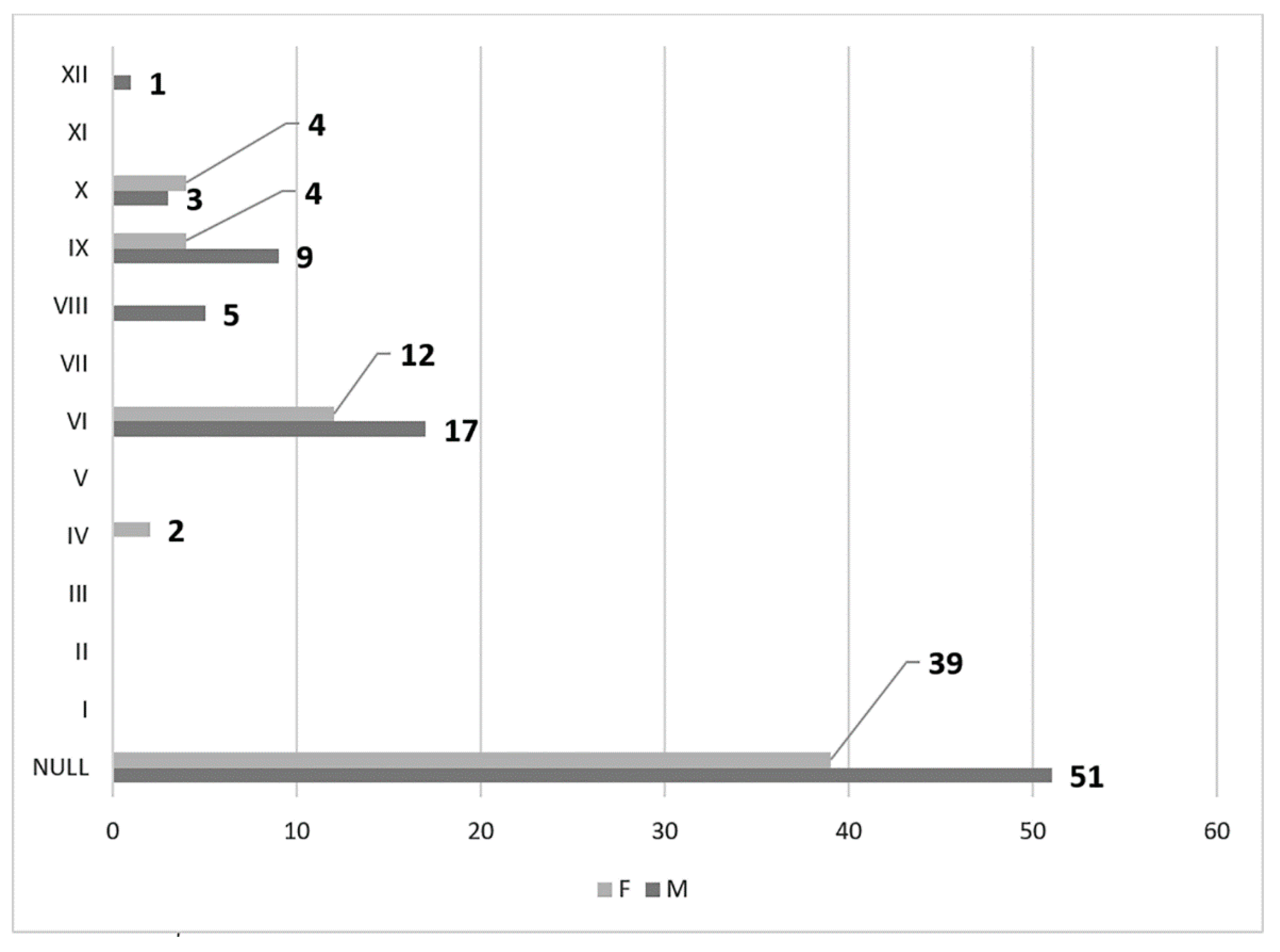
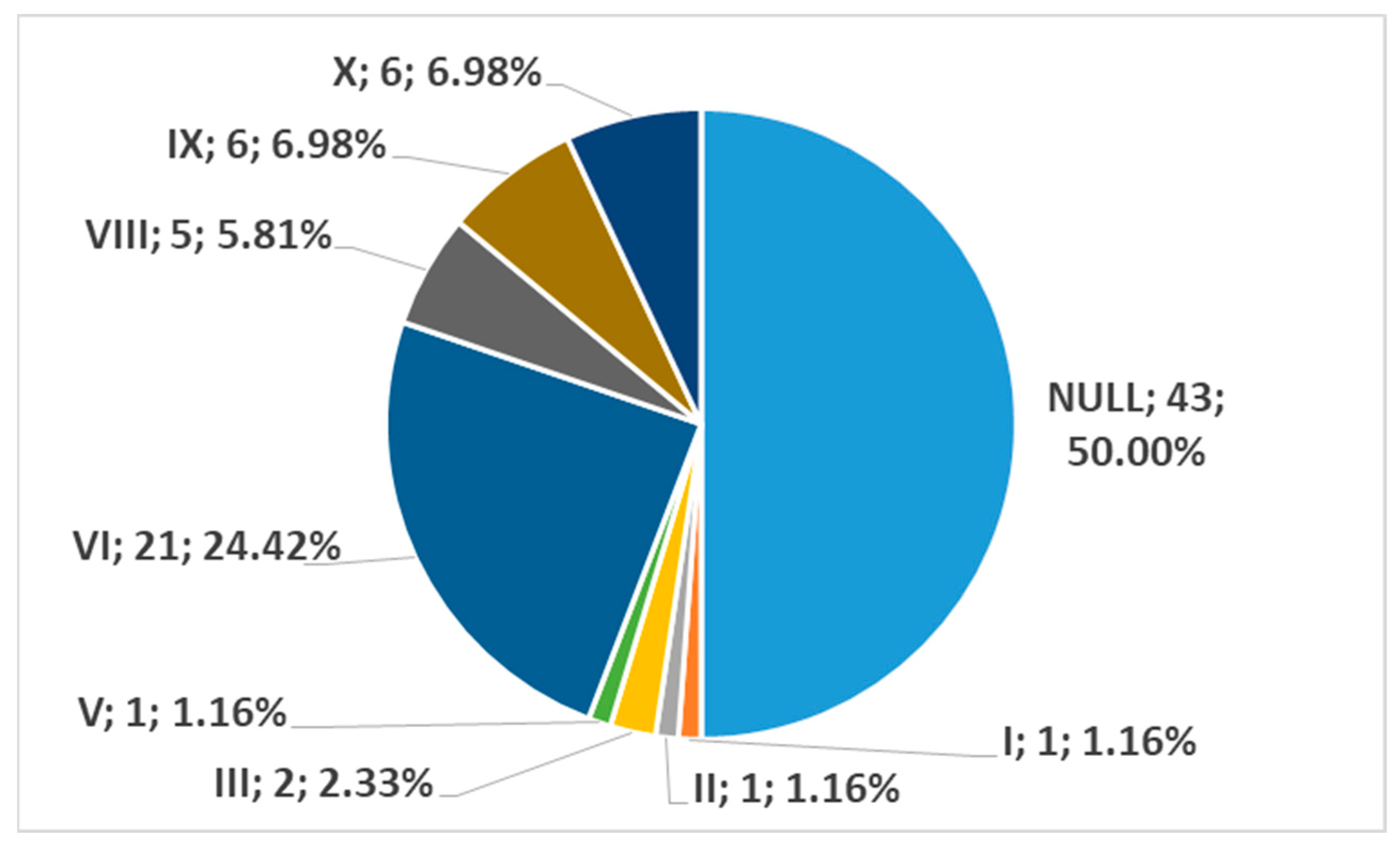
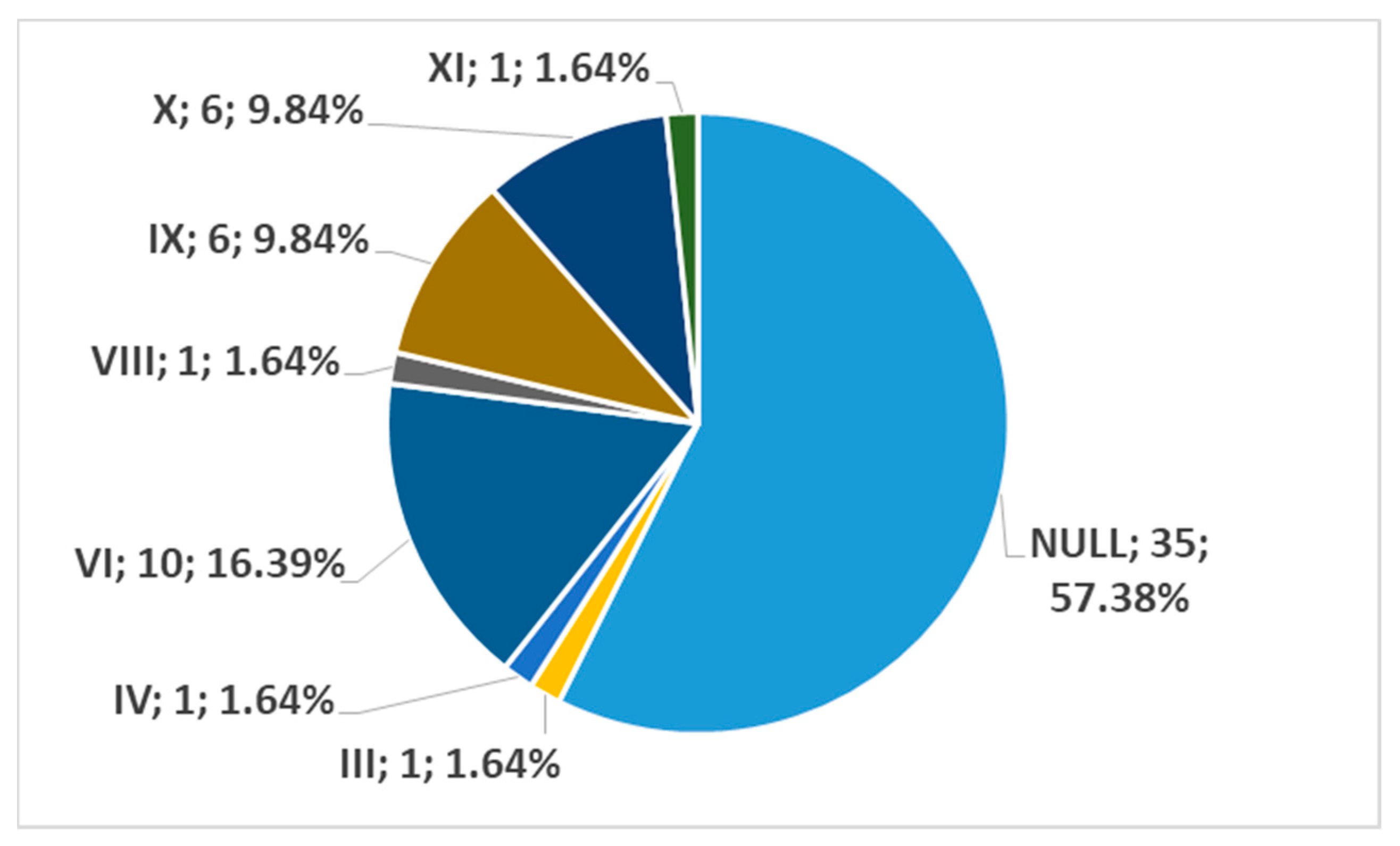
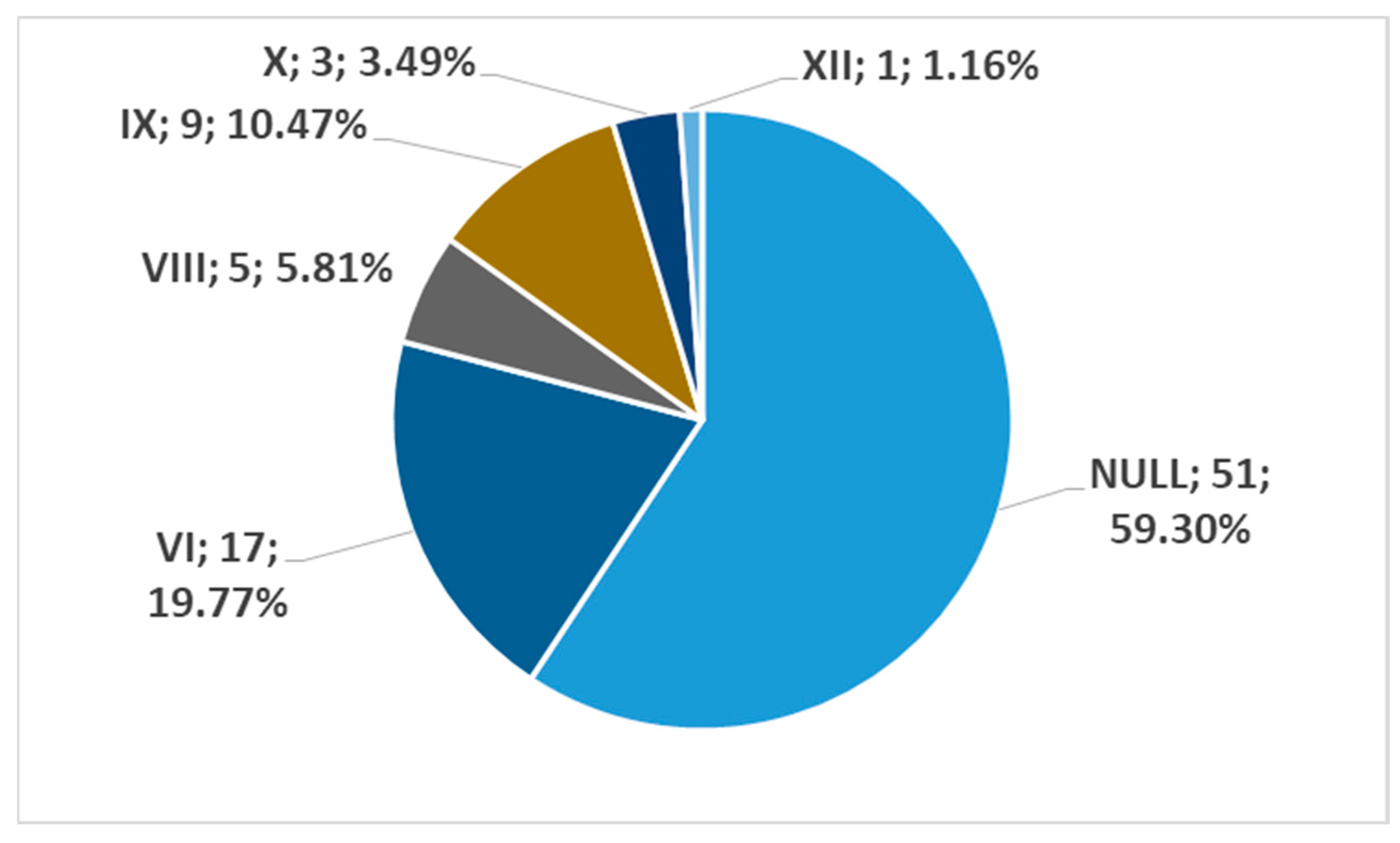
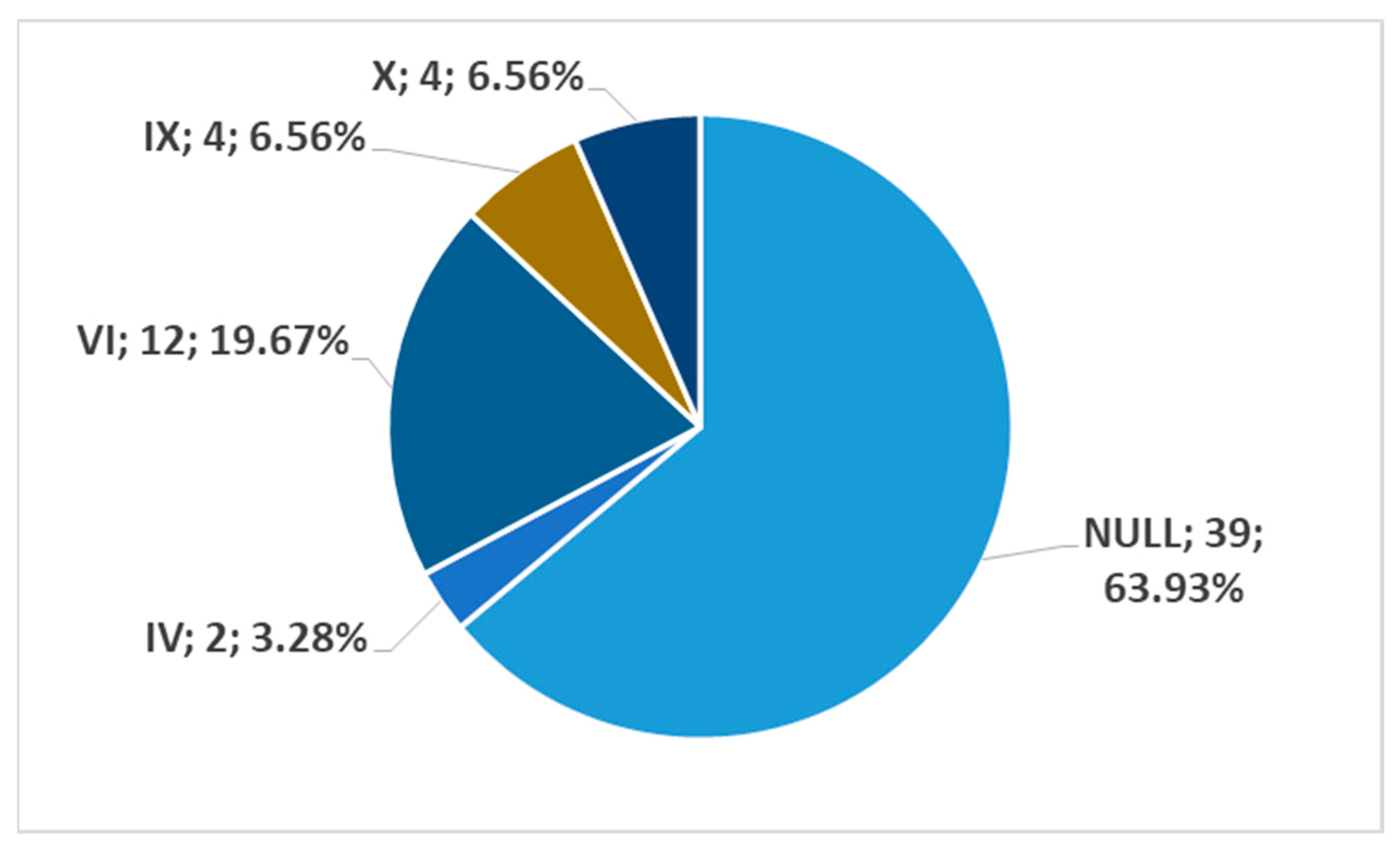
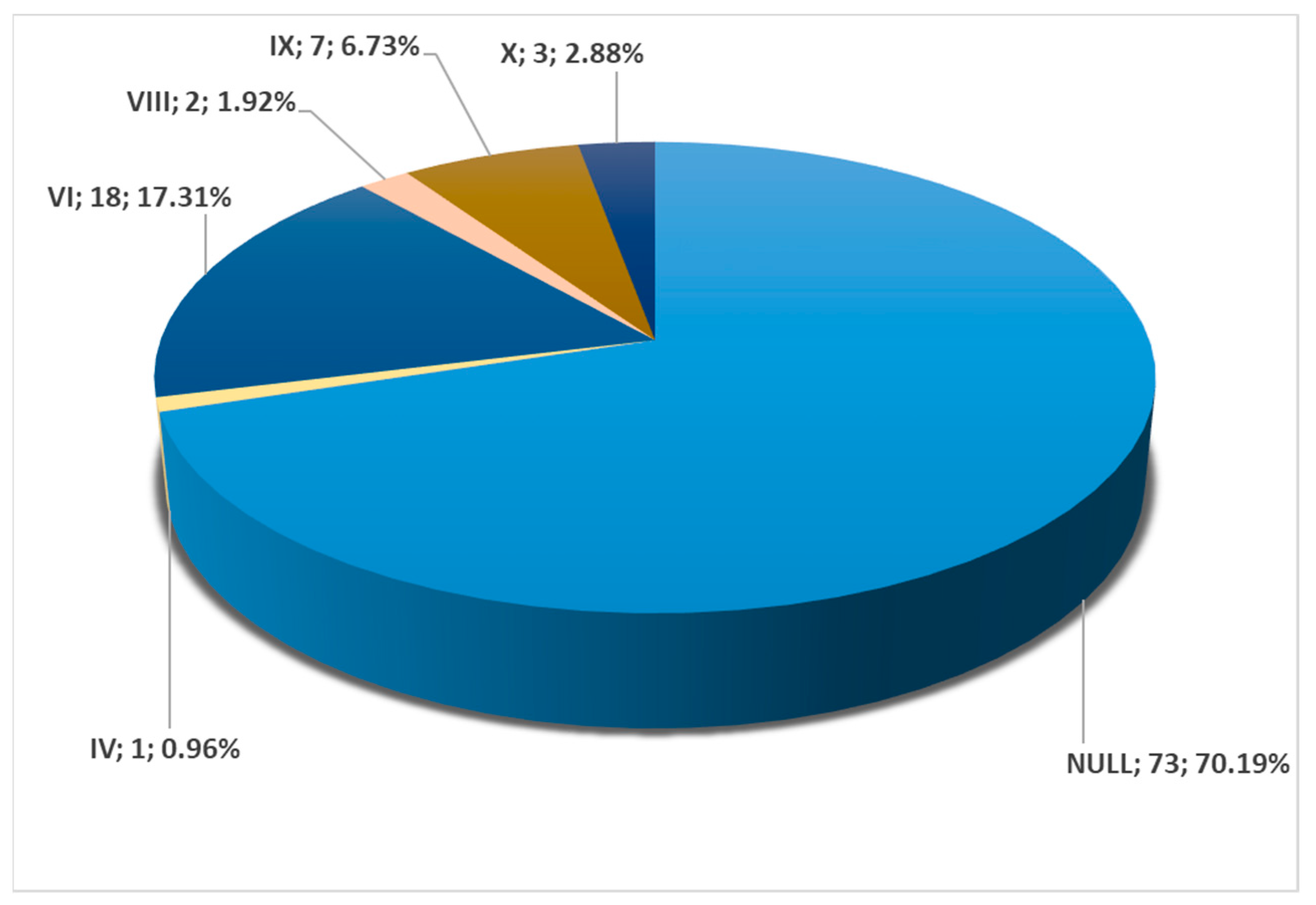
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zumre, O.; Salbacak, A.; Cicekcibasi, A.E.; Tuncer, I.; Seker, M. Investigation of the bifurcation level of the common carotid artery and variations of the branches of the external carotid artery in human fetuses. Ann. Anat. 2005, 187, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Anangwe, D.; Saidi, H.; Ogeng’o, J.; Awori, K.O. Anatomical variations of the carotid arteries in adult Kenyans. East. Afr. Med. J. 2008, 85, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Subbiah, N.K. A Cadaveric Study on the Course of the Cervical Segment of the Internal Carotid Artery and Its Variations. Cureus 2020, 12, e7663. [Google Scholar] [CrossRef]
- Klosek, S.K.; Rungruang, T. Topography of carotid bifurcation: Considerations for neck examination. Surg. Radiol. Anat. 2008, 30, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Lieber, B.B.; Wakhloo, A.K. Morphological age-dependent development of the human carotid bifurcation. J. Biomech. 2005, 38, 453–465. [Google Scholar] [CrossRef]
- Gray, H.; Standring, S.; Anand, N.; Birch, R.; Collins, P.; Crossman, A.; Gleeson, M.; Jawaheer, G.; Smith, A.L.; Spratt, J.D.; et al. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Al-Rafiah, A.; AA, E.L.-H.; Aal, I.H.; Zaki, A.I. Anatomical study of the carotid bifurcation and origin variations of the ascending pharyngeal and superior thyroid arteries. Folia Morphol. 2011, 70, 47–55. [Google Scholar]
- Nayak, S.; Kumar, N. Multiple Loops of External and Internal Carotid Arteries Vulnerable in Surgical and Radiological Procedures. Balk. Med. J. 2018, 35, 285–286. [Google Scholar] [CrossRef]
- Nayak, S.B.; Shetty, S.D. Surgical and embryological perspective of a big loop of internal carotid artery extending laterally beyond internal jugular vein. Surg. Radiol. Anat. 2021, 43, 413–416. [Google Scholar] [CrossRef]
- Paulsen, F.; Tillmann, B.; Christofides, C.; Richter, W.; Koebke, J. Curving and looping of the internal carotid artery in relation to the pharynx: Frequency, embryology and clinical implications. J. Anat. 2000, 197 Pt 3, 373–381. [Google Scholar] [CrossRef]
- Uno, M.; Yagi, K.; Takai, H.; Hara, K.; Oyama, N.; Yagita, Y.; Matsubara, S. Diagnosis and Operative Management of Carotid Endarterectomy in Patients with Twisted Carotid Bifurcation. Neurol. Med. Chir. 2020, 60, 383–389. [Google Scholar] [CrossRef]
- Tokugawa, J.; Kudo, K.; Mitsuhashi, T.; Mitsuhashi, T.; Hishii, M. Older age, carotid artery stenosis, and female sex as factors correlated with twisted carotid bifurcation based on 457 angiographic studies. Clin. Neurol. Neurosurg. 2023, 233, 107902. [Google Scholar] [CrossRef]
- Lukins, D.E.; Pilati, S.; Escott, E.J. The Moving Carotid Artery: A Retrospective Review of the Retropharyngeal Carotid Artery and the Incidence of Positional Changes on Serial Studies. AJNR Am. J. Neuroradiol. 2016, 37, 336–341. [Google Scholar] [CrossRef]
- Rusu, M.C.; Jianu, A.M.; Manta, B.A.; Hostiuc, S. Aortic Origins of the Celiac Trunk and Superior Mesenteric Artery. Diagnostics 2021, 11, 1111. [Google Scholar] [CrossRef]
- Minca, D.I.; Rusu, M.C.; Radoi, P.M.; Vrapciu, A.D.; Hostiuc, S.; Toader, C. The Infraoptic or Infrachiasmatic Course of the Anterior Cerebral Artery Emerging an Elongated Internal Carotid Artery. Tomography 2022, 8, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Larsen, J.L. On the symmetry and asymmetry of the bifurcation of the common carotid artery: A study of bilateral carotid angiograms in 100 adults. Neuroradiology 1979, 17, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Uithoven, K.E.; Ryder, J.R.; Brown, R.; Rudser, K.D.; Evanoff, N.G.; Dengel, D.R.; Kelly, A.S. Determination of Bilateral Symmetry of Carotid Artery Structure and Function in Children and Adolescents. J. Vasc. Diagn. Interv. 2017, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, N.; Puymerail, L.; Michel, J.; Santini, L.; Lebreton-Chakour, C.; Robert, D.; Giovanni, A.; Adalian, P.; Dessi, P. Analysis of hyoid bone using 3D geometric morphometrics: An anatomical study and discussion of potential clinical implications. Dysphagia 2013, 28, 435–445. [Google Scholar] [CrossRef]
- Lemaire, V.; Jacquemin, G.; Nelissen, X.; Heymans, O. Tip of the greater horn of the hyoid bone: A landmark for cervical surgery. Surg. Radiol. Anat. 2005, 27, 33–36. [Google Scholar] [CrossRef]
- Kolbel, T.; Holst, J.; Lindh, M.; Matzsch, T. Carotid artery entrapment by the hyoid bone. J. Vasc. Surg. 2008, 48, 1022–1024. [Google Scholar] [CrossRef]
- Plotkin, A.; Bartley, M.G.; Bowser, K.E.; Yi, J.A.; Magee, G.A. Carotid Artery Entrapment by the Hyoid Bone-A Rare Cause of Recurrent Strokes in a Young Patient. Ann. Vasc. Surg. 2019, 57, 48.e7–48.e11. [Google Scholar] [CrossRef]
- Martinelli, O.; Fresilli, M.; Jabbour, J.; Di Girolamo, A.; Irace, L. Internal Carotid Stenosis Associated with Compression by Hyoid Bone. Ann. Vasc. Surg. 2019, 58, 379.e1–379.e3. [Google Scholar] [CrossRef] [PubMed]
- Kho, L.K.; Bates, T.R.; Thompson, A.; Dharsono, F.; Prentice, D. Cerebral embolism and carotid-hyoid impingement syndrome. J. Clin. Neurosci. 2019, 64, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Nezami, N.; Dardik, A.; Nassiri, N. Hyoid bone impingement contributing to symptomatic atherosclerosis of the carotid bifurcation. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, K.; Uehara, T.; Kanamaru, H.; Kataoka, H.; Saito, K.; Ishibashi-Ueda, H.; Shobatake, R.; Yamamoto, Y.; Toyoda, K. Repetitive Artery-to-Artery Embolism Caused by Dynamic Movement of the Internal Carotid Artery and Mechanical Stimulation by the Hyoid Bone. Circulation 2015, 132, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Renard, D.; Freitag, C. Hyoid-related internal carotid artery dissection. J. Neurol. 2012, 259, 2501–2502. [Google Scholar] [CrossRef]
- Mori, M.; Yamamoto, H.; Koga, M.; Okatsu, H.; Shono, Y.; Toyoda, K.; Fukuda, K.; Iihara, K.; Yamada, N.; Minematsu, K. Hyoid bone compression-induced repetitive occlusion and recanalization of the internal carotid artery in a patient with ipsilateral brain and retinal ischemia. Arch. Neurol. 2011, 68, 258–259. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, T.J.; Lee, J.S.; Lee, J.S. Neurological picture. Repetitive internal carotid artery compression of the hyoid: A new mechanism of golfer’s stroke? J. Neurol. Neurosurg. Psychiatry 2011, 82, 233–234. [Google Scholar] [CrossRef]
- Renard, D.; Rougier, M.; Aichoun, I.; Labauge, P. Hyoid bone-related focal carotid vasculopathy. J. Neurol. 2011, 258, 1540–1541. [Google Scholar] [CrossRef]
- Yukawa, S.; Yamamoto, S.; Hara, H. Carotid artery dissection associated with an elongated hyoid bone. J. Stroke Cerebrovasc. Dis. 2014, 23, e411–e412. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Chu, C.; Ren, Y.; Hua, Y.; Ji, X.; Song, H. Hyoid Elongation May Be a Rare Cause of Recurrent Ischemic Stroke in Youth-A Case Report and Literature Review. Front. Neurol. 2021, 12, 653471. [Google Scholar] [CrossRef]
- Schneider, C.G.; Kortmann, H. Pseudoaneurysm of the common carotid artery due to ongoing trauma from the hyoid bone. J. Vasc. Surg. 2007, 45, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Koreckij, J.; Alvi, H.; Gibly, R.; Pang, E.; Hsu, W.K. Incidence and risk factors of the retropharyngeal carotid artery on cervical magnetic resonance imaging. Spine 2013, 38, E109–E112. [Google Scholar] [CrossRef] [PubMed]
- Ferracci, F.X.; Heuze, D.; Sacco, R.; Curado, J.; Monnot, A.; Duparc, F.; Ould-Slimane, M. Common carotid artery medialization and fracture dislocation of the cervical spine. Surg. Radiol. Anat. 2022, 44, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.E.; Konsky, G.; Renner, C.; Moreno-De-Luca, A.; Gutman, D.; Cucchiara, B.; Messe, S.R. Carotid artery atherosclerosis is not associated with hyoid proximity: Results from a cross-sectional and longitudinal cohort study. Clin. Imaging 2019, 58, 39–45. [Google Scholar] [CrossRef]
- Abdelaziz, O.S.; Ogilvy, C.S.; Lev, M. Is there a potential role for hyoid bone compression in pathogenesis of carotid artery stenosis? Surg. Neurol. 1999, 51, 650–653. [Google Scholar] [CrossRef]
- McConnel, C.S., Jr.; Marlowe, F.I. Postoperative perforation of the carotid artery by the hyoid bone. Arch. Otolaryngol. 1972, 95, 282–283. [Google Scholar] [CrossRef]
- Nadig, S.; Barnwell, S.; Wax, M.K. Pseudoaneurysm of the external carotid artery–review of literature. Head Neck 2009, 31, 136–139. [Google Scholar] [CrossRef]
- Campbell, A.S.; Butler, A.P.; Grandas, O.H. A case of external carotid artery pseudoaneurysm from hyoid bone fracture. Am. Surg. 2003, 69, 534–535. [Google Scholar] [CrossRef]
- Bernardes, M.N.D.; Cascudo, N.C.M.; El Cheikh, M.R.; Goncalves, V.F.; Lamounier, P.; Ramos, H.V.L.; Costa, C.C. Aberrant common and internal carotid arteries and their surgical implications: A case report. Braz. J. Otorhinolaryngol. 2021, 87, 366–369. [Google Scholar] [CrossRef]
- Kumar, S.; Ferrari, R.; Narayan, Y. Cervical muscle response to head rotation in whiplash-type left lateral impacts. Spine 2005, 30, 536–541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manta, M.D.; Rusu, M.C.; Hostiuc, S.; Vrapciu, A.D.; Manta, B.A.; Jianu, A.M. The Carotid–Hyoid Topography Is Variable. Medicina 2023, 59, 1494. https://doi.org/10.3390/medicina59081494
Manta MD, Rusu MC, Hostiuc S, Vrapciu AD, Manta BA, Jianu AM. The Carotid–Hyoid Topography Is Variable. Medicina. 2023; 59(8):1494. https://doi.org/10.3390/medicina59081494
Chicago/Turabian StyleManta, Mihaela Daniela, Mugurel Constantin Rusu, Sorin Hostiuc, Alexandra Diana Vrapciu, Bogdan Adrian Manta, and Adelina Maria Jianu. 2023. "The Carotid–Hyoid Topography Is Variable" Medicina 59, no. 8: 1494. https://doi.org/10.3390/medicina59081494
APA StyleManta, M. D., Rusu, M. C., Hostiuc, S., Vrapciu, A. D., Manta, B. A., & Jianu, A. M. (2023). The Carotid–Hyoid Topography Is Variable. Medicina, 59(8), 1494. https://doi.org/10.3390/medicina59081494







