Intramuscular Electrical Stimulation for the Treatment of Trigger Points in Patients with Chronic Migraine: A Protocol for a Pilot Study Using a Single-Case Experimental Design
Abstract
1. Introduction
2. Methods
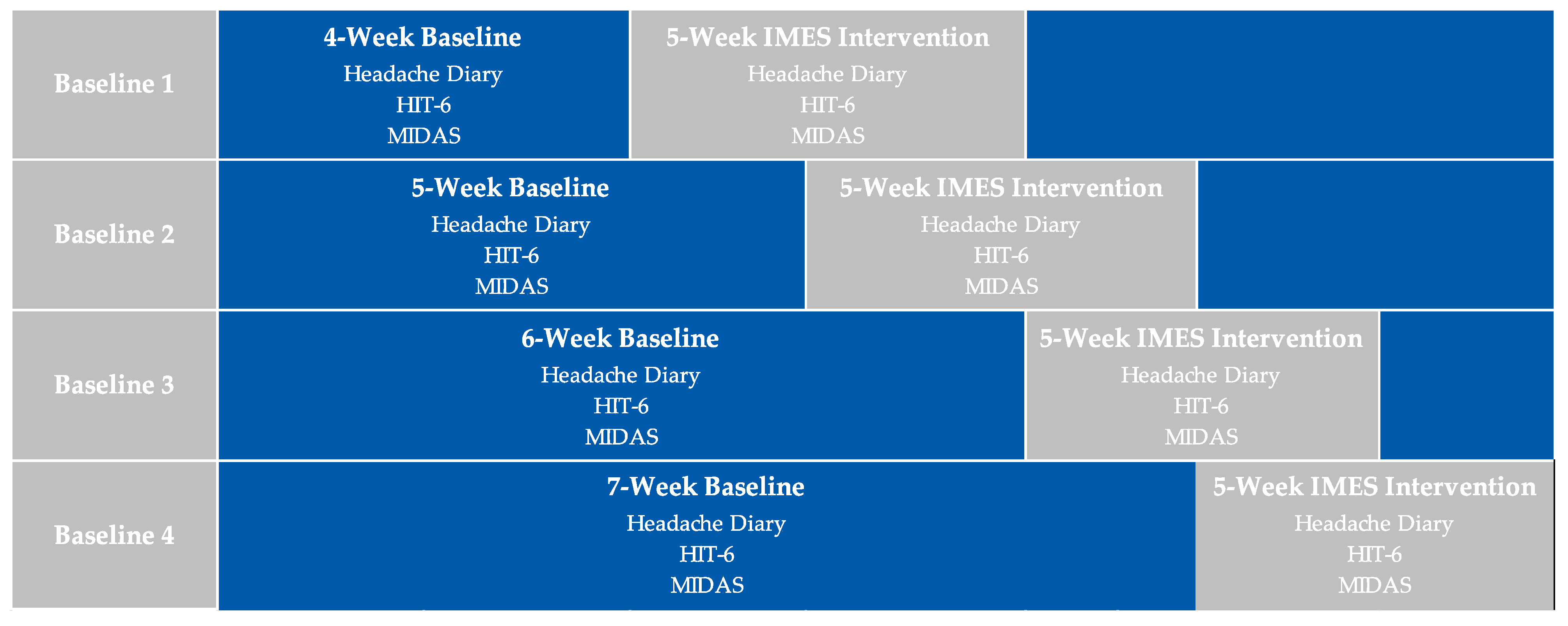
2.1. Study Design
2.2. Study Setting and Participants
2.3. Participant Selection
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Participant Evaluation
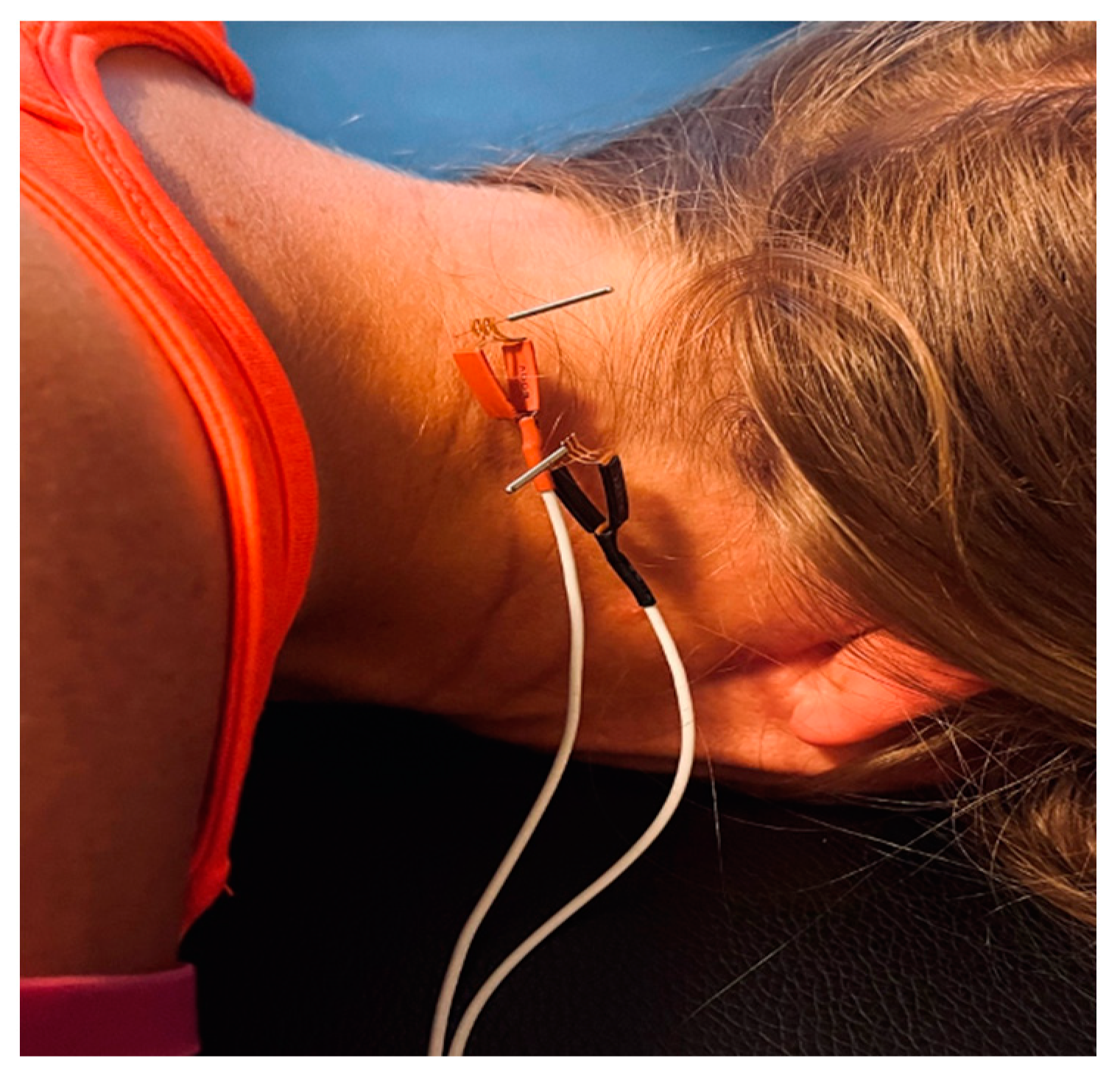
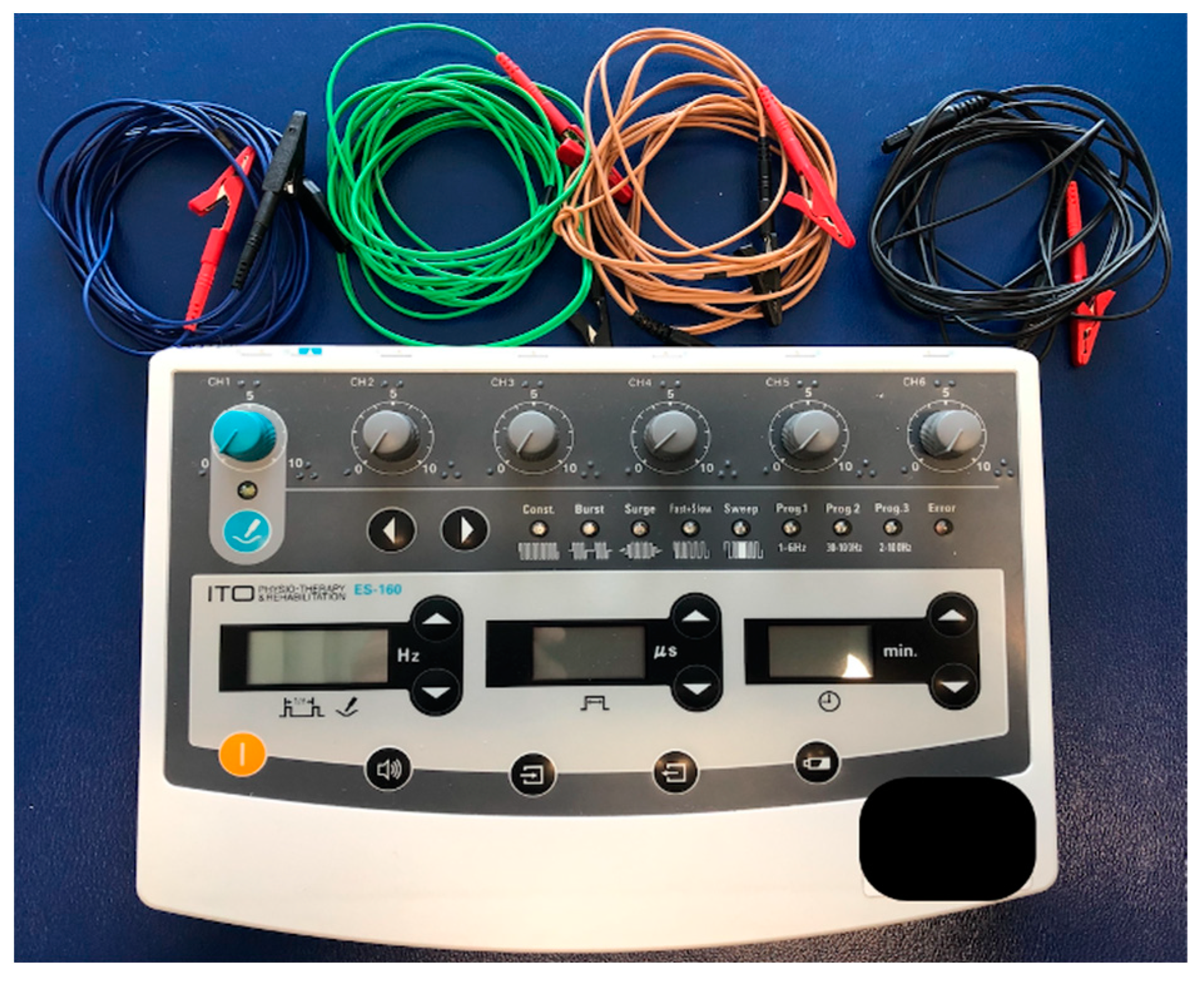
2.5. Interventions
2.6. Outcome Measures
2.6.1. Primary Outcome Measure
2.6.2. Secondary Outcome Measures
2.7. Data Analysis
2.7.1. Visual Analysis
2.7.2. Statistical Analysis
2.8. Blinding
2.9. Adverse Events and Monitoring
3. Discussion
3.1. Study Implications
3.2. Limitations
3.3. Potential Contributions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Global, regional, and national burden of migraine and tension-type headache, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [CrossRef]
- Steiner, T.J.; Stovner, L.J. Global epidemiology of migraine and its implications for public health and health policy. Nat. Rev. Neurol. 2023, 19, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Smitherman, T.A.; Burch, R.; Sheikh, H.; Loder, E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: A review of statistics from national surveillance studies. Headache 2013, 53, 427–436. [Google Scholar] [CrossRef]
- Katsarava, Z.; Buse, D.C.; Manack, A.N.; Lipton, R.B. Defining the differences between episodic migraine and chronic migraine. Curr. Pain Headache Rep. 2012, 16, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Natoli, J.L.; Manack, A.; Dean, B.; Butler, Q.; Turkel, C.C.; Stovner, L.; Lipton, R.B. Global prevalence of chronic migraine: A systematic review. Cephalalgia 2010, 30, 599–609. [Google Scholar] [CrossRef]
- Charles, A. The pathophysiology of migraine: Implications for clinical management. Lancet Neurol. 2018, 17, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazali, H.M.; Younis, S.; Al-Sayegh, Z.; Ashina, S.; Ashina, M.; Schytz, H.W. Prevalence of neck pain in migraine: A systematic review and meta-analysis. Cephalalgia 2022, 42, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Thomas, L.; Jull, G.; Treleaven, J. Cervical musculoskeletal impairments in migraine. Arch. Physiother. 2021, 11, 27. [Google Scholar] [CrossRef]
- Blaschek, A.; Decke, S.; Albers, L.; Schroeder, A.S.; Lehmann, S.; Straube, A.; Landgraf, M.N.; Heinen, F.; von Kries, R. Self-reported neck pain is associated with migraine but not with tension-type headache in adolescents. Cephalalgia 2014, 34, 895–903. [Google Scholar] [CrossRef]
- Bragatto, M.M.; Bevilaqua-Grossi, D.; Benatto, M.T.; Lodovichi, S.S.; Pinheiro, C.F.; Carvalho, G.F.; Dach, F.; Fernández-de-Las-Peñas, C.; Florencio, L.L. Is the presence of neck pain associated with more severe clinical presentation in patients with migraine? A cross-sectional study. Cephalalgia 2019, 39, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, K.; May, A. Stratifying migraine patients based on dynamic pain provocation over the upper cervical spine. J. Headache Pain 2017, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Do, T.P.; Heldarskard, G.F.; Kolding, L.T.; Hvedstrup, J.; Schytz, H.W. Myofascial trigger points in migraine and tension-type headache. J. Headache Pain 2018, 19, 84. [Google Scholar] [CrossRef]
- Di Antonio, S.; Arendt-Nielsen, L.; Ponzano, M.; Bovis, F.; Torelli, P.; Pelosin, E.; Finocchi, C.; Castaldo, M. Migraine patients with and without neck pain: Differences in clinical characteristics, sensitization, musculoskeletal impairments, and psychological burden. Musculoskelet. Sci. Pract. 2023, 102800. [Google Scholar] [CrossRef]
- Bron, C.; Dommerholt, J.D. Etiology of myofascial trigger points. Curr. Pain Headache Rep. 2012, 16, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Ge, H.Y.; Wang, Y.H.; Yue, S.W. A afferent fibers are involved in the pathology of central changes in the spinal dorsal horn associated with myofascial trigger spots in rats. Exp. Brain Res. 2015, 233, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Calandre, E.P.; Hidalgo, J.; García-Leiva, J.M.; Rico-Villademoros, F. Trigger point evaluation in migraine patients: An indication of peripheral sensitization linked to migraine predisposition? Eur. J. Neurol. 2006, 13, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Ceña, M.; Ferracini, G.N.; Florencio, L.L.; Ruíz, M.; Guerrero, Á.L.; Arendt-Nielsen, L.; Fernández-de-Las-Peñas, C. The Number of Active But Not Latent Trigger Points Associated with Widespread Pressure Pain Hypersensitivity in Women with Episodic Migraines. Pain Med. 2017, 18, 2485–2491. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Cuadrado, M.L.; Pareja, J.A. Myofascial trigger points, neck mobility and forward head posture in unilateral migraine. Cephalalgia 2006, 26, 1061–1070. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C. Myofascial Head Pain. Curr. Pain Headache Rep. 2015, 19, 28. [Google Scholar] [CrossRef]
- Rezaeian, T.; Mosallanezhad, Z.; Nourbakhsh, M.R.; Noroozi, M.; Sajedi, F. Effects of Dry Needling Technique Into Trigger Points of the Sternocleidomastoid Muscle in Migraine Headache: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2020, 99, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, M.A.; Tafuri, E.; Savini, A.; Fabrizio, A.; Affaitati, G.; Lerza, R.; Di Ianni, L.; Lapenna, D.; Mezzetti, A. Contribution of myofascial trigger points to migraine symptoms. J. Pain 2007, 8, 869–878. [Google Scholar] [CrossRef]
- Hesse, J.; Møgelvang, B.; Simonsen, H. Acupuncture versus metoprolol in migraine prophylaxis: A randomized trial of trigger point inactivation. J. Intern Med. 1994, 235, 451–456. [Google Scholar] [CrossRef]
- Pourahmadi, M.; Dommerholt, J.; Fernández-de-Las-Peñas, C.; Koes, B.W.; Mohseni-Bandpei, M.A.; Mansournia, M.A.; Delavari, S.; Keshtkar, A.; Bahramian, M. Dry Needling for the Treatment of Tension-Type, Cervicogenic, or Migraine Headaches: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab068. [Google Scholar] [CrossRef]
- Vázquez-Justes, D.; Yarzábal-Rodríguez, R.; Doménech-García, V.; Herrero, P.; Bellosta-López, P. Effectiveness of dry needling for headache: A systematic review. Neurologia 2020, 37, 806–815. [Google Scholar] [CrossRef]
- Hadizadeh, M.; Bashardoust Tajali, S.; Attarbashi Moghadam, B.; Jalaie Sh, B.M. Effects of Intramuscular Elec-trical Stimulation on Symptoms Following Trigger Points; A Controlled Pilot Study. J. Mod. Rehabil. 2017, 11, 31–36. [Google Scholar] [CrossRef]
- Perreault, T.; Ball, A.; Dommerholt, J.; Theiss, R.; Fernández-de-Las-Peñas, C.; Butts, R. Intramuscular Electrical Stimulation to Trigger Points: Insights into Mechanisms and Clinical Applications-A Scoping Review. J. Clin. Med. 2022, 11, 6039. [Google Scholar] [CrossRef]
- Niddam, D.M.; Chan, R.C.; Lee, S.H.; Yeh, T.C.; Hsieh, J.C. Central modulation of pain evoked from myofascial trigger point. Clin. J. Pain 2007, 23, 440–448. [Google Scholar] [CrossRef]
- Tanious, R.; Onghena, P. Randomized Single-Case Experimental Designs in Healthcare Research: What, Why, and How? Healthcare 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Nikles, J.; Evans, K.; Hams, A.; Sterling, M. A systematic review of N-of-1 trials and single case experimental designs in physiotherapy for musculoskeletal conditions. Musculoskelet Sci. Pract. 2022, 62, 102639. [Google Scholar] [CrossRef] [PubMed]
- Krasny-Pacini, A.; Evans, J. Single-case experimental designs to assess intervention effectiveness in rehabilitation: A practical guide. Ann. Phys. Rehabil. Med. 2018, 61, 164–179. [Google Scholar] [CrossRef]
- Lobo, M.A.; Moeyaert, M.; Baraldi Cunha, A.; Babik, I. Single-Case Design, Analysis, and Quality Assessment for Intervention Research. J. Neurol. Phys. Ther. 2017, 41, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, S.; Jongerling, J. Power of a randomization test in a single case multiple baseline AB design. PLoS ONE 2020, 15, e0228355. [Google Scholar] [CrossRef]
- Hovaguimian, A.; Roth, J. Management of chronic migraine. BMJ 2022, 379, e067670. [Google Scholar] [CrossRef]
- Tate, R.L.; Perdices, M.; Rosenkoetter, U.; Shadish, W.; Vohra, S.; Barlow, D.H.; Horner, R.; Kazdin, A.; Kratochwill, T.; McDonald, S.; et al. The Single-Case Reporting Guideline In BEhavioural Interventions (SCRIBE) 2016 Statement. Phys. Ther. 2016, 96, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Tassorelli, C.; Diener, H.C.; Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; Ashina, M.; Becker, W.J.; Ferrari, M.D.; Goadsby, P.J.; Pozo-Rosich, P.; et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia 2018, 38, 815–832. [Google Scholar] [CrossRef] [PubMed]
- Tanious, R.; Onghena, P. A systematic review of applied single-case research published between 2016 and 2018: Study designs, randomization, data aspects, and data analysis. Behav. Res. Methods 2021, 53, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Mohd Ali, K.; Ramli, A.; Rajadurai, S.; Mohan, V.; Justine, M.; Mohd Rasdi, H.F. Fear of needles does not influence pain tolerance and sympathetic responses among patients during a therapeutic needling. Pol. Ann. Med. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Dommerholt, J. International Consensus on Diagnostic Criteria and Clinical Considerations of Myofascial Trigger Points: A Delphi Study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef]
- Li, L.; Stoop, R.; Clijsen, R.; Hohenauer, E.; Fernández-de-Las-Peñas, C.; Huang, Q.; Barbero, M. Criteria Used for the Diagnosis of Myofascial Trigger Points in Clinical Trials on Physical Therapy: Updated Systematic Review. Clin. J. Pain 2020, 36, 955–967. [Google Scholar] [CrossRef]
- Szikszay, T.M.; Hoenick, S.; von Korn, K.; Meise, R.; Schwarz, A.; Starke, W.; Luedtke, K. Which Examination Tests Detect Differences in Cervical Musculoskeletal Impairments in People With Migraine? A Systematic Review and Meta-Analysis. Phys. Ther. 2019, 99, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Castien, R.F.; Coppieters, M.W.; Durge, T.S.C.; Scholten-Peeters, G.G.M. High concurrent validity between digital and analogue algometers to measure pressure pain thresholds in healthy participants and people with migraine: A cross-sectional study. J. Headache Pain 2021, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Nahman-Averbuch, H.; Shefi, T.; Schneider, V.J., 2nd; Li, D.; Ding, L.; King, C.D.; Coghill, R.C. Quantitative sensory testing in patients with migraine: A systematic review and meta-analysis. Pain 2018, 159, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Florencio, L.L.; Giantomassi, M.C.; Carvalho, G.F.; Gonçalves, M.C.; Dach, F.; Fernández-de-Las-Peñas, C.; Bevilaqua-Grossi, D. Generalized Pressure Pain Hypersensitivity in the Cervical Muscles in Women with Migraine. Pain Med. 2015, 16, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Florencio, L.L.; Martins, J.; Bragatto, M.M.; Fernández-de-Las-Penãs, C.; Dach, F.; Bevilaqua-Grossi, D. Craniocervical flexion test in patients with migraine: Discriminative validity and accuracy. Int. J. Clin. Pract. 2021, 75, e14248. [Google Scholar] [CrossRef] [PubMed]
- Anarte-Lazo, E.; Carvalho, G.F.; Schwarz, A.; Luedtke, K.; Falla, D. Differentiating migraine, cervicogenic headache and asymptomatic individuals based on physical examination findings: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2021, 22, 755. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.N.; Mohseni Bandpei, M.A.; Ali, M.; Khan, G.A. Reliability of the universal goniometer for assessing active cervical range of motion in asymptomatic healthy persons. Pak. J. Med. Sci. 2016, 32, 457–461. [Google Scholar] [CrossRef]
- Williams, M.A.; McCarthy, C.J.; Chorti, A.; Cooke, M.W.; Gates, S. A systematic review of reliability and validity studies of methods for measuring active and passive cervical range of motion. J. Manip. Physiol. Ther. 2010, 33, 138–155. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Downey, C.; Miangolarra-Page, J.C. Validity of the lateral gliding test as tool for the diagnosis of intervertebral joint dysfunction in the lower cervical spine. J. Manip. Physiol. Ther. 2005, 28, 610–616. [Google Scholar] [CrossRef]
- Bakhtadze, M.A.; Patijn, J.; Galaguza, V.N.; Bolotov, D.A.; Popov, A.A. Inter-examiner reproducibility of the segmental motion palpation springing test for side bending at level C2–C3. Int. Musculoskelet. Med. 2011, 33, 8–14. [Google Scholar] [CrossRef]
- Hariharan, K.V.; Timko, M.G.; Bise, C.G.; Sundaram, M.; Schneider, M.J. Inter-examinerreliability study of physical examination procedures to assess the cervical spine. Chiropr. Man. Therap. 2021, 29, 20. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Souza, A.I.S.; Florencio, L.L.; Carvalho, G.F.; Fernández-De-Las-Peñas, C.; Dach, F.; Bevilaqua-Grossi, D. Reduced flexion rotation test in women with chronic and episodic migraine. Braz. J. Phys. Ther. 2019, 23, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Harry Von, P.; Maloul, R.; Hoffmann, M.; Hall, T.; Ruch, M.M.; Ballenberger, N. Diagnostic accuracy and validity of three manual examination tests to identify alar ligament lesions: Results of a blinded case-control study. J. Man. Manip. Ther. 2019, 27, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Hutting, N.; Scholten-Peeters, G.G.; Vijverman, V.; Keesenberg, M.D.; Verhagen, A.P. Diagnostic accuracy of upper cervical spine instability tests: A systematic review. Phys. Ther. 2013, 93, 1686–1695. [Google Scholar] [CrossRef]
- Osmotherly, P.G.; Rivett, D.A.; Rowe, L.J. The anterior shear and distraction tests for craniocervical instability. An evaluation using magnetic resonance imaging. Man. Ther. 2012, 17, 416–421. [Google Scholar] [CrossRef]
- Rupp, R.; Biering-Sørensen, F.; Burns, S.P.; Graves, D.E.; Guest, J.; Jones, L.; Read, M.S.; Rodriguez, G.M.; Schuld, C.; Tansey-Md, K.E.; et al. International Standards for Neurological Classification of Spinal Cord Injury: Revised 2019. Top Spinal Cord Inj. Rehabil. 2021, 27, 1–22. [Google Scholar] [CrossRef]
- Franz, S.; Kirshblum, S.C.; Weidner, N.; Rupp, R.; Schuld, C. Motor levels in high cervical spinal cord injuries: Implications for the International Standards for Neurological Classification of Spinal Cord Injury. J. Spinal. Cord. Med. 2016, 39, 513–517. [Google Scholar] [CrossRef]
- Perreault, T.; Dunning, J.; Butts, R. The local twitch response during trigger point dry needling: Is it necessary for successful outcomes? J. Bodyw. Mov. Ther. 2017, 21, 940–947. [Google Scholar] [CrossRef]
- Hong, C.Z. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am. J. Phys. Med. Rehabil. 1994, 73, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.A.; Hooper, T.L.; Brismée, J.M.; Allen, B.; Lierly, M.; Gilbert, K.K.; Pendergrass, T.J.; Edwards, D. Influence of clinical experience on accuracy and safety of obliquus capitus inferior dry needling in unembalmed cadavers. Physiother. Theory Pract. 2022, 38, 2052–2061. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Mesa-Jiménez, J.A.; Paredes-Mancilla, J.A.; Koppenhaver, S.L.; Fernández-Carnero, S. Cadaveric and Ultrasonographic Validation of Needling Placement in the Cervical Multifidus Muscle. J. Manip. Physiol. Ther. 2017, 40, 365–370. [Google Scholar] [CrossRef]
- Chao, L.; Gonçalves, A.S.; Campos, A.C.P.; Assis, D.V.; Jerônimo, R.; Kuroki, M.A.; Sant’Anna, F.M.; Meas, Y.; Rouxeville, Y.; Hsing, W.; et al. Comparative effect of dense-and-disperse versus non-repetitive and non-sequential frequencies in electroacupuncture-induced analgesia in a rodent model of peripheral neuropathic pain. Acupunct. Med. 2022, 40, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Han, J.S. All three types of opioid receptors in the spinal cord are important for 2/15 Hz electroacupuncture analgesia. Eur. J. Pharmacol. 1992, 211, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Han, J.S. Analgesia induced by electroacupuncture of different frequencies is mediated by different types of opioid receptors: Another cross-tolerance study. Behav. Brain Res. 1992, 47, 143–149. [Google Scholar] [CrossRef]
- Baeumler, P.I.; Fleckenstein, J.; Benedikt, F.; Bader, J.; Irnich, D. Acupuncture-induced changes of pressure pain threshold are mediated by segmental inhibition—A randomized controlled trial. Pain 2015, 156, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M. Safety aspects of electroacupuncture. Acupunct. Med. 2011, 29, 83–85. [Google Scholar] [CrossRef]
- Panizza, M.; Nilsson, J.; Roth, B.J.; Basser, P.J.; Hallett, M. Relevance of stimulus duration for activation of motor and sensory fibers: Implications for the study of H-reflexes and magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Romita, V.V.; Suk, A.; Henry, J.L. Parametric studies on electroacupuncture-like stimulation in a rat model: Effects of intensity, frequency, and duration of stimulation on evoked antinociception. Brain Res. Bull. 1997, 42, 289–296. [Google Scholar] [CrossRef]
- Ahmed, S.; Haddad, C.; Subramaniam, S.; Khattab, S.; Kumbhare, D. The Effect of Electric Stimulation Techniques on Pain and Tenderness at the Myofascial Trigger Point: A Systematic Review. Pain Med. 2019, 20, 1774–1788. [Google Scholar] [CrossRef]
- Plaza-Manzano, G.; Gómez-Chiguano, G.F.; Cleland, J.A.; Arías-Buría, J.L.; Fernández-de-Las-Peñas, C.; Navarro-Santana, M.J. Effectiveness of percutaneous electrical nerve stimulation for musculoskeletal pain: A systematic review and meta-analysis. Eur. J. Pain 2020, 24, 1023–1044. [Google Scholar] [CrossRef]
- Luedtke, K.; Basener, A.; Bedei, S.; Castien, R.; Chaibi, A.; Falla, D.; Fernández-de-Las-Peñas, C.; Gustafsson, M.; Hall, T.; Jull, G.; et al. Outcome measures for assessing the effectiveness of non-pharmacological interventions in frequent episodic or chronic migraine: A Delphi study. BMJ Open 2020, 10, e029855. [Google Scholar] [CrossRef]
- Vo, P.; Paris, N.; Bilitou, A.; Valena, T.; Fang, J.; Naujoks, C.; Cameron, A.; de Reydet de Vulpillieres, F.; Cadiou, F. Burden of Migraine in Europe Using Self-Reported Digital Diary Data from the Migraine Buddy© Application. Neurol. Ther. 2018, 7, 321–332. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Constantin, L.; Ebel-Bitoun, C.; Igracki Turudic, I.; Hitier, S.; Amand-Bourdon, C.; Stewart, A. Multinational descriptive analysis of the real-world burden of headache using the Migraine Buddy application. Eur. J. Neurol. 2021, 28, 4184–4193. [Google Scholar] [CrossRef]
- Smelt, A.F.; Assendelft, W.J.; Terwee, C.B.; Ferrari, M.D.; Blom, J.W. What is a clinically relevant change on the HIT-6 questionnaire? An estimation in a primary-care population of migraine patients. Cephalalgia 2014, 34, 29–36. [Google Scholar] [CrossRef]
- Bigal, M.E.; Rapoport, A.M.; Lipton, R.B.; Tepper, S.J.; Sheftell, F.D. Assessment of migraine disability using the migraine disability assessment (MIDAS) questionnaire: A comparison of chronic migraine with episodic migraine. Headache 2003, 43, 336–342. [Google Scholar] [CrossRef]
- Castien, R.F.; van der Wouden, J.C.; De Hertogh, W. Pressure pain thresholds over the cranio-cervical region in headache: A systematic review and meta-analysis. J. Headache Pain 2018, 19, 9. [Google Scholar] [CrossRef]
- Kwong, W.J.; Pathak, D.S. Validation of the eleven-point pain scale in the measurement of migraine headache pain. Cephalalgia 2007, 27, 336–342. [Google Scholar] [CrossRef]
- Lane, J.D.; Gast, D.L. Visual analysis in single case experimental design studies: Brief review and guidelines. Neuropsychol. Rehabil. 2014, 24, 445–463. [Google Scholar] [CrossRef]
- Manolov, R.; Moeyaert, M.; Fingerhut, J.E. A Priori Justification for Effect Measures in Single-Case Experimental Designs. Perspect. Behav. Sci. 2022, 45, 153–186. [Google Scholar] [CrossRef]
- Parker, R.I.; Vannest, K. An improved effect size for single-case research: Nonoverlap of all pairs. Behav. Ther. 2009, 40, 357–367. [Google Scholar] [CrossRef] [PubMed]
- DeHart, W.B.; Kaplan, B.A. Applying mixed-effects modeling to single-subject designs: An introduction. J. Exp. Anal. Behav. 2019, 111, 192–206. [Google Scholar] [CrossRef]
- Brady, S.; McEvoy, J.; Dommerholt, J.; Doody, C. Adverse events following trigger point dry needling: A prospective survey of chartered physiotherapists. J. Man. Manip. Ther. 2014, 22, 134–140. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.; Jo, D.J. An acute cervical epidural hematoma as a complication of dry needling. Spine 2011, 36, E891–E893. [Google Scholar] [CrossRef]
- Berrigan, W.A.; Whitehair, C.L.; Zorowitz, R.D. Acute Spinal Epidural Hematoma as a Complication of Dry Needling: A Case Report. PMR 2019, 11, 313–316. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Nijs, J.; Cagnie, B.; Gerwin, R.D.; Plaza-Manzano, G.; Valera-Calero, J.A.; Arendt-Nielsen, L. Myofascial Pain Syndrome: A Nociceptive Condition Comorbid with Neuropathic or Nociplastic Pain. Life 2023, 13, 694. [Google Scholar] [CrossRef]
- Bartsch, T.; Goadsby, P.J. Central mechanisms of peripheral nerve stimulation in headache disorders. Prog. Neurol. Surg. 2011, 24, 16–26. [Google Scholar] [CrossRef]
- Scherer, S.S.; Schiraldi, L.; Sapino, G.; Cambiaso-Daniel, J.; Gualdi, A.; Peled, Z.M.; Hagan, R.; Pietramaggiori, G. The Greater Occipital Nerve and Obliquus Capitis Inferior Muscle: Anatomical Interactions and Implications for Occipital Pain Syndromes. Plast. Reconstr. Surg. 2019, 144, 730–736. [Google Scholar] [CrossRef]
- Braithwaite, F.A.; Walters, J.L.; Li, L.S.K.; Moseley, G.L.; Williams, M.T.; McEvoy, M.P. Effectiveness and adequacy of blinding in the moderation of pain outcomes: Systematic review and meta-analyses of dry needling trials. PeerJ 2018, 6, e5318. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, F.A.; Walters, J.L.; Moseley, G.L.; Williams, M.T.; McEvoy, M.P. Towards more homogenous and rigorous methods in sham-controlled dry needling trials: Two Delphi surveys. Physiotherapy 2020, 106, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Souza, A.I.S.; Carvalho, G.F.; Florêncio, L.L.; Fernández-de-Las-Peñas, C.; Dach, F.; Bevilaqua-Grossi, D. Intrarater and Interrater Reliability of the Flexion Rotation Test and Cervical Range of Motion in People With Migraine. J. Manip. Physiol. Ther. 2020, 43, 874–881. [Google Scholar] [CrossRef]
- Hepp, Z.; Dodick, D.W.; Varon, S.F.; Gillard, P.; Hansen, R.N.; Devine, E.B. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia 2015, 35, 478–488. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perreault, T.; Arendt-Nielson, L.; Fernández-de-las-Peñas, C.; Dommerholt, J.; Herrero, P.; Hubbard, R. Intramuscular Electrical Stimulation for the Treatment of Trigger Points in Patients with Chronic Migraine: A Protocol for a Pilot Study Using a Single-Case Experimental Design. Medicina 2023, 59, 1380. https://doi.org/10.3390/medicina59081380
Perreault T, Arendt-Nielson L, Fernández-de-las-Peñas C, Dommerholt J, Herrero P, Hubbard R. Intramuscular Electrical Stimulation for the Treatment of Trigger Points in Patients with Chronic Migraine: A Protocol for a Pilot Study Using a Single-Case Experimental Design. Medicina. 2023; 59(8):1380. https://doi.org/10.3390/medicina59081380
Chicago/Turabian StylePerreault, Thomas, Lars Arendt-Nielson, César Fernández-de-las-Peñas, Jan Dommerholt, Pablo Herrero, and Ryan Hubbard. 2023. "Intramuscular Electrical Stimulation for the Treatment of Trigger Points in Patients with Chronic Migraine: A Protocol for a Pilot Study Using a Single-Case Experimental Design" Medicina 59, no. 8: 1380. https://doi.org/10.3390/medicina59081380
APA StylePerreault, T., Arendt-Nielson, L., Fernández-de-las-Peñas, C., Dommerholt, J., Herrero, P., & Hubbard, R. (2023). Intramuscular Electrical Stimulation for the Treatment of Trigger Points in Patients with Chronic Migraine: A Protocol for a Pilot Study Using a Single-Case Experimental Design. Medicina, 59(8), 1380. https://doi.org/10.3390/medicina59081380






