Using Hormone Data and Age to Pinpoint Cycle Day within the Menstrual Cycle
Abstract
1. Introduction
2. Materials and Methods
2.1. Hormone Monitoring
2.2. Study Design
2.3. Defining Ovulation and Cycle Length
2.4. Statistical Analysis
3. Results
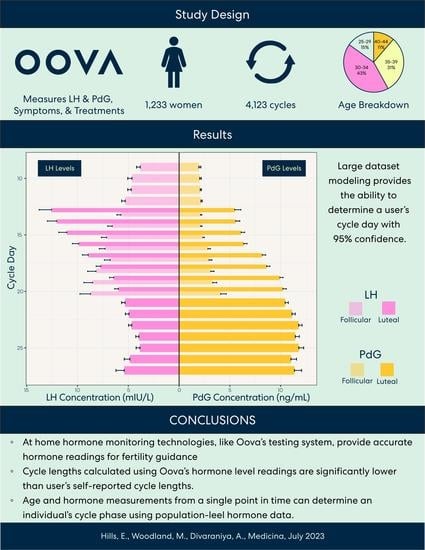
3.1. Result 1: Patient Demographic
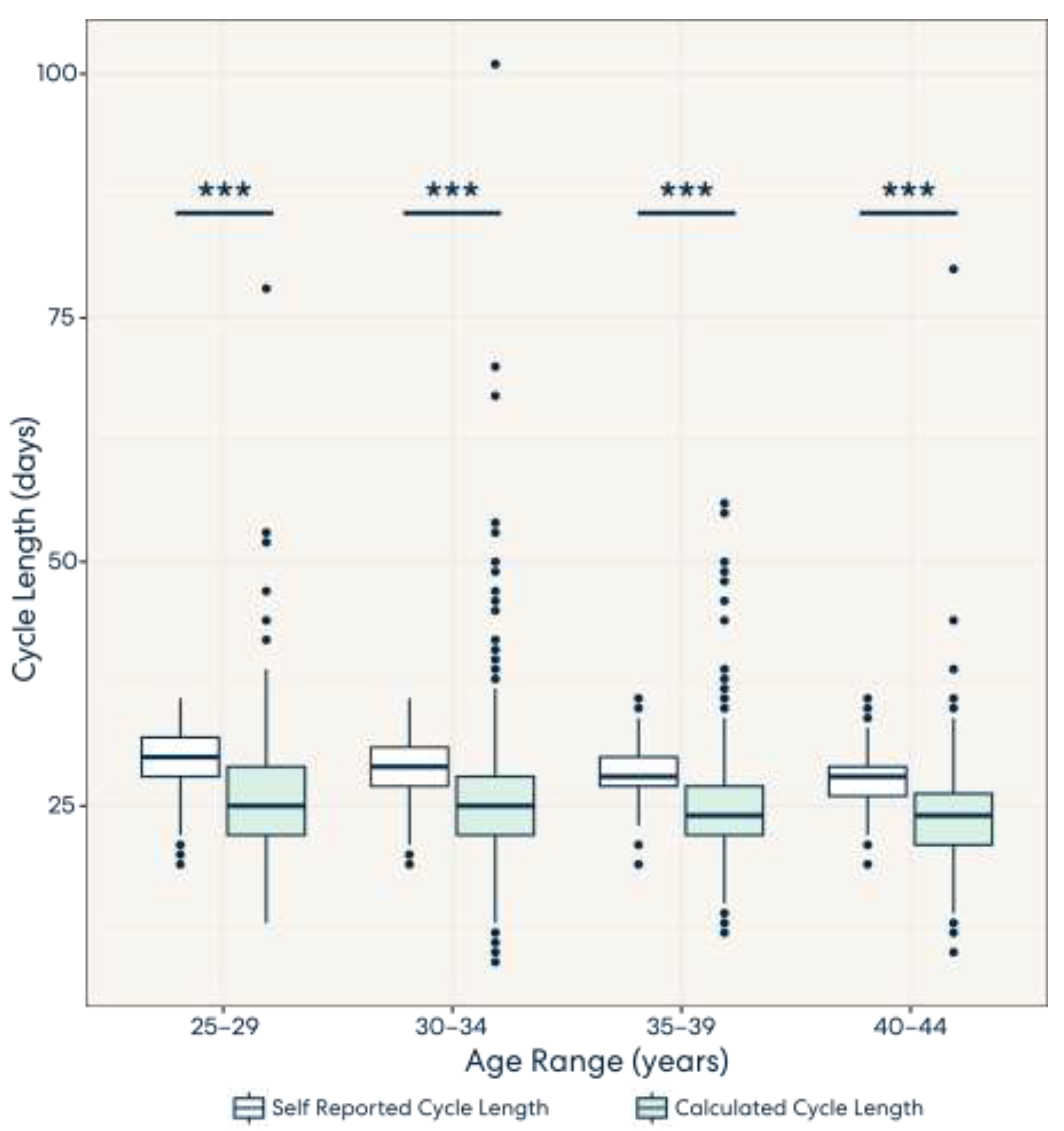
3.2. Result 2: Calculated Cycle Lengths Tend to Be Shorter Than Self-Reported Cycle Lengths
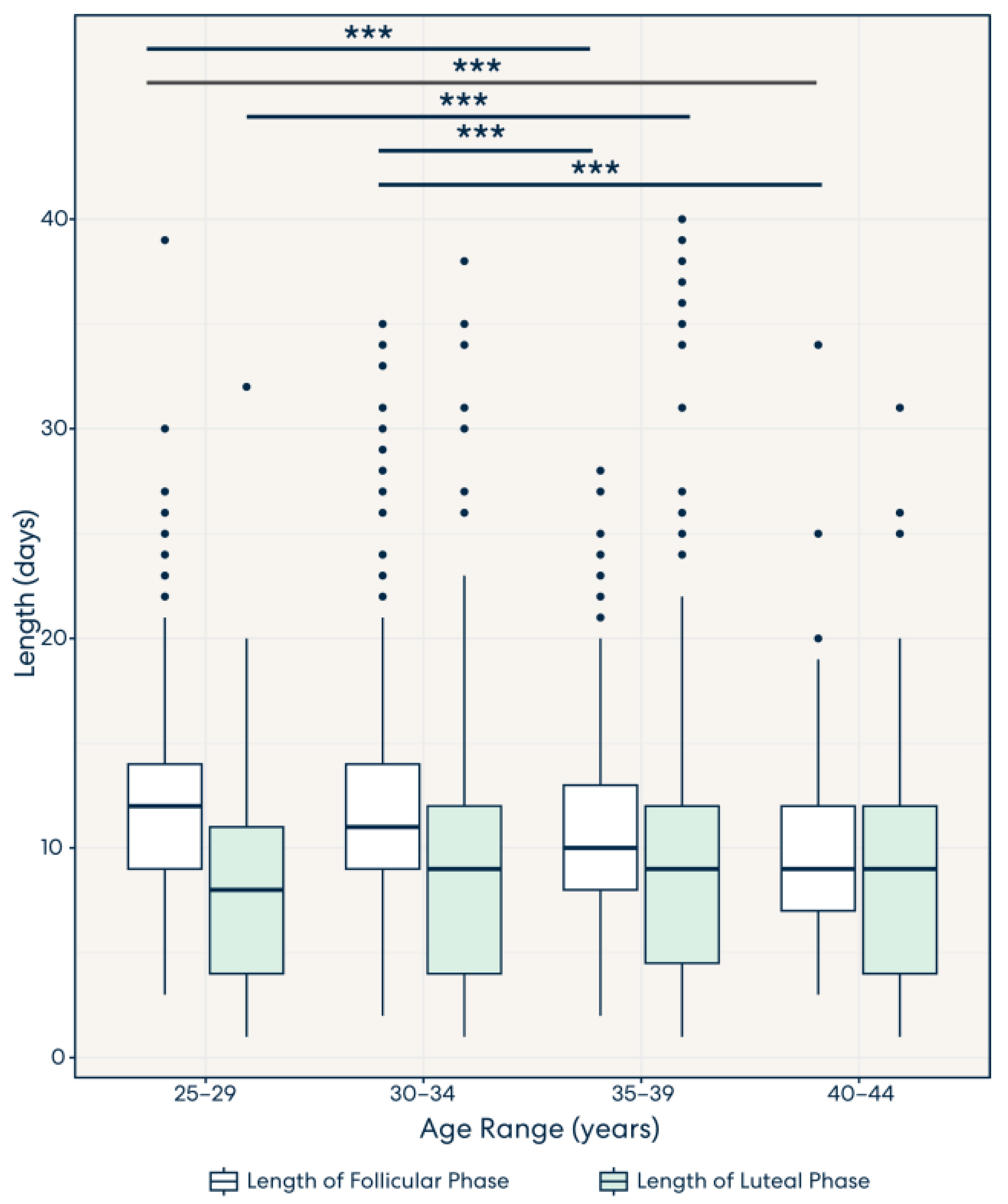
3.3. Result 3: Lengths of the Follicular Phase and Luteal Phase Decrease and Increase, Respectively, across Age Groups
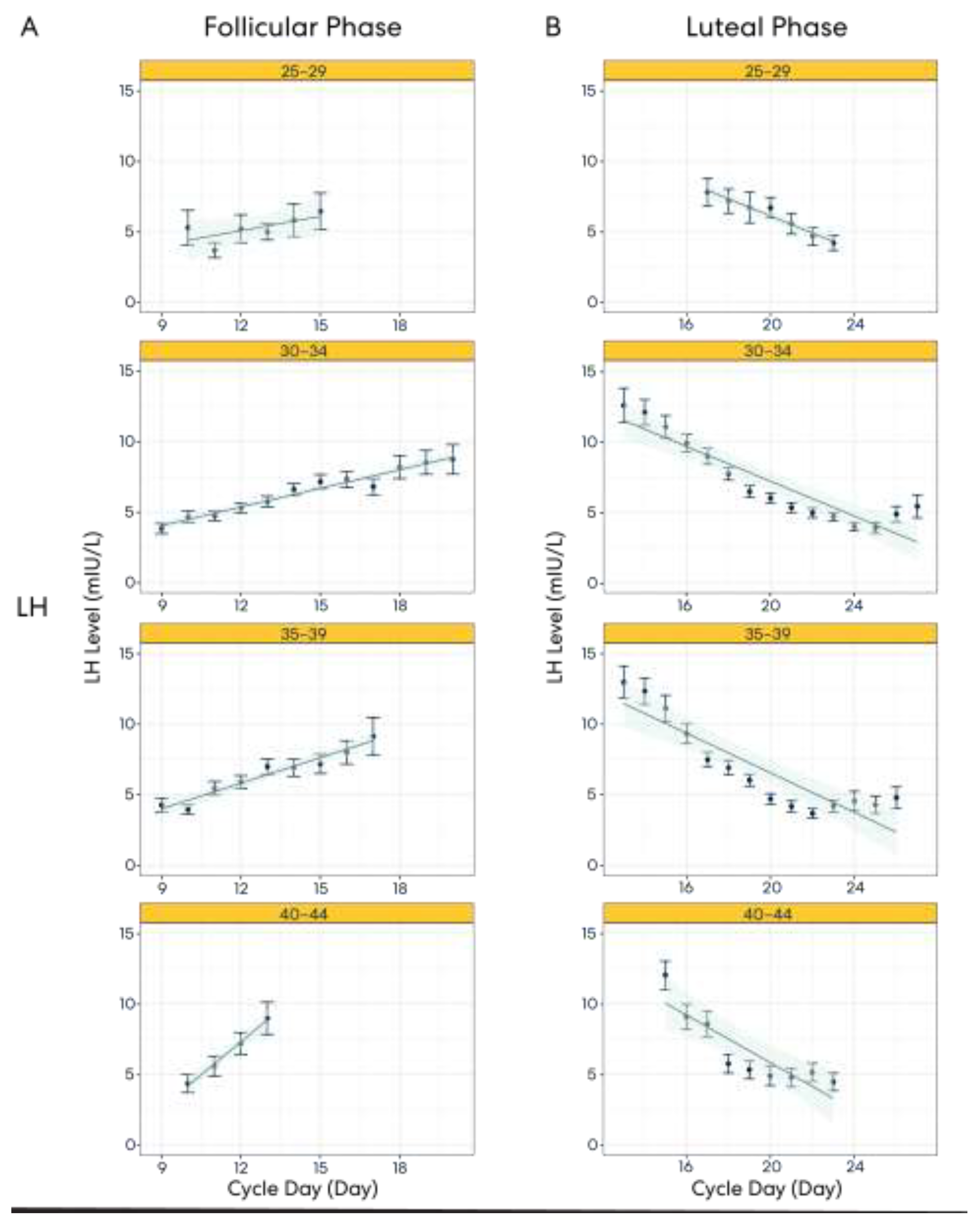
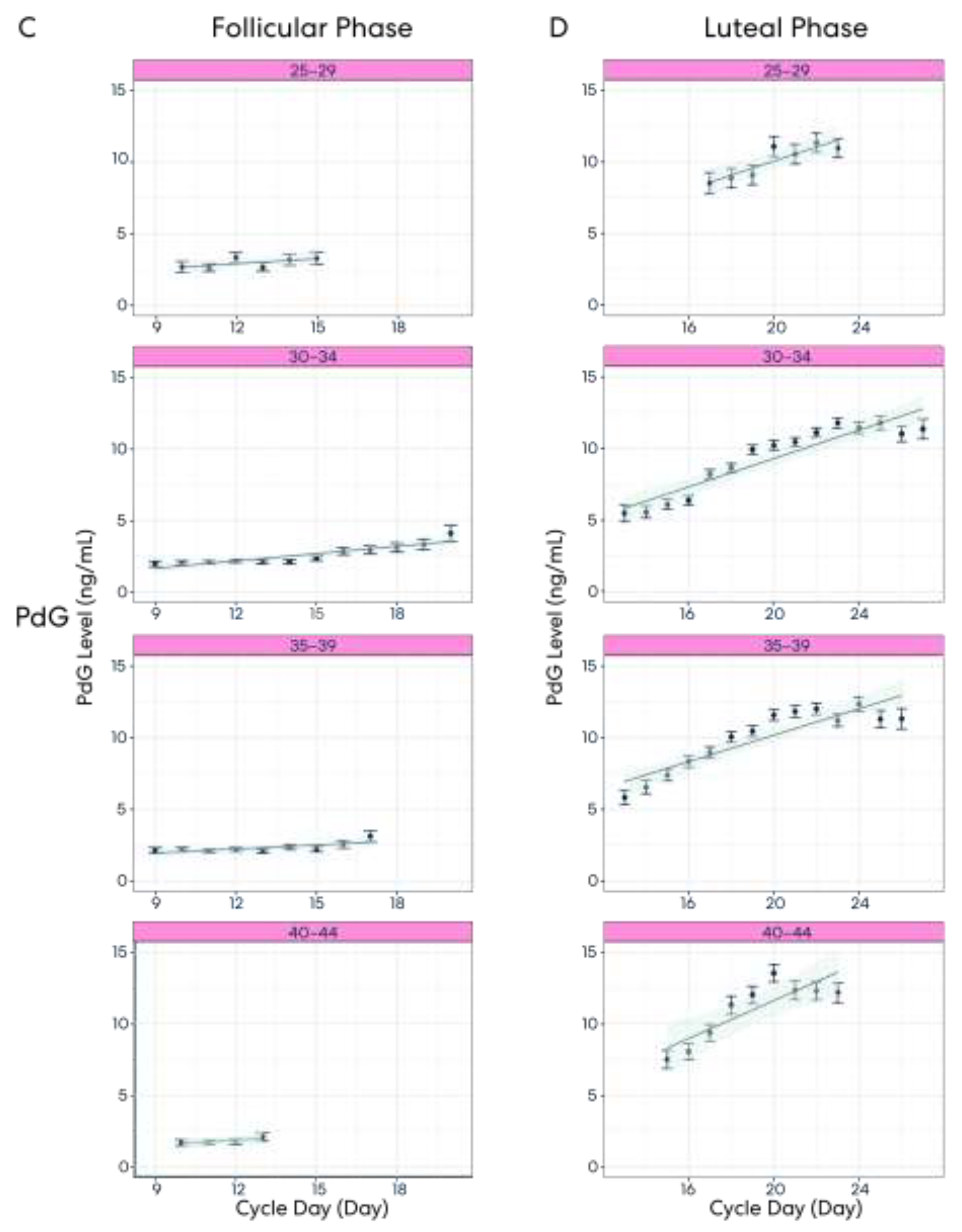
3.4. Result 4: Hormone Pattern Variability in the Follicular and Luteal Phases
3.5. Result 5: Hormone Patterns Enable Identification of the User’s Particular Cycle Day Based on Age
4. Discussion
4.1. Implications of Findings beyond Tracking Fertility
4.2. Further Directions
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawkins, S.M.; Matzuk, M.M. The Menstrual Cycle. Ann. N. Y. Acad. Sci. 2008, 1135, 10–18. [Google Scholar] [CrossRef]
- Thiyagarajan, D.K.; Basit, H.; Jeanmonod, R. Physiology, Menstrual Cycle; StatPearls: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Reed, B.G.; Carr, B.R. The Normal Menstrual Cycle and the Control of Ovulation; Endotext: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Shechter, A.; Varin, F.; Boivin, D.B. Circadian Variation of Sleep During the Follicular and Luteal Phases of the Menstrual Cycle. Sleep 2010, 33, 647–656. [Google Scholar] [CrossRef]
- Boivin, D.B.; Shechter, A. Sleep, Hormones, and Circadian Rhythms throughout the Menstrual Cycle in Healthy Women and Women with Premenstrual Dysphoric Disorder. Int. J. Endocrinol. 2010, 2010, 259345. [Google Scholar] [CrossRef]
- Kiesner, J. One woman’s low is another woman’s high: Paradoxical effects of the menstrual cycle. Psychoneuroendocrinology 2011, 36, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Biggs, W.; Demuth, R. Premenstrual syndrome and premenstrual dysphoric disorder. Am. Fam. Phys. 2011, 84, 918–924. Available online: https://pubmed.ncbi.nlm.nih.gov/22010771/ (accessed on 9 April 2023).
- Draper, C.F.; Duisters, K.; Weger, B.; Chakrabarti, A.; Harms, A.C.; Brennan, L.; Hankemeier, T.; Goulet, L.; Konz, T.; Martin, F.P.; et al. Menstrual cycle rhythmicity: Metabolic patterns in healthy women. Sci. Rep. 2018, 8, 14568. [Google Scholar] [CrossRef]
- Calloway, D.H.; Kurzer, M.S. Menstrual Cycle and Protein Requirements of Women. J. Nutr. 1982, 112, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, A.M.; Sjaarda, L.A.; Mitchell, E.M.; Perkins, N.J.; Schliep, K.C.; Wactawski-Wende, J.; Mumford, S.L. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur. J. Nutr. 2016, 55, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Handy, A.B.; Greenfield, S.F.; Yonkers, K.A.; Payne, L.A. Psychiatric Symptoms Across the Menstrual Cycle in Adult Women: A Comprehensive Review. Harv. Rev. Psychiatry 2022, 30, 100. [Google Scholar] [CrossRef]
- Statham, G. Understanding the effects of the menstrual cycle on training and performance in elite athletes: A preliminary study. Prog. Brain Res. 2020, 253, 25–58. [Google Scholar] [CrossRef]
- Carmichael, M.A.; Thomson, R.L.; Moran, L.J.; Wycherley, T.P. The Impact of Menstrual Cycle Phase on Athletes’ Performance: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 1667. [Google Scholar] [CrossRef]
- Grieger, J.A.; Norman, R.J. Menstrual Cycle Length and Patterns in a Global Cohort of Women Using a Mobile Phone App: Retrospective Cohort Study. J. Med. Internet Res. 2020, 22, e17109. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: A committee opinion. Fertil. Steril. 2015, 103, e44–e50. [Google Scholar] [CrossRef]
- Schmalenberger, K.M.; Tauseef, H.A.; Barone, J.C.; Owens, S.A.; Lieberman, L.; Jarczok, M.N.; Girdler, S.S.; Kiesner, J.; Ditzen, B.; Eisenlohr-Moul, T.A. How to study the menstrual cycle: Practical tools and recommendations. Psychoneuroendocrinology 2021, 123, 104895. [Google Scholar] [CrossRef] [PubMed]
- Direito, A.; Bailly, S.; Mariani, A.; Ecochard, R. Relationships between the luteinizing hormone surge and other characteristics of the menstrual cycle in normally ovulating women. Fertil. Steril. 2013, 99, 279–285.e3. [Google Scholar] [CrossRef] [PubMed]
- Zinaman, M.; Johnson, S.; Ellis, J.; Ledger, W. Accuracy of perception of ovulation day in women trying to conceive. Curr. Med. Res. Opin. 2012, 28, 749–754. [Google Scholar] [CrossRef]
- Worsfold, L.; Marriott, L.; Johnson, S.; Harper, J.C. Period tracker applications: What menstrual cycle information are they giving women? Women’s Health 2021, 17, 17455065211049905. [Google Scholar] [CrossRef]
- Bull, J.R.; Rowland, S.P.; Scherwitzl, E.B.; Scherwitzl, R.; Danielsson, K.G.; Harper, J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit. Med. 2019, 2, 83. [Google Scholar] [CrossRef]
- Fehring, R.J.; Schneider, M.; Raviele, K. Variability in the phases of the menstrual cycle. JOGNN 2006, 35, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, L.; Cooke, D.; Brown, S. Self-Monitoring of Fertility Hormones. Linacre Q. 2018, 85, 26–34. [Google Scholar] [CrossRef]
- Broad, A.; Biswakarma, R.; Harper, J.C. A survey of women’s experiences of using period tracker applications: Attitudes, ovulation prediction and how the accuracy of the app in predicting period start dates affects their feelings and behaviours. Women’s Health 2022, 18, 17455057221095246. [Google Scholar] [CrossRef] [PubMed]
- Ecochard, R.; Leiva, R.; Bouchard, T.; Boehringer, H.; Direito, A.; Mariani, A.; Fehring, R. Use of urinary pregnanediol 3-glucuronide to confirm ovulation. Steroids 2013, 78, 1035–1040. [Google Scholar] [CrossRef]
- Su, H.; Yi, Y.; Wei, T.; Chang, T.; Cheng, C. Detection of ovulation, a review of currently available methods. Bioeng. Transl. Med. 2017, 2, 238. [Google Scholar] [CrossRef]
- Soumpasis, I.; Grace, B.; Johnson, S. Real-life insights on menstrual cycles and ovulation using big data. Hum. Reprod. Open 2020, 2020, hoaa011. [Google Scholar] [CrossRef] [PubMed]
- Mesen, T.B.; Young, S.L. Progesterone and the Luteal Phase: A Requisite to Reproduction. Obstet. Gynecol. Clin. North. Am. 2015, 42, 135. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.; Hero, M.; Alfthan, H.; Passioni, A.; Tapanainen, J.S.; Stenman, U.H. Identification of the LH surge by measuring intact and total immunoreactivity in urine for prediction of ovulation time. Hormones 2022, 21, 413. [Google Scholar] [CrossRef]
- Pattnaik, S.; Das, D.; Venkatesan, V.A. Validation of urinary reproductive hormone measurements using a novel smartphone connected reader. Sci. Rep. 2023, 13, 9227. [Google Scholar] [CrossRef] [PubMed]
- Dozortsev, D.I.; Diamond, M.P. Luteinizing hormone–independent rise of progesterone as the physiological trigger of the ovulatory gonadotropins surge in the human. Fertil. Steril. 2020, 114, 191–199. [Google Scholar] [CrossRef]
- Klein, N.A.; Harper, A.J.; Houmard, B.S.; Sluss, P.M.; Soules, M.R. Is the Short Follicular Phase in Older Women Secondary to Advanced or Accelerated Dominant Follicle Development? J. Clin. Endocrinol. Metab. 2002, 87, 5746–5750. [Google Scholar] [CrossRef] [PubMed]
- Lenton, E.A.; De Kretser, D.M.; Woodward, A.J.; Robertson, D.M. Inhibin concentrations throughout the menstrual cycles of normal, infertile, and older women compared with those during spontaneous conception cycles. J. Clin. Endocrinol. Metab. 1991, 73, 1180–1190. [Google Scholar] [CrossRef]
- Klein, N.A.; Battaglia, D.E.; Fujimoto, V.Y.; Davis, G.S.; Bremner, W.J.; Soules, M.R. Reproductive aging: Accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J. Clin. Endocrinol. Metab. 1996, 81, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Steiner, A.Z.; Pollack, A.Z.; Perkins, N.J.; Filiberto, A.C.; Albert, P.S.; Mattison, D.R.; Wactawski-Wende, J.; Schisterman, E.F. The Utility of Menstrual Cycle Length as an Indicator of Cumulative Hormonal Exposure. J. Clin. Endocrinol. Metab. 2012, 97, E1871. [Google Scholar] [CrossRef] [PubMed]
- Vigil, P.; Lyon, C.; Flores, B.; Rioseco, H.; Serrano, F. Ovulation, A Sign of Health. Linacre Q. 2017, 84, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.A.; Vitzthum, V.J. The extent and causes of natural variation in menstrual cycles: Integrating empirically-based models of ovarian cycling into research on women’s health. Drug Discov. Today Dis. Model. 2020, 32, 41–49. [Google Scholar] [CrossRef]
- Halleran, M.; Chernoff, A.; Gordon, J.L. Fertility Knowledge Among Women Struggling to Conceive Without Medical Intervention: A Brief Report. Front. Glob. Women’s Health 2022, 3, 828052. [Google Scholar] [CrossRef]
- García, D.; Brazal, S.; Rodríguez, A.; Prat, A.; Vassena, R. Knowledge of age-related fertility decline in women: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 109–118. [Google Scholar] [CrossRef]
- Homer, H.A. The Role of Oocyte Quality in Explaining Unexplained Infertility. Semin. Reprod. Med. 2020, 38, 21–28. [Google Scholar] [CrossRef]
- Toner, J.P. Age = egg quality, FSH level = egg quantity. Fertil. Steril. 2003, 79, 491. [Google Scholar] [CrossRef]
- Nasseri, A.; Grifo, J.A. Genetics, age, and infertility. Maturitas 1998, 30, 189–192. [Google Scholar] [CrossRef]
- Johnson, S.; Weddell, S.; Godbert, S.; Freundl, G.; Roos, J.; Gnoth, C. Development of the first urinary reproductive hormone ranges referenced to independently determined ovulation day. Clin. Chem. Lab. Med. 2015, 53, 1099–1108. [Google Scholar] [CrossRef]
- Martinez, A.R.; van Hooff, M.H.A.; Schoute, E.; van der Meer, M.; Broekmans, F.J.M.; Hompes, P.G.A. The reliability, acceptability and applications of basal body temperature (BBT) records in the diagnosis and treatment of infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 47, 121–127. [Google Scholar] [CrossRef]
- Tatsumi, T.M.; Sampei, M.R.; Saito, K.M.; Honda, Y.; Okazaki, Y.; Arata, N.M.; Narumi, K.; Morisaki, N.M.; Ishikawa, T.M.; Narumi, S.M. Age-Dependent and Seasonal Changes in Menstrual Cycle Length and Body Temperature Based on Big Data. Obstet. Gynecol. 2020, 136, 666–674. [Google Scholar] [CrossRef]
- Kamel, R.M. Management of the infertile couple: An evidence-based protocol. Reprod. Biol. Endocrinol. 2010, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, C.I.; Lynch, A.M.; Morin, A.K. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann. Pharmacother. 2008, 42, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Bäckström, T.; Haage, D.; Löfgren, M.; Johansson, I.; Strömberg, J.; Nyberg, S.; Andréen, L.; Ossewaarde, L.; van Wingen, G.; Turkmen, S.; et al. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience 2011, 191, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sundström Poromaa, I.; Smith, S.; Gulinello, M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch. Womens Ment. Health 2003, 6, 23–41. [Google Scholar] [CrossRef] [PubMed]
| Follicular | Luteal | |||||
|---|---|---|---|---|---|---|
| Cycle Day | N | LH | PdG | N | LH | PdG |
| 9 | 255 | 3.83 ± 0.36 | 1.95 ± 0.20 | |||
| 10 | 405 | 4.64 ± 0.41 | 2.03 ± 0.15 | |||
| 11 | 485 | 4.72 ± 0.33 | 2.08 ± 0.12 | |||
| 12 | 514 | 5.30 ± 0.35 | 2.15 ± 0.13 | |||
| 13 | 489 | 5.74 ± 0.39 | 2.11 ± 0.60 | 115 | 12.57 ± 1.22 | 5.48 ± 0.60 |
| 14 | 459 | 6.59 ± 0.44 | 2.11 ± 0.14 | 194 | 12.06 ± 0.91 | 5.57 ± 0.41 |
| 15 | 378 | 7.15 ± 0.50 | 2.33 ± 0.15 | 264 | 11.05 ± 0.79 | 6.11 ± 0.36 |
| 16 | 301 | 7.29 ± 0.57 | 2.84 ± 0.24 | 359 | 9.89 ± 0.62 | 6.37 ± 0.32 |
| 17 | 247 | 6.79 ± 0.57 | 2.94 ± 0.30 | 433 | 8.95 ± 0.57 | 8.19 ± 0.34 |
| 18 | 211 | 8.19 ± 0.82 | 3.11 ± 0.33 | 477 | 7.71 ± 0.45 | 8.63 ± 0.32 |
| 19 | 157 | 8.52 ± 0.85 | 3.32 ± 0.39 | 504 | 6.44 ± 0.41 | 9.92 ± 0.33 |
| 20 | 109 | 8.72 ± 1.04 | 4.10 ± 0.55 | 515 | 5.98 ± 0.37 | 10.23 ± 0.33 |
| 21 | 533 | 5.32 ± 0.39 | 10.44 ± 0.32 | |||
| 22 | 518 | 4.94 ± 0.35 | 11.12 ± 0.33 | |||
| 23 | 479 | 4.67 ± 0.29 | 11.78 ± 0.34 | |||
| 24 | 361 | 3.96 ± 0.30 | 11.45 ± 0.39 | |||
| 25 | 248 | 3.85 ± 0.38 | 11.79 ± 0.48 | |||
| 26 | 183 | 4.85 ± 0.55 | 11.02 ± 0.55 | |||
| 27 | 126 | 5.40 ± 0.81 | 11.36 ± 0.70 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hills, E.; Woodland, M.B.; Divaraniya, A. Using Hormone Data and Age to Pinpoint Cycle Day within the Menstrual Cycle. Medicina 2023, 59, 1348. https://doi.org/10.3390/medicina59071348
Hills E, Woodland MB, Divaraniya A. Using Hormone Data and Age to Pinpoint Cycle Day within the Menstrual Cycle. Medicina. 2023; 59(7):1348. https://doi.org/10.3390/medicina59071348
Chicago/Turabian StyleHills, Elinor, Mark B. Woodland, and Aparna Divaraniya. 2023. "Using Hormone Data and Age to Pinpoint Cycle Day within the Menstrual Cycle" Medicina 59, no. 7: 1348. https://doi.org/10.3390/medicina59071348
APA StyleHills, E., Woodland, M. B., & Divaraniya, A. (2023). Using Hormone Data and Age to Pinpoint Cycle Day within the Menstrual Cycle. Medicina, 59(7), 1348. https://doi.org/10.3390/medicina59071348