Abstract
Introduction: Methotrexate (MTX) reduces rheumatoid arthritis activity and ameliorates the long-term functional status in these patients. To achieve this aim, patients need to take their medication regularly. Nevertheless, non-adherence to MTX still remains a considerable issue in the management of rheumatoid arthritis. Objective: This study aimed to estimate the adherence to methotrexate in patients with rheumatoid arthritis and to identify specific non-adherence risk factors. Methods: A cross-sectional study included 111 patients (mean age 56.2 ± 10.6 years, 78.4% female, and mean disease duration 6 years (3–13)). Three adherence self-assessment questionnaires were used: the Compliance-Questionnaire-Rheumatology (CQR19), the Medication Adherence Reports Scale (MARS-5), and the Visual Analogue Scale (VAS). We also collected demographic data, disease and treatment characteristics, and anxiety/depression estimation results (Hospital Anxiety and Depression Scale, HADS). Results: Adherence was identified in 48.6% of patients (COR19), 70.3% of patients (MARS-5), and 82.9% of patients (VAS questionnaire). All three questionnaires displayed a significant positive mutual correlation: CQR19 with MARS-5 and VAS (r = 0.364, r = 0.329, respectively, p < 0.001 for both) and between the VAS and MARS-5 scores (r = 0.496, p < 0.001). A significant positive prediction was shown for urban residence (0.347 (0.134–0.901), p = 0.030) using the MARS-5, female sex (0.264 (0.095–0.730), p = 0.010) according to the CQR19, and for a dose of methotrexate (0.881 (0.783–0.992), p = 0.036) using the VAS, while negative predictions were shown for comorbidity number (3.062 (1.057–8.874), p = 0.039) and depression (1.142 (1.010–1.293), p = 0.035) using the MARS-5 and for older age (1.041 (1.003–1.081), p = 0.034) according to the CQR19. The use of steroids was a significant positive predictor in all three questionnaires and remained an independent predictor for methotrexate adherence in the multivariate logistic regression. Conclusions: We showed non-adherence to methotrexate in a significant number of patients using all three questionnaires. Concomitant steroid therapy emerged as an independent positive predictor for adherence.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease characterized by symmetric peripheral polyarthritis and progressive joint damage. Disease-modifying antirheumatic drugs (DMARDs) reduce disease activity and radiologic progression and ameliorate the long-term functional status in RA patients []. To achieve this aim, patients need to take their medication regularly.
Methotrexate (MTX) is a synthetic DMARD recommended as the first-line treatment in a patient with RA [,] due to its safety and efficacy, different methods of application (parenteral and oral), and drug dose titration possibility [,]. It can be used as a monotherapy or combined with other synthetic or biological DMARDs []. However, not all patients display a good response to MTX, which can be partially attributed to therapy noncompliance [,]. Optimal utilization of MTX requires starting therapy immediately and adherence to a prescribed dose and regimen until a satisfactory response is obtained, followed by complying with a maintenance dose.
Non-adherence is defined as the avoidance of therapy onset, intentional or unintentional disregard for the physician’s recommendations, or non-persistence with treatment []. Several studies have been conducted concerning the adherence to MTX in RA patients. The results were inconsistent due to different criteria for establishing adherence, the vast heterogeneity of participant samples, the employment of different types of measurements, and follow-up length. A literature analysis revealed a large variation in adherence, with its rate range spanning from 59% to 107%, where excessive MTX use was also considered an adherence problem [,]. In previous studies, the association of certain demographic and disease characteristics, socio-economic status, mental health, and patients’ beliefs regarding medication with adherence to MTX was found [,]. Hope et al. concluded that the mild course of the disease, depression absence, patients’ confidence in the efficacy and necessity of the therapy, as well as MTX monotherapy are potential predictors of adherence to MTX []. According to the literature, more than 200 variables have an influence on adherence, but none of them showed correlative consistency in different studies [], indicating that no uniform non-adherence profile could be made [].
In order to enhance adherence, the priority is to identify individuals displaying non-adherent behavior []. Along with the substantial subjective and objective measurement approaches, no strategy is rated as optimal. A multi-method that combines self-assessment and reasonable objective measurements is currently the most adequate procedure to estimate adherent behavior. Still, the most feasible manner of detecting non-adherent patients in clinical practice is a self-reporting method.
In addition to the need for the better education of patients, there are still numerous issues in adherence improvement [,]. Considering the existence of a still-significant number of non-adherent patients, innovative interventions to optimize the use of MTX are needed to improve the disease’s final outcome []. Therefore, this research aimed to estimate adherence to MTX and identify specific socio-demographic, clinical, and psychological risk factors for non-adherence.
2. Materials and Methods
2.1. Study Patients
This cross-sectional study included patients treated in the Rheumatology Clinic of the Military Medical Academy in Belgrade during 2016 and 2017 who met the following requirements: RA diagnosis based on the classification criteria ACR/EULAR2010 [], age ≥ 18 years, and prescribed MTX with minimum two months’ continuance. Exclusion criteria were the presence of another connective tissue systemic disease, psychiatric disorders, and antidepressant therapy within the last month, as well as recent infections and surgical procedures.
This is a single-center study approved by the institutional ethics committee and it conformed to the Declaration of Helsinki. All participants have signed informed consent.
Literature-based questionnaires were used to collect data about patients and disease characteristics. One part of the questionnaire was related to socio-demographic characteristics (sex, age, place of residence, education, employment, and smoking status) and the other to clinical aspects of the disease (disease duration, current MTX dose, maximal MTX dose, therapy side effects, concomitant use of steroids, other DMARDs or biologics, comorbidity presence, frequency of blood laboratory analysis, and the annual number of physician visits).
The following inflammatory markers were analyzed: erythrocyte sedimentation (ESR) rate according to Westergreen (mm/h), C reactive protein (CRP) using nephelometry (range 0–5 mg/L), and interleukin 6 (IL-6) serum level engaging enzyme-linked immunosorbent assay (ELISA, Bionova, Madrid, Spain) according to the manufacturer instructions (range 0–5.9 pg/mL).
RA activity was assessed by Disease Activity Score 28-joint count (DAS28-ESR) [] and Clinical Disease Activity Index (CDAI) [,,]. Disease activity was defined as remission (DAS28 < 2.6, CDAI ≤ 2.8), low disease activity (DAS28 2.6–3.2, CDAI 2.8–10), moderate disease activity (DAS28 3.2–5.1, CDAI 10–22), and high disease activity for DAS28 over 5.1 and CDAI over 22. The functional ability was estimated using a questionnaire for assessing patients’ health status, the Health Assessment Questionnaire (HAQ), more accurately the disability index HAQ-DI []. An HAQ score from 0–1 represents mild to moderate difficulties, 1–2 moderate to heavy disabilities, and 2–3 severely heavy disabilities.
2.2. Adherence Assessment
Adherence to MTX therapy was conveyed employing three literature-based questionnaires: the Compliance-Questionnaire-Rheumatology (CQR19), the Medication Adherence Reports Scale (MARS-5), and freely estimated adherence on the Visual Analogue Scale (VAS) ranging from 0 to 10 cm.
The CQR19 [,] is composed of 19 statement questions regarding specific barriers in taking prescribed medication and the patient grades the level of agreement according to a Likert scale from 1 to 4 (1 point = strongly disagree, 2 points = disagree, 3 points = agree, and 4 points = strongly agree). Out of the 19 questions, 6 were formulated in negation (numbered questions 4, 8, 9, 11, 12, and 19); therefore, reverse scoring had to be applied. The overall score was obtained by adding up all points and subtracting 19 from the total sum. The new calculated value was divided by 0.57. This ensures that the complete CQR19 result can vary from 0 (no adherence) to 100 (perfect adherence) [,,]. In agreement with published data, a less than 80% score represents low adherence.
The MARS-5 is an adherence questionnaire developed for patients with different chronic diseases []. The questionnaire is composed of 5 questions regarding certain aspects of non-adherent behavior. Each question has multiple-choice answers: 1 = always, 2 = often, 3 = sometimes, 4 = rarely, and 5 = never. The total MARS-5 score can take values from 5 to 25, with a higher rating indicating elevated adherence levels. Different studies suggest that in the majority of measuring scales, a score above 80% is considered satisfactory. Therefore, in this study, patients with MARS-5 scores above 23 were qualified as adherents [].
The Visual Analogue Scale (VAS) ranges from 0% to 100%. On a scale span from 0 cm to 10 cm, a patient solely determines the level of adherence by marking a number or position that most adequately suits their subjective estimation of compliance to therapy. Values beyond 80% were considered a high level of therapy adherence.
2.3. Mental Health Assessment
Mental health assessment was conveyed by the Hospital Anxiety and Depression Scale (HADS) questionnaire, recommended by The National Institute for Health and Care Excellence (NICE) in order to diagnose depression and anxiety []. The questionnaire was comprised of 14 questions, with half of them (7) related to anxiety and the other half (7 related) to depression. Each question was answered according to a Likert scale with 4 possible answers (0–3). The overall score for each scale ranged from 0 to 21 and was categorized as 0–7 = normal finding, 8–10 = borderline anxiety/depression, and 11–21 = abnormal, presence of anxiety/depression [,]. The specificity of these scores is 0.78 and 0.79 for anxiety and depression, respectively, while score sensitivity values corresponding to anxiety and depression are 0.9 and 0.83 [].
2.4. Statistical Data Analysis
Statistical analysis was performed using IBM SPSS version 26.0 (IBM Corp.Released 2019. IBM SPSS Statistics for Windows, version 26.0 Armonk, NY: IBM Corp). Descriptive values were presented as average values with standard deviation or as median with an interquartile range (25–75th percentile). Distribution was verified by the Kolmogorov–Smirnov test. Attribute variables were presented in the form of frequencies of individual categories. Statistical significance was estimated by the Chi-square test or Fisher’s exact test for attributive variables. Continuous variables were tested using Student’s t-test for (in) dependent samples or nonparametric alternative Mann–Whitney test and Wilcoxon test. The correlation was estimated by Pearson correlation. Predictive value exploration for the chosen variables in the assessment of adherence to MTX in the group of patients with RA was conveyed by univariate and multivariate logistic regression analyses. A p-value less than 0.05 for variables in the univariate analysis was used for inclusion in the multivariate analysis. The results of the logistic regression analysis were presented as odds ratio with 95% confidence interval (OR (95% CI). A p-value less than 0.05 was considered statistically significant for all analyses.
3. Results
Our study included 111 RA patients with an average age of 56.2 ± 10.6 years. Females made up the majority of the survey sample (78.4%), almost half of the patients (48.6%) were non-smokers, and 35.1% were employed. The majority of respondents lived in the city (79.3%) and had a high school education (68.5%) (Table 1).

Table 1.
Participant characteristics and comparison between demographic, clinical, and psychological features of adherent and non-adherent patients.
Comorbidities were marked in 74 (66.7%) patients, and 23 (20.7%) of them had three or more conditions. The median disease duration in our sample population from the establishment of diagnosis was 6 years (IQR: 3–13.5). The number of annual visits to a rheumatologist in our patients was 4 (IQR: 3–6), while the average laboratory analysis was conducted 4 times a year.
In combined therapy adjacent to MTX, 96 patients (86.5%) were taking steroids and he median dose was 6 mg. Antimalarials were used by 33 (29.7%) patients, and 34 study participants (30.6%) were on biological therapy. A third of the subjects had side effects: gastrointestinal tract discomforts (13.5%), leukopenia, and elevated liver enzymes (10.8%).
According to the CDAI, low disease activity was detected in 29.7% of patients, moderate was observed in 32.4%, and high in 33.3%. Similar results were obtained by DAS28 scoring: 36.9% of study participants had high and 34.2% moderate disease activity. The average HAQ scale score was 0.89 ± 0.48.
The mental health analysis revealed that 18% of patients had elevated scores on the HADS scale for depression, while 10.8% of study participants had increased scores on the HADS scale for anxiety. About a quarter of patients accomplished a borderline HADS score for depression and anxiety.
Adherence to MTX measured by three scales (CQR19, MARS-5, and VAS) was represented as a dichotomous variable (Table 2). The average score for adherence to MTX on the CQR19 scale was 76.9 ± 13.5, while a non-adherence level to MTX was detected in 56 (50.5%) patients. According to the MARS-5 criteria, a non-adherence level was found in 32 study partakers (28.8%) (average score 22.63 ± 2.58). On the VAS ranging from 0 to 100 mm, patients graded self-reported adherence to MTX as 87.44 ± 16.49. Non-adherence to MTX (<80%) according to the VAS was detected in 19 of the study participants (17.1%) (Table 2).

Table 2.
The medication adherence rate to methotrexate.
The analysis of all three scores used to measure adherence showed a statistically positive correlation of the CQR19 score with the MARS-5 score (r = 0.364, p < 0.001) and the VAS score (r = 0.329, p < 0.001). An even stronger positive correlation was observed between the VAS and MARS-5 scores (r = 0.496, p < 0.001) (Figure 1).
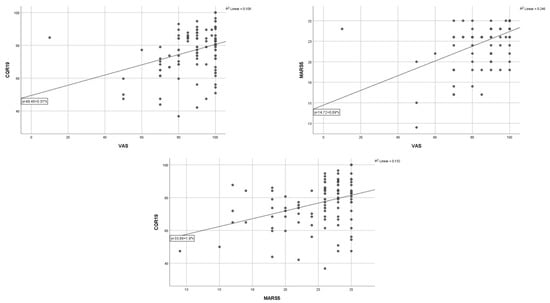
Figure 1.
Correlation between three questionnaires of quality of life in patients with rheumatoid arthritis.
In the analysis of adherence indicators, the CQR19, the MARS-5, and the VAS score identified significant differences in adherence to MTX regarding sex, age, and urban residence. Also, a prominent dissimilarity was observed with respect to the number of present chronic diseases, number of annual physician visits, MTX dose, and co-medication with steroids. Patients on corticosteroid therapy had markedly enhanced adherence expressed with the VAS (p = 0.049) and CQR19 score (p = 0.022) (Table 1).
In the univariate logistic regression, statistical significance for non-adherence measured by the VAS was obtained for MTX dose (0.881 (0.783–0.992), p = 0.036) and concomitant steroid use (0.273 (0.078–0.956), p = 0.042). Patients who used a lower dose of MTX and those without concomitant steroid therapy had a higher degree of non-adherence (Table 3).

Table 3.
Univariate logistic regression: prediction of non-adherent behavior.
Using the MARS-5, statistical significance for non-adherence was obtained for place of residence (0.347 (0.134–0.901), p = 0.030), where a higher degree of non-adherence was obtained for those living in rural areas.
Statistical significance was also obtained for the number of comorbidities (3.062 (1.057–8.874), p = 0.039), where patients with a higher number of comorbidities were more non-adherent. Additionally, the presence of depression (1.142 (1.010–1.293), p = 0.035) leads to a higher degree of non-adherence. As observed in the previous questionnaire, a statistically significant parameter for non-adherence was the concomitant use of steroids (0.306 (0.094–0.998). p = 0.050). This means that patients who were not using steroids had a higher degree of non-adherence (Table 3).
In the multivariate logistic regression, it was found that a statistically significant independent predictor for non-adherence was the concomitant use of steroids (Table 4). The concomitant use of corticosteroids reduces the chance of poor non-adherence by 4.4 times.

Table 4.
Multivariate logistic regression analysis: predictors of non-adherence measured by the MARS-5.
For non-adherence measured by the CQR scale by univariate regression, statistical significance was obtained for sex (0.264 (0.095–0.730), p = 0.010), where males have a higher degree of non-adherence. Statistical significance was detected both for age (1.041 (1.003–1.081), p = 0.034), meaning older patients were more non-adherent, as well as for the concomitant use of steroids (0.157 (0.033–0.746), p = 0.020).
The multivariate logistic regression identified gender and the concomitant use of steroids as statistically significant independent predictors of non-adherence (Table 5). The concomitant use of corticosteroids diminishes the odds of non-adherence by 5.1 times, while the female gender reduces this chance by approximately 3.9.

Table 5.
Multivariate logistic regression analysis: predictors of non-adherence measured by the CQR19.
Finally, the composite score is shown as non-adherence in at least two of the three tested scores. The multivariate logistic regression identified comorbidities and the concomitant use of steroids as statistically significant predictors of non-adherence (Table 6). The concomitant use of corticosteroids diminishes the odds of non-adherence by 4.65 times, while a smaller number of comorbidities reduces this chance by approximately 4.1.

Table 6.
Univariate and multivariate logistic regression analysis: predictors of non-adherence measured by the composite score #.
4. Discussion
In this study, the employment of three self-assessment questionnaires showed that 48.6% (CQR19), 70.3% (MARS-5), and 82.9% (VAS) of patients were adherent. The logistic regression showed that the concomitant use of corticosteroids is an independent predictor of adherence to MTX in all three questionnaires. Current studies exhibit significant variation in the degree of adherence using different measuring instruments. The latest research on adherence assessment by the CQR questionnaire found that 78% and 85.7% of patients were adherent [,]. De Cuyper et al. demonstrated that only 24.2% of patients were adherent when the MARS-5 questionnaire estimation tool was used, while the VAS score delivered more promising results, showing adherent behavior in 94% of the subjects. This observation can be explained by the absence of an existing standard for adherence measurement and the probability of overestimation in the self-reporting approach. The CQR was chosen as the only validated questionnaire in the rheumatology field. For the MARS-5 questionnaire, the latest studies did not use the same cut-off point for the dichotomization of the MARS-5, limiting the possibility of data comparison.
The statistical difference was detected between adherent and non-adherent patients in terms of gender, age, place of residence, number of annual visits to a physician, MTX dose, and steroid co-medication. In our cohort, younger patients had a higher degree of adherence, which is consistent with the data from van den Bemt et al. [].
This could be clarified by the association of older age with comorbidities and therefore polypharmacy, increasing the probability of side effect incidence. However, other research groups report opposing results that show adherence in the elderly patient population [,,], succeeding to the conclusion that age can affect adherence differentially. The female gender impact on better adherence was previously observed [,,], while some authors report inconsistency [,].
The positive influence of comorbidities on non-adherence is in accordance with published research [,], where two or more comorbidities represent a predictor of non-adherence. When multiple comorbidities are present, complexity and the extent of therapy carry a risk of forgetfulness and unintentional non-adherence []. In our study, patients who had a higher number of annual visits to a physician were more adherent. This is understandable since doctor–patient relations have a big effect on the efficacy of therapeutic regimens. Occasionally, non-adherence can occur due to a lack of conscientiousness in patients regarding medication necessity []. Educating patients about their condition and therapy, as well as the doctor–patient confidence level, can diversly affect adherence [].
In the univariate logistic regression following the VAS, the current MTX dose was a significant predictor of non-adherence, while the MARS-5 questionnaire showed a marked prediction of adherence for depression and comorbidities. This was also shown for gender and age in the CQR19.
The concomitant use of steroids was a statistically significant predictor of non-adherence, according to the results of all three questionnaires. The majority of studies did not find an association between the dose of MTX and adherence, and only one study highlighted a connection between the higher dose of MTX with improved adherence [], but the supportive validation from other research groups is missing [,].
The negative association between worse mental health and adequate adherence has also been shown in studies by other authors [,], which is consistent with our research. Patients suffering from depression have a 2–3 times higher rate of non-adherence compared to those without this disorder [].
In our study, the multivariate analysis revealed that the concomitant use of steroids is an independent positive predictor of adherence, measured by the MARS-5 and CQR19. According to our knowledge, the influence of the concomitant use of steroids on MTX adherence was analyzed in only a few studies and the results are inconsistent [,,]. For example, H. Bliddal et al. did not find any influence of prednisolone therapy on MTX adherence [], while the negative impact of concomitant steroid use was shown by Alrubaye et al. []. On the other hand, Hoekstra M et al. showed that the concomitant use of prednisolone remained significantly related to MTX therapy in a multivariate analysis, which is consistent with our results [].
This result may be a consequence of the fact that our patients had more frequent relapses of the disease, which required the use of steroids and thus affected the patients’ belief in the necessity of MTX therapy. Perhaps the large number of patients who received steroids in comparison with other studies where this number was significantly smaller influenced this result.
Several research studies showed that the lack of comprehension related to MTX’s slow-acting effects can impact adherence []. Presumably, steroid administration with rapid and efficient action could have altered adherence. Treharna et al. found that adherence was better in a group of patients taking steroids because the absence of therapy can lead to a significant worsening of symptoms in the active form of the disease []. These results could also be the explanation for the better adherence to MTX in our group of patients on steroids, where steroids enhance the importance of MTX itself in the treatment of RA. On the other hand, the side effects of steroids cause concern in patients and could be diminished with MTX as a steroid-sparing drug.
Since our results show that the concomitant use of steroids can increase adherence to MTX, this may have a clinical implication in patients who have discontinued steroids (due to less disease activity), as they require additional attention in their adherence to MTX therapy.
Since all three scores showed a good correlation, a composite score was created. It showed similar results as for each score individually. Therefore, it can be recommended that each of these three scores can be equally used separately or together in the same patient. This is very important for the further clinical implication of these adherence tests.
The main limitations of this study are its cross-sectional design and monocentric character. A cross-sectional design enables adherence-related variable identification but with inconclusive results. An additional problem is a bias selection since all included subjects were recruited from the same institution and had an opportunity for more frequent follow-ups, which could have diminished the inclusion of non-adherent patients. Also, a small patient sample size does not have enough statistical power to determine the influence of the examined variables on adherence. The utilization of the MARS-5 questionnaire without a clearly defined cut-off point for the separation of adherent and non-adherent study participants may result differentially. Commonly, the use of scale dichotomization and cut-off points can lead to the loss of important information due to the simplification of the examined characteristics. Also, employing self-assessment scales can result in the overestimation of adherence due to social desirability and giving adjusted answers. The research did not convey a follow-up over time, and it is known that adherence is a dynamic process that can be influenced by several factors and that patient behavior can change over time.
This study’s strength is using three measuring scales that exhibited significant mutual correlation, especially that observed between the VAS and MARS-5. To our knowledge, DeCuyper et al. were the only other authors that used all three self-reported questionnaires assessing adherence to MTX, and they have shown similar results. In accordance with the research of these authors, this would indicate the importance of VAS application in everyday use for the rapid assessment of adherence to MTX in patients with RA [].
5. Conclusions
Our study showed that a significant percentage of patients with RA are non-adherent. Furthermore, extensive variation in the level of adherence can be observed depending on the measuring tool. A curious fact is that the concomitant use of steroids was singled out as an independent predictor of non-adherence. Since adherence to MTX in patients with RA is still suboptimal and measurements are not standardized, they are nevertheless necessary for daily clinical practice. The use of self-reporting questionnaires may have clinical relevance for the rapid detection of adherence. Given the above facts, further research is needed to discover non-adherent patients and to explain the reasons for non-adherence, as well as to develop adapted and possibly highly personalized interventions to improve adherence.
Author Contributions
Conceptualization, J.C., D.K.T. and G.R.; methodology, J.C. and G.R.; software, M.C., N.R. and M.M.; validation, J.C.; formal analysis, M.C., M.M. and N.R.; investigation, M.P., J.C., M.C. and J.C.; resources, J.C.; data curation, J.C.; writing—original draft preparation, all authors; writing—review and editing, G.R. and N.R.; visualization, J.C.; supervision, G.R. and D.K.T.; project administration, J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Belgrade, Serbia (protocol code 29/III-16, date 13 March 2017) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data sets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to express their sincere gratitude to Slobodan Mišeljić, Commonwealth Radiology Associates General Hospital, 295 Varnum Avenue, Lowell, MA, 01854, for his careful reading of the text and revision of the article in terms of the content clarity, writing style, and English grammar.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Jones, G.; Halbert, J.; Crotty, M.; Shanahan, E.M.; Batterham, M.; Ahern, M. The effect of treatment on radiological progression in rheumatoid arthritis: A systematic review of randomized placebo-controlled trials. Rheumatology 2003, 42, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Combe, B.; Landewe, R.; Daien, C.I.; Hua, C.; Aletaha, D.; Álvaro-Gracia, J.M.; Bakkers, M.; Brodin, N.; Burmester, G.R.; Codreanu, C.; et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann. Rheum. Dis. 2017, 76, 948–959. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. The Management of Rheumatoid Arthritis in Adults; National Institute for Health and Care Excellence (NICE): London, UK, 2018. [Google Scholar]
- Taylor, P.C.; Balsa Criado, A.; Mongey, A.B.; Avouac, J.; Marotte, H.; Mueller, R.B. How to get the most from methotrexate (MTX) treatment for your rheumatoid arthritis patient?-MTX in the treat-to-target strategy. J. Clin. Med. 2019, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Wollenhaupt, J.; Albrecht, K.; Alten, R.; Backhaus, M.; Baerwald, C.; Bolten, W.; Braun, J.; Burkhardt, H.; Burmester, G.; et al. European League of Associations for Rheumatology (EULAR). S1-Leitlinie der DGRh zur sequenziellen medikamentösen Therapie der rheumatoiden Arthritis 2012. Adaptierte EULAR-Empfehlungen und aktualisierter Therapiealgorithmus [German 2012 guidelines for the sequential medical treatment of rheumatoid arthritis. Adapted EULAR recommendations and updated treatment algorithm]. Z. Rheumatol. 2012, 71, 592–603. [Google Scholar] [PubMed]
- O’Dell, J.R.; Curtis, J.R.; Mikuls, T.R.; Cofield, S.S.; Bridges, S.L., Jr.; Ranganath, V.K.; Moreland, L.W.; TEAR Trial Investigators. Validation of the methotrexate-first strategy in patients with early, poor-prognosis rheumatoid arthritis: Results from a two-year randomized, double-blind trial. Arthritis Rheum. 2013, 65, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H.; Smith, C.M.; Farewell, V.; Walker, D.; Hassell, A.; Chau, L.; Scott, D.L.; CARDERA (Combination Anti-Rheumatic Drugs in Early Rheumatoid Arthritis) Trial Group. Factorial randomised controlled trial of glucocorticoids and combination disease modifying drugs in early rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 656–663. [Google Scholar] [CrossRef]
- Hider, S.L.; Silman, A.; Bunn, D.; Manning, S.; Symmons, D.; Lunt, M. Comparing the long-term clinical outcome of treatment with methotrexate or sulfasalazine prescribed as the first disease-modifying antirheumatic drug in patients with inflammatory polyarthritis. Ann. Rheum. Dis. 2006, 65, 1449–1455. [Google Scholar] [CrossRef]
- Brodtkorb, E.; Samsonsen, C.; Sund, J.K.; Bråthen, G.; Helde, G.; Reimers, A. Treatment non-adherence in pseudo-refractory epilepsy. Epilepsy Res. 2016, 122, 1–6. [Google Scholar] [CrossRef]
- Hope, H.F.; Bluett, J.; Barton, A.; Hyrich, K.L.; Cordingley, L.; Verstappen, S.M. Psychological factors predict adherence to methotrexate in rheumatoid arthritis; findings from a systematic review of rates, predictors and associations with patient-reported and clinical outcomes. RMD Open 2016, 2, e000171. [Google Scholar] [CrossRef]
- Curtis, J.R.; Bykerk, V.P.; Aassi, M.; Schiff, M. Adherence and Persistence with Methotrexate in Rheumatoid Arthritis: A Systematic Review. J. Rheumatol. 2016, 43, 1997–2009. [Google Scholar] [CrossRef]
- Cannon, G.W.; Mikuls, T.R.; Hayden, C.L.; Ying, J.; Curtis, J.R.; Reimold, A.M.; Caplan, L.; Kerr, G.S.; Richards, J.S.; Johnson, D.S.; et al. Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res. 2011, 63, 1680–1690. [Google Scholar] [CrossRef]
- De Cuyper, E.; De Gucht, V.; Maes, S.; Van Camp, Y.; De Clerck, L.S. Determinants of methotrexate adherence in rheumatoid arthritis patients. Clin. Rheumatol. 2016, 35, 1335–1339. [Google Scholar] [CrossRef]
- de Klerk, E.; van der Heijde, D.; Landewé, R.; van der Tempel, H.; Urquhart, J.; van der Linden, S. Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J. Rheumatol. 2003, 30, 44–54. [Google Scholar]
- van den Bemt, B.J.; Zwikker, H.E.; van den Ende, C.H. Medication adherence in patients with rheumatoid arthritis: A critical appraisal of the existing literature. Expert Rev. Clin. Immunol. 2012, 8, 337–351. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 1999, 47, 555–567. [Google Scholar] [CrossRef]
- Zangi, H.A.; Ndosi, M.; Adams, J.; Andersen, L.; Bode, C.; Boström, C.; Van Eijk-Hustings, Y.; Gossec, L.; Korandová, J.; Mendes, G.; et al. European League Against Rheumatism (EULAR). EULAR recommendations for patient education for people with inflammatory arthritis. Ann. Rheum. Dis. 2015, 74, 954–962. [Google Scholar] [CrossRef]
- Sowden, E.; Hassan, W.; Gooden, A.; Jepson, B.; Kausor, T.; Shafait, I.; Haque, S.; Brockbank, J.E.; Ley, R.W.; Teh, L.-S. Limited end-user knowledge of methotrexate despite patient education: An assessment of rheumatologic preventive practice and effectiveness. J. Clin. Rheumatol. 2012, 18, 130–133. [Google Scholar] [CrossRef]
- Horne, R. Compliance, adherence, and concordance. In Pharmacy Practice; Taylor, K., Harding, G., Eds.; Taylor & Francis: London, UK, 2001; pp. 148–167. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van ‘t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D. Scores for all seasons: SDAI and CDAI. Clin. Exp. Rheumatol. 2014, 32 (Suppl. 85), S75–S79. [Google Scholar]
- Aletaha, D.; Smolen, J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): A review of their usefulness and validity in rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23 (Suppl. 39), S100–S108. [Google Scholar] [PubMed]
- Smolen, J.S.; Breedveld, F.C.; Schiff, M.H.; Kalden, J.R.; Emery, P.; Eberl, G.; van Riel, P.L.; Tugwell, P. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 2003, 42, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Bruce, B.; Fries, J.F. The Stanford Health Assessment Questionnaire: Dimensions and practical applications. Health Qual. Life Outcomes 2003, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, E.; van der Heijde, D.; van der Tempel, H.; van der Linden, S. Development of a questionnaire to investigate patient compliance with antirheumatic drug therapy. J. Rheumatol. 1999, 26, 2635–2641. [Google Scholar] [PubMed]
- de Klerk, E.; van der Heijde, D.; Landewé, R.; van der Tempel, H.; van der Linden, S. The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: A validation study. J. Rheumatol. 2003, 30, 2469–2475. [Google Scholar]
- Cinar, F.I.; Cinar, M.; Yilmaz, S.; Acikel, C.; Erdem, H.; Pay, S.; Simsek, I. Cross-Cultural Adaptation, Reliability, and Validity of the Turkish Version of the Compliance Questionnaire on Rheumatology in Patients With Behçet’s Disease. J. Transcult. Nurs. 2016, 27, 480–486. [Google Scholar] [CrossRef]
- Salt, E.; Hall, L.; Peden, A.R.; Home, R. Psychometric properties of three medication adherence scales in patients with rheumatoid arthritis. J. Nurs. Meas. 2012, 20, 59–72. [Google Scholar] [CrossRef]
- Garfield, S.; Clifford, S.; Eliasson, L.; Barber, N.; Willson, A. Suitability of measures of self-reported medication adherence for routine clinical use: A systematic review. BMC Med. Res. Methodol. 2011, 11, 149. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health. Common Mental Health Disorders: The NICE Guideline on Identification and Pathways to Care. National Clinical Guideline Number 123. Available online: http://www.nice.org.uk/nicemedia/live/13476/54604/54604.pdf (accessed on 15 February 2014).
- Katchamart, W.; Narongroeknawin, P.; Chanapai, W.; Thaweeratthakul, P.; Srisomnuek, A. Prevalence of and factors associated with depression and anxiety in patients with rheumatoid arthritis: A multicenter prospective cross-sectional study. Int. J. Rheum. Dis. 2020, 23, 302–308. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- de Thurah, A.; Nørgaard, M.; Johansen, M.B.; Stengaard-Pedersen, K. Methotrexate compliance among patients with rheumatoid arthritis: The influence of disease activity, disease duration, and co-morbidity in a 10-year longitudinal study. Scand. J. Rheumatol. 2010, 39, 197–205. [Google Scholar] [CrossRef]
- Viller, F.; Guillemin, F.; Briançon, S.; Moum, T.; Suurmeijer, T.; van den Heuvel, W. Compliance to drug treatment of patients with rheumatoid arthritis: A 3 year longitudinal study. J. Rheumatol. 1999, 26, 2114–2122. [Google Scholar]
- Park, D.C.; Hertzog, C.; Leventhal, H.; Morrell, R.W.; Leventhal, E.; Birchmore, D.; Martin, M.; Bennett, J. Medication adherence in rheumatoid arthritis patients: Older is wiser. J. Am. Geriatr. Soc. 1999, 47, 172–183. [Google Scholar] [CrossRef]
- Yajima, N.; Kawaguchi, T.; Takahashi, R.; Nishiwaki, H.; Toyoshima, Y.; Oh, K.; Odai, T.; Kanai, T.; Morisky, D.E.; Yamaguchi, T.; et al. Adherence to methotrexate and associated factors considering social desirability in patients with rheumatoid arthritis: A multicenter cross-sectional study. BMC Rheumatol. 2022, 6, 75. [Google Scholar] [CrossRef]
- Hurkmans, E.J.; Maes, S.; de Gucht, V.; Knittle, K.; Peeters, A.J.; Ronday, H.K.; Vlieland, T.P. Motivation as a determinant of physical activity in patients with rheumatoid arthritis. Arthritis Care Res. 2010, 62, 371–377. [Google Scholar] [CrossRef]
- Borah, B.J.; Huang, X.; Zarotsky, V.; Globe, D. Trends in RA patients’ adherence to subcutaneous anti-TNF therapies and costs. Curr. Med. Res. Opin. 2009, 25, 1365–1377. [Google Scholar] [CrossRef]
- Berner, C.; Erlacher, L.; Fenzl, K.H.; Dorner, T.E. Medication Adherence and Coping Strategies in Patients with Rheumatoid Arthritis: A Cross-Sectional Study. Int. J. Rheumatol. 2019, 2019, 4709645. [Google Scholar] [CrossRef]
- Choudhry, N.K.; Fischer, M.A.; Avorn, J.; Liberman, J.N.; Schneeweiss, S.; Pakes, J.; Brennan, T.A.; Shrank, W.H. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch. Intern. Med. 2011, 171, 814–822. [Google Scholar] [CrossRef]
- Katchamart, W.; Narongroeknawin, P.; Sukprasert, N.; Chanapai, W.; Srisomnuek, A. Rate and causes of noncompliance with disease-modifying antirheumatic drug regimens in patients with rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 1291–1298. [Google Scholar] [CrossRef]
- Goh, H.; Kwan, Y.H.; Seah, Y.; Low, L.L.; Fong, W.; Thumboo, J. A systematic review of the barriers affecting medication adherence in patients with rheumatic diseases. Rheumatol. Int. 2017, 37, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Smolen, J.S. Effectiveness profiles and dose dependent retention of traditional disease modifying antirheumatic drugs for rheumatoid arthritis. An observational study. J. Rheumatol. 2002, 29, 1631–1638. [Google Scholar] [PubMed]
- Ideguchi, H.; Ohno, S.; Ishigatsubo, Y. Risk factors associated with the cumulative survival of low-dose methotrexate in 273 Japanese patients with rheumatoid arthritis. J. Clin. Rheumatol. 2007, 13, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Marroquín, R.; Contreras-Yáñez, I.; Alcocer-Castillejos, N.; Pascual-Ramos, V. Major depressive episodes are associated with poor concordance with therapy in rheumatoid arthritis patients: The impact on disease outcomes. Clin. Exp. Rheumatol. 2014, 32, 904–913. [Google Scholar]
- Hoekstra, M.; van de Laar, M.A.; Bernelot Moens, H.J.; Kruijsen, M.W.; Haagsma, C.J. Longterm observational study of methotrexate use in a Dutch cohort of 1022 patients with rheumatoid arthritis. J. Rheumatol. 2003, 30, 2325–2329. [Google Scholar]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef]
- Alrubaye Yasir, S.J.; Mohammed Al-Juboori, B.M.; Al-Humairi Ameer, K. The Causes of Non-Adherence to Methotrexate in Patients with Rheumatoid Arthritis. Res. J. Pharm. Technol. 2021, 14, 769–774. [Google Scholar] [CrossRef]
- Treharne, G.; Lyons, A.; Kitas, G. Medication adherence in Rheumatoid arthritis: Effects of psychosocial factors. Psychol. Health Med. 2004, 9, 337–349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).