Abstract
Background and Objectives: Sperm DNA fragmentation refers to any break in one or both of the strands of DNA in the head of a sperm. The most widely used methodologies for assessing sperm DNA fragmentation are the sperm chromatin structure assay (SCSA), the sperm chromatin dispersion assay (SCD), the single-cell gel electrophoresis assay (SCGE–comet), and the terminal-deoxynucleotidyl-transferase (TdT)-mediated dUTP nick end labelling (TUNEL) assay. The aim of this study was to compare the efficiency and sensitivity of the analysis of sperm DNA fragmentation using TUNEL via fluorescence microscopy, and flow cytometry. Materials and Methods: Semen samples were collected and analyzed for standard characteristics using light microscopy, and for sperm DNA fragmentation using both TUNEL via fluorescence microscopy, and flow cytometry. Results: There were no significant differences in the values of the sperm DNA fragmentation index (DFI) obtained when the analysis was performed using TUNEL or flow cytometry (p = 0.543). Spearman’s correlation analysis revealed a significant negative correlation between sperm motility (%) and sperm DNA fragmentation (p < 0.01), as well as between sperm concentration and sperm DNA fragmentation (p < 0.05). The Mann–Whitney U test showed no significant difference in the DFI among couples with repeated implantation failure (RIF) and miscarriages (p = 0.352). Conclusions: Both methods (TUNEL via fluorescence microscopy, and flow cytometry) have a high efficiency and sensitivity in accurately detecting sperm DNA fragmentation, and can be effectively used to assess male fertility.
1. Introduction
One in six couples is affected by infertility and, in a third of these cases, the cause is of male origin [1,2,3,4,5,6,7]. The most common way to evaluate male fertility is standard semen analysis, according to the WHO guidelines [8]. Nevertheless, subtle sperm defects, such as breaks in the DNA, cannot be identified using a standard semen analysis. Since the spermatozoon must deliver an intact genome for normal fertilization, the initiation of cleavage, and normal development, it is possible that DNA defects may influence reproductive outcomes [9,10]. High levels of sperm DNA fragmentation have been associated with lower fertilization rates, poor embryo quality, and delayed cleavage [11].
Sperm DNA fragmentation has not yet been shown to be a good predictor of positive HCG, clinical pregnancy, miscarriage, and live births during ICSI or IVF cycles [12]. A retrospective analysis of clinical outcomes in vitrified–warmed single-blastocyst transfer cycles, in relation to sperm DNA fragmentation, revealed no straight correlation between a positive hCG rate, a clinical pregnancy rate, and first trimester miscarriage, and DFI levels, suggesting that ART outcomes are not affected by sperm DNA fragmentation independently of the gamete quality [13]. A recent systematic review and meta-analysis on the impact of a very short abstinence period on sperm parameters and DNA fragmentation showed a significant increase in sperm concentration and motility in the second ejaculation, and a significant decrease in sperm DNA fragmentation [14]. A second consecutive ejaculation after a very short time from the first one could therefore be an easy and effective strategy for collecting better-quality spermatozoa.
Much attention has also been given to the assay used to evaluate sperm DNA fragmentation. The most widely used methodologies are the sperm chromatin structure assay (SCSA), the sperm chromatin dispersion assay (SCD), the single-cell gel electrophoresis assay (SCGE–comet), and the terminal-deoxynucleotidyl-transferase (TdT)-mediated dUTP nick end labelling (TUNEL) assay [15,16,17,18,19,20,21,22,23]. All of these tests are commercially available, but have different levels of sensitivity and specificity. There are studies that use either one assay or the other, jeopardizing the reliability of the results of other researchers who use different sperm DNA fragmentation tests. Even in large meta-analyses, the inclusion of studies with different sperm DNA fragmentation assays presents an obstacle to drawing solid conclusions [24].
The terminal-deoxynucleotidyl-transferase (TdT)-mediated dUTP nick end labelling (TUNEL) assay is one of the most reliable and sensitive methods for evaluating sperm DNA fragmentation [25,26]. The enzyme TdT is used to add labelled nucleotides to free 3′ OH ends of DNA strands, resulting in single-strand poly-U extensions. It can simultaneously detect single- and double-strand breaks, which constitutes an advantage of the test, and it can be performed via either fluorescence microscopy or flow cytometry [16,17,18,19,25,26]. In general, flow cytometry has been characterized as an automated, rapid, and sensitive method for evaluating male infertility [27,28,29]. Due to its multiparametric capability, it can be used to measure concentration in sperm samples, while the incorporation of DNA dyes differentiates haploid round spermatids and diploid cells from actually haploid mature spermatozoa. At the same time, it assesses spermatogenesis, motility, and viability [27,28,29]. Propidium iodide (PI), in combination with carboxyfluorescein-diacetate succinimidylester and SYBR-14 constitute the most common viability stains, which enter the spermatozoa emitting red or green fluorescence, respectively [17,18]. The aim of this study was to compare the efficiency and sensitivity of sperm DNA fragmentation analyses using TUNEL via fluorescence microscopy and flow cytometry.
2. Materials and Methods
Semen samples were collected and analyzed for standard characteristics using light microscopy at Fertilia by Genesis, Thessaloniki, Greece. The sperm DNA fragmentation was assessed using TUNEL, both via fluorescence microscopy, and via flow cytometry, at the Genetics Unit and the Department of Immunology and Histocompatibility at Papageorgiou Hospital, Thessaloniki, Greece. This study was approved by the Bioethics Committee of the Aristotle University Medical School (1.30/21 November 2018) and Genesis (01/7-2/3056). All analyses were performed following the patients’ informed consent.
2.1. Standard Semen Analysis
The semen analysis was performed according to the World Health Organization (WHO) criteria. The lower reference limits were: for volume, 1.5 mL; for concentration, 15 millions/mL; for progressive motility A + B, 32%; and for normal morphology, 4% [8].
2.2. Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) via Fluorescence Microscopy
The evaluation of sperm DNA fragmentation using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) via fluorescence microscopy was performed according to Chatzimeletiou et al. [19,30]. Ten microliters of fixed sperm suspensions in 3: 1 methanol: acetic acid (Sigma-Aldrich, Taufkirchen, Germany) were spread on polysine slides (Gerhard Menzel Braunschweig, Germany–Thermo Fisher Scientific, Singapore), and incubated in 0.1 M Tris/DTT swelling solution (Sigma-Aldrich, Taufkirchen, Germany) for 30 min, then washed in phosphate-buffered saline (PBS) (Sigma-Aldrich, Taufkirchen, Germany) and H2O, primed with TdT buffer and CoCl2 (Roche Diagnostics GmbH, Mannheim, Germany), and incubated with TdT buffer, CoCl2TdT enzyme, and dUTP (Roche Diagnostics GmbH, Mannheim, Germany) for 60 min in the dark. The slides were then placed in stop buffer, and were washed twice in PBS (Sigma-Aldrich, Taufkirchen, Germany). After staining with Texas Red (Vector Laboratories, Burlingame, CA, USA), the slides were washed in PBS (Sigma-Aldrich, Taufkirchen, Germany), air dried, mounted in Vectarshield antifade medium with DAPI (4, 6-diamidino-2-phenylidole; Vector Laboratories, Burlingame, CA, USA) under a coverslip, and sealed with nail varnish. Fragmented sperm head nuclei were assessed via fluorescence microscopy, using the Zeiss Imager.Z1 fluorescence microscope, equipped with TRITC (red) and DAPI (blue) filters, and the images were captured using Isis software (Metasystems, Altlussheim, Germany) [19,30]. The total number of sperm analyzed per sample was 1000. All sperm were stained blue with DAPI, but the fragmented sperm were additionally stained red with Texas Red. The calculation of the sperm DNA fragmentation index (DFI) using TUNEL via fluorescence microscopy was conducted by dividing the number of fragmented sperm head nuclei (red-stained) by the total number of sperm head nuclei (DAPI-stained). DFI= Number of fragmented sperm/1000.
2.3. Flow Cytometry
The flow cytometry was performed according to Chatzimeletiou et al. [31]. An aliquot of 100 μL of sperm samples was washed in PBS (Sigma-Aldrich Taufkirchen, Germany), and then centrifuged for 5 min at 300 g. After the removal of the supernatant, the precipitant was incubated in TNE buffer (NaCl (0.15 Μ), Tris HCL (0.01 Μ), EDTA (0.0011 Μ) pH 7.4) (Bioline Scientific, Athens, Greece) and detergent solution (NaCl 0.15 Μ, TRITON X-100) (Bioline Scientific, Athens, Greece) for 5 min. Acridine orange (Bioline Scientific Athens Greece) was added, and a further 5 min incubation followed, in the dark. The samples were finally analyzed via flow cytometry (Beckman Coulter, FC 500, South Kraemer Boulevard Brea, CA, USA), separating the intact sperm (green) from the fragmented ones (red), based on the change in color due to the acridine orange inserted into the fragmented portion of the sperm. The separation of sperm based on the FS/SS characteristics and gating on the viable cells removed any unwanted events, and allowed the calculation of the sperm DNA fragmentation index (DFI) in the viable portion of the cells. The calculation of the DFI using flow cytometry was performed by dividing the red fluorescence by the total red and green fluorescence. The DFI = red fluorescence/total red and green fluorescence.
2.4. Statistical Analysis
The statistical analysis of the results was carried out using the SPSS version 28.0.1.0 statistical package for Windows (IBM, New York, NY, USA).The whole group of patients (Group A, N = 35) was subdivided into two sub-groups: patients with a DNA fragmentation index (DFI) below 30% (detected by both methods) were allocated to Group B (N = 25), whereas patients with a DFI above 30% (detected by both methods) were allocated to Group C (N = 10). The normality of the data was assessed using the Shapiro–Wilk test. The non-parametric Wilcoxon signed-rank test was used to compare the medians of the DFI values obtained using the TUNEL and flow cytometry methods in both Group A and Group C. A paired-samples t-test was used to compare the means of the DFI values obtained using the TUNEL and flow cytometry methods in Group B. The Mann–Whitney U test was used to determine whether there were significant differences in the DFI between the infertility cases associated with repeated implantation failure (RIF), and those associated with miscarriage. Spearman’s test was used for the correlation analysis between the sperm concentration and DFI, and the sperm motility (%) and DFI. For the correlation analysis, each patient’s DFI was calculated as the average of the values obtained using both the TUNEL and flow cytometry methods. The statistical significance was set at p < 0.05. Box and whisker plots, and scatter plots were generated using Microsoft Excel software (version 2302, Microsoft Corporation, Redmond, WA, USA).
3. Results
The standard semen analysis and sperm DNA fragmentation assessed using both TUNEL and flow cytometry are shown in Table 1.

Table 1.
The sperm DNA fragmentation assessment using TUNEL and flow cytometry, and standard semen analysis. DFI, DNA fragmentation index; RIF, repeated implantation failure (lower reference limits: for concentration/mL, ≥15 × 106; for motility A + B, ≥32%; for DFI, ≤29).
The descriptive statistics of the various sperm parameters in the different groups of patients are shown in Table 2.

Table 2.
The descriptive statistics of various sperm parameters, between the groups of patients. Groups B and C are sub-groups of Group A. The sperm DNA fragmentation (%DFI) was assessed using both TUNEL and flow cytometry.
The association of different causes of infertility with sperm DNA fragmentation is shown in Table 3.

Table 3.
The association of different causes of infertility with sperm DNA fragmentation.
The results show no significant differences in the values of sperm DNA fragmentation index (DFI) obtained when the analysis was performed using TUNEL or flow cytometry (p = 0.543) (Figure 1 and Figure 2, Table 2). Spearman’s correlation analysis revealed a significant negative correlation between sperm motility (%) and sperm DNA fragmentation (rho = −0.464, p < 0.01; Figure 3), as well as between sperm concentration and sperm DNA fragmentation (rho = −0.405, p < 0.05; Figure 4). The Mann–Whitney U test showed no significant difference in the DFI between couples with repeated implantation failure (RIF) and miscarriages (p = 0.352) (Table 3).
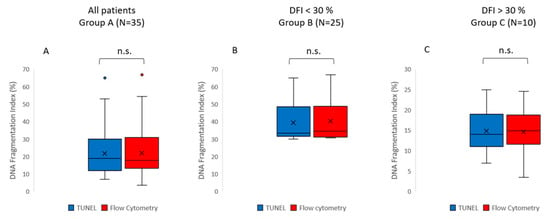
Figure 1.
Box and whisker plots displaying the distribution of the data for sperm DNA fragmentation (DFI). The DFI values were obtained using either the TUNEL method (blue) or the flow cytometry method (red). (A) Group A includes all patients (N = 35); (B) Group B (N = 25) is a subgroup including the patients with DFI values below 30%, as detected by both methods; (C) Group C (N = 10) is a subgroup including the patients with DFI values above 30%, as detected by both methods. The box represents the interquartile range (IQR), which contains the middle 50% of the data. The line within the box represents the median. The whiskers extend from the top and bottom of the box to the minimum and maximum values within 1.5 times the IQR, respectively. Outliers are represented as individual points. The statistical analysis of all data (Group A) showed no significant difference in the DFI when assessed using TUNEL or flow cytometry (Wilcoxon signed rank test, p = 0.543). No significant differences in the DFI between TUNEL and flow cytometry were found in either Group B (paired samples t-test, p = 0.547) or Group C (Wilcoxon signed rank test, p = 0.169).
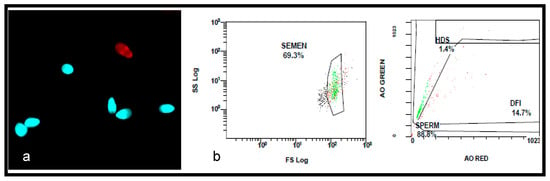
Figure 2.
The assessment of sperm DNA fragmentation using TUNEL and flow cytometry. (a) A photomicrograph showing the TUNEL-labelled sperm. The normal spermatozoa are stained in blue with 4, 6-diamidino-2-phenylidole (DAPI), and the fragmented sperm is stained in red with Texas Red. The DFI was 14%. (b) The flow cytometry, showing 14.7% DFI.
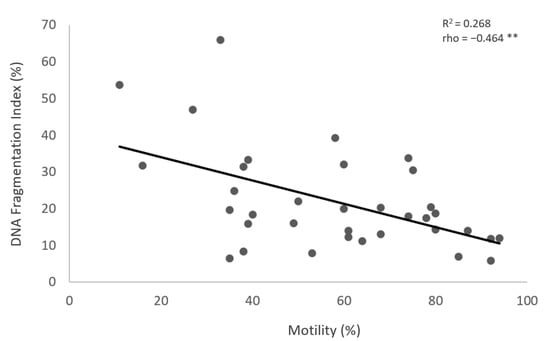
Figure 3.
The correlation between the sperm motility (%) (X-axis) and the DFI (Y-axis). Individual data points and the regression line are shown. There is a significant negative correlation between the sperm motility (%) and the DFI. Spearman’s correlation coefficient (rho) = −0.464, p-value < 0.01. The notation (**) indicates that the p-value is less than 0.01.
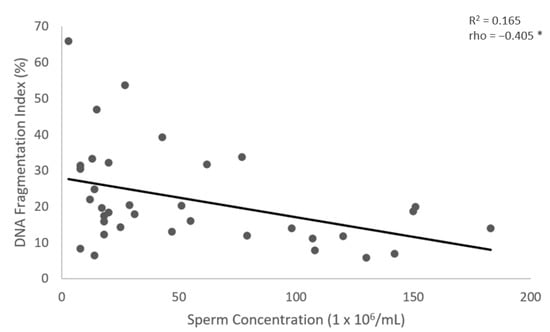
Figure 4.
The correlation between the sperm concentration (X-axis) and the DFI (Y-axis). Individual data points and the regression line are shown. There is a significant negative correlation between the sperm concentration and the DFI. Spearman’s correlation coefficient (rho) = −0.405, p-value < 0.05. The notation (*) indicates that the p-value is less than 0.05.
4. Discussion
Sperm DNA fragmentation refers to any break in one or both of the strands of DNA in the head of a sperm [20]. Breaks in the DNA may play a crucial role in gamete function and, as a consequence may affect fertilization, embryo development, and the reproductive outcome [9,10,11,12,13]. Aspects of sperm function that can be disrupted include motility and sperm–zona recognition. Sperm transcripts and proteins are involved in acrosome reaction and fusion and, once released into the oocyte, can influence embryo development. The impact DNA fragmentation may have on the success of assisted reproduction cycles highly depends on the balance between the extent of the DNA breaks, and the ability of the oocyte to repair this damage. This diversity in sperm DNA damage and the repair capacity of the oocyte may explain why some fragmented sperm retain their fertilizing ability [9,10,11,12,13,20,26].
The most widely used methodologies for assessing sperm DNA fragmentation are the sperm chromatin structure assay (SCSA), the sperm chromatin dispersion assay (SCD), the single-cell gel electrophoresis assay (SCGE–comet), and the terminal-deoxynucleotidyl-transferase (TdT)-mediated dUTP nick end labelling (TUNEL) assay [16,17,18,19,20,21,22,23]. Our study compared the efficiency and sensitivity of sperm DNA fragmentation analysis using TUNEL via fluorescence microscopy, and flow cytometry, and showed no significant differences in the values of the sperm DNA fragmentation index (DFI) obtained when analysis was performed using either of the two methods. Additionally, our study revealed a significant negative correlation between the sperm motility and sperm DNA fragmentation, as well as between the sperm concentration and sperm DNA fragmentation. Our results are in agreement with previous studies, confirming the reliability of both methods in assessing sperm DNA fragmentation [16,17,18,19,21,22,23,24,25,26,27,28,29,30,31]. The reliable measurement of sperm DNA fragmentation is of utmost importance to the identification of the causes of male infertility, and a deep understanding of the mechanisms leading to DNA fragmentation may provide new management strategies for overcoming male infertility.
Various mechanisms have been proposed as leading to sperm DNA fragmentation, including (a) the abortive apoptosis theory, (b) the defective maturation theory, and (c) oxidative stress [32,33,34,35,36]. The abortive apoptosis theory suggests that DNA fragmentation may originate in the testis, as part of the normal process of apoptosis, or as a consequence of different insults during transit in the genital tract, and that it is induced by activated endonucleases, which mostly lead to DNA double-stranded breaks [32,33]. According to this theory, fragmented sperm in the ejaculate may be derived from germinal cells whose apoptotic process was not completed in the testis [32,33]. On the other hand, the defective maturation theory suggests that DNA fragmentation may occur during chromatin compaction, as a result of histones’ replacement by protamines [34]. However, these two mechanisms cannot provide a full explanation for the occurrence of DNA fragmentation in the ejaculate, especially as higher levels of DNA fragmentation have been observed in the caudal epididymis and the ejaculate than in testicular sperm [35,36]. The generation of reactive oxygen species (oxidative stress) appears to be the main cause of DNA fragmentation, following release from the testis. Genitourinary infections, varicocele, and immature spermatozoa retaining cytoplasmic droplets may lead to excessive intrinsic reactive oxygen species production, increasing sperm DNA fragmentation [35,36].
The modern lifestyle may also predispose men to increased sperm DNA fragmentation [1,2,3]. Cigarette smoking, excessive alcohol consumption, an unhealthy diet leading to obesity which can, in turn, be linked to diabetes, a lack of exercise, exposure to environmental pollutants, an elevated testicular temperature from computers/laptops, hot tubs, and tight-fitting underwear are all contributing factors [1,2,3,6,37,38,39]. Sperm with fragmented DNA look normal in morphology, are motile and viable, and can successfully fertilize oocytes. However, embryonic development and subsequent implantation may be impaired in embryos derived from sperm with fragmented DNA. Although oocytes have the machinery to repair DNA damage, factors such as the quality of the oocyte itself and the type of sperm DNA damage may influence the extent to which this repair occurs [40,41,42]. Therefore, it is of utmost importance to accurately diagnose DNA fragmentation in sperm and, in men with high levels of sperm DNA fragmentation, suggest suitable treatments before the initiation of their assisted reproduction cycle. Given that new sperm are generated every 72 days, decreasing exposure to oxidative stress by making lifestyle changes, avoiding smoking, limiting alcohol intake, and incorporating a healthy diet and exercise, as well as considering the use of supplements containing vitamins and antioxidants, may decrease the degree of sperm DNA fragmentation [43,44]. Surgical repair, in cases of varicocele, may also be considered, if proved to be necessary [45,46].
In general, apoptosis and sperm chromatin maturation defects are believed to act in the testis, and cause the DNA breaks found in non-viable ejaculated spermatozoa [47,48]. On the other hand, oxidative stress induces sperm DNA fragmentation following release from the testis, during the transit through the male genital tract, and causes the DNA breaks found in viable spermatozoa in the ejaculate [47,48,49]. Oxidative stress is also suggested to be the main mechanism inducing sperm DNA fragmentation after ejaculation during in vitro manipulation [50]. All assisted reproductive technologies (ARTs), including intrauterine insemination (IUI), conventional in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI), require the handling and micromanipulation of sperm. The most widely used methodologies for sperm selection during ART treatments are density-gradient centrifugation (DGC) and swim-up, which enable the selection of the most highly motile and morphologically normal spermatozoa [50,51,52]. Sperm may sustain damage both during the selection process, and when remaining for an extended time in the incubator before insemination. Spermatozoa may also acquire additional damage when selected with more advanced technologies, using high magnification (IMSI), as they remain exposed to light for longer before insemination [50]. Any damage of this type may alter sperm characteristics and functions. The motility, morphology, mitochondrial function, and ability to undergo the acrosome reaction may be altered, affecting fertilization rates, embryo quality, and subsequent embryonic development. Whether or not mature spermatozoa are able to trigger apoptotic pathways warrants further investigation [45,46,47,48,49].
In the current study, we compared the efficiency and sensitivity of sperm DNA fragmentation analysis using TUNEL via fluorescence microscopy, and flow cytometry. Our results showed no significant differences in the sperm DNA fragmentation index (DFI) values obtained when analysis was performed using TUNEL or flow cytometry and, additionally, revealed a significant negative correlation between the sperm motility and sperm DNA fragmentation, as well as between the sperm concentration and sperm DNA fragmentation. These results are in agreement with previous studies, confirming the reliability of both methods [16,17,18,19,21,22,23,24,25,26,27,28,29,30,31,45]. In contrast, the comet and SCD–HALO tests, which measure only a limited number of sperm (50–200) per sample, suffer from their lacking the statistical robustness of flow cytometric or TUNEL measurements [16,19,22,30,31,53].
5. Conclusions
We conclude that both methods used in this study (TUNEL via fluorescence microscopy, and flow cytometry) have a high level of efficiency and sensitivity in accurately detecting sperm DNA fragmentation. No statistically significant differences in sperm DNA fragmentation were obtained when the analysis was performed either using TUNEL or flow cytometry, and no straight correlation was observed amongst different couple’s indications, and DNA fragmentation. Larger-scale studies are needed, to elucidate any potential associations between fragmentation levels, repeated implantation failure (RIF), and miscarriages. The reliable measurement of sperm DNA fragmentation is of utmost importance in identifying the causes of male infertility, and a deep understanding of the mechanisms leading to DNA fragmentation may open new horizons for the therapeutic treatment of infertile males.
Author Contributions
Conceptualization, K.C.; methodology, K.C. and A.F.; software, K.C.; validation, K.C., A.F. and M.M.; formal analysis, K.C., T.-T.N. and A.F.; investigation, K.C.; resources, K.C. and G.A.; writing—original draft preparation, K.C.; writing—review and editing, T.-T.N., G.A., G.Z., K.P., A.G. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Bioethics Committee of the Aristotle University Medical School (1.30/21 November 2018) and Genesis (01/7-2/3056).
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szabó, A.; Váncsa, S.; Hegyi, P.; Váradi, A.; Forintos, A.; Filipov, T.; Ács, J.; Ács, N.; Szarvas, T.; Nyirády, P.; et al. Lifestyle-, environmental-, and additional health factors associated with an increased sperm DNA fragmentation: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. RBE 2023, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Bliatka, D.; Nigdelis, M.P.; Chatzimeletiou, K.; Mastorakos, G.; Lymperi, S.; Goulis, D.G. The effects of postnatal exposure of endocrine disruptors on testicular function: A systematic review and a meta-analysis. Hormones 2020, 19, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Chatzimeletiou, K.; Morrison, E.E.; Prapas, N.; Prapas, Y.; Handyside, A.H. Symposium: Genetic and epigenetic aspects of assisted reproduction. The centrosome and early embryogenesis: Clinical insights. Reprod. Biomed. Online 2008, 16, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. The changing tide of human fertility. Hum. Reprod. 2022, 37, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Tramontano, L.; Esteves, S.C. Effects of mobile phone radiofrequency radiation on sperm quality. Zygote 2022, 30, 159–168. [Google Scholar] [CrossRef]
- Vallet-Buisan, M.; Mecca, R.; Jones, C.; Coward, K.; Yeste, M. Contribution of semen to early embryo development: Fertilization and beyond. Hum. Reprod. Update 2023, 29, 395–433. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Zini, A.; Boman, J.M.; Belzile, E.; Ciampi, A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum. Reprod. 2008, 23, 2663–2668. [Google Scholar] [CrossRef]
- Simon, L.; Castillo, J.; Oliva, R.; Lewis, S.E. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod. Biomed. Online 2011, 23, 724–734. [Google Scholar] [CrossRef]
- Esbert, M.; Pacheco, A.; Soares, S.R.; Amorós, D.; Florensa, M.; Ballesteros, A.; Meseguer, M. High sperm DNA fragmentation delays human embryo kinetics when oocytes from young and healthy donors are microinjected. Andrology 2018, 6, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Ten, J.; Guerrero, J.; Linares, Á.; Rodríguez-Arnedo, A.; Morales, R.; Lledó, B.; Llácer, J.; Bernabeu, R. Sperm DNA fragmentation on the day of fertilisation is not associated with assisted reproductive technique outcome independently of gamete quality. Hum. Fertil. 2022, 25, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Wang, H.; Zhou, W.; Zheng, Y.; Ye, D. Does sperm DNA fragmentation affect clinical outcomes during vitrified-warmed single-blastocyst transfer cycles? A retrospective analysis of 2034 vitrified-warmed single-blastocyst transfer cycles. J. Assist. Reprod. Genet. 2022, 39, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Cannarella, R.; Crafa, A.; Manna, C.; La Vignera, S.; Condorelli, R.A.; Calogero, A.E. The Impact of a Very Short Abstinence Period on Conventional Sperm Parameters and Sperm DNA Fragmentation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7303. [Google Scholar] [CrossRef]
- Lin, H.T.; Wu, M.H.; Wu, W.L.; Tsai, L.C.; Chen, Y.Y.; Hung, K.H.; Wu, P.H.; Chen, T.S.; Ou, H.T.; Cheng, Y.S. Incorporating sperm DNA fragmentation index with computer-assisted semen morphokinematic parameters as a better window to male fertility. Chin. J. Physiol. 2022, 65, 143–150. [Google Scholar]
- Sharma, R.; Iovine, C.; Agarwal, A.; Henkel, R. TUNEL assay-Standardized method for testing sperm DNA fragmentation. Andrologia 2021, 53, e13738. [Google Scholar] [CrossRef]
- Sharma, R.; Ahmad, G.; Esteves, S.C.; Agarwal, A. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: Protocol, reference values, and quality control. J. Assist. Reprod. Genet. 2016, 33, 291–300. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Mata-Campuzano, M.; Alvarez-Rodríguez, M.; Alvarez, M.; Anel, L.; de Paz, P. Probes and techniques for sperm evaluation by flow cytometry. Reprod. Domest. Anim. 2010, 45 (Suppl. S2), 67–78. [Google Scholar] [CrossRef]
- Chatzimeletiou, K.; Sioga, A.; Oikonomou, L.; Charalampidou, S.; Kantartzi, P.D.; Zournatzi, V.; Panidis, D.; Goulis, D.G.; Papadimas, I.; Tarlatzis, B.C. Semen analysis by electron and fluorescence microscopy in a case of partial hydatidiform mole reveals a high incidence of abnormal morphology, diploidy and tetraploidy. Fertil. Steril. 2011, 95, 2430.e1–2430.e5. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Zini, A.; Coward, R.M.; Evenson, D.P.; Gosálvez, J.; Lewis, S.E.M.; Sharma, R.; Humaidan, P. Sperm DNA fragmentation testing: Summary evidence and clinical practice recommendations. Andrologia 2021, 53, e13874. [Google Scholar] [CrossRef]
- Evenson, D.P.; Djira, G.; Kasperson, K.; Christianson, J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil. Steril. 2020, 114, 311–320. [Google Scholar] [CrossRef]
- Evenson, D.P. The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef]
- Jerre, E.; Bungum, M.; Evenson, D.; Giwercman, A. Sperm chromatin structure assay high DNA stainability sperm as a marker of early miscarriage after intracytoplasmic sperm injection. Fertil. Steril. 2019, 112, 46–53.e2. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Zini, A.; Dyachenko, A.; Ciampi, A.; Carrell, D.T. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 2017, 19, 80–90. [Google Scholar] [PubMed]
- Baskaran, S.; Agarwal, A.; Panner Selvam, M.K.; Finelli, R.; Robert, K.A.; Iovine, C.; Pushparaj, P.N.; Samanta, L.; Harlev, A.; Henkel, R. Tracking research trends and hotspots in sperm DNA fragmentation testing for the evaluation of male infertility: A scientometric analysis. Reprod. Biol. Endocrinol. RBE 2019, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Cissen, M.; Wely, M.V.; Scholten, I.; Mansell, S.; Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef]
- Garrido, N.; Meseguer, M.; Remohi, J.; Pellicer, A.; Simón, C. Flow cytometry in human reproductive biology. Gynecol. Endocrinol. 2002, 16, 505–521. [Google Scholar] [CrossRef]
- Levek-Motola, N.; Soffer, Y.; Shochat, L.; Raziel, A.; Lewin, L.M.; Golan, R. Flow cytometry of human semen: A preliminary study of a non-invasive method for the detection of spermatogenetic defects. Hum. Reprod. 2005, 20, 3469–3475. [Google Scholar] [CrossRef]
- Eustache, F.; Jouannet, P.; Auger, J. Evaluation of flow cytometric methods to measure human sperm concentration. J. Androl. 2001, 22, 558–567. [Google Scholar]
- Chatzimeletiou, K.; Galanis, N.; Karagiannidis, A.; Sioga, A.; Pados, G.; Goulis, D.; Kalpatsanidis, A.; Tarlatzis, B.C. Fertility potential in a man with ankylosing spondylitis as revealed by semen analysis by light, electron and fluorescence microscopy. SAGE Open Med. Case Rep. 2018, 6, 2050313X18759898. [Google Scholar] [CrossRef]
- Chatzimeletiou, K.; Fleva, A.; Sioga, A.; Georgiou, I.; Nikolopoulos, T.T.; Markopoulou, M.; Petrogiannis, N.; Anifandis, G.; Patrikiou, A.; Kolibianakis, E.; et al. Effects of Different Drug Therapies and COVID-19 mRNA Vaccinationon Semen Quality in a Man with Ankylosing Spondylitis: A Case Report. Medicina 2022, 58, 173. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Mariethoz, E.; Manicardi, G.; Bizzaro, D.; Bianchi, P.G.; Bianchi, U. Origin of DNA damage in ejaculated human spermatozoa. Rev. Reprod. 1999, 4, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Mariethoz, E.; St John, J.C. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp. Cell Res. 1999, 251, 350–355. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Longo, F.J. Chromatin structure-function alterations during mammalian spermatogenesis: DNA nicking and repair in elongating spermatids. Eur. J. Histochem. 1993, 37, 109–128. [Google Scholar]
- Aitken, J.; Krausz, C.; Buckingham, D. Relationships between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation, and the presence of leukocytes and precursor germ cells in human sperm suspensions. Mol. Reprod. Dev. 1994, 39, 268–279. [Google Scholar] [CrossRef]
- Moskovtsev, S.I.; Jarvi, K.; Mullen, J.B.; Cadesky, K.I.; Hannam, T.; Lo, K.C. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil. Steril. 2010, 93, 1142–1146. [Google Scholar] [CrossRef]
- Donkin, I.; Barrès, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Kim, J. Are genes destiny? Exploring the role of intrauterine environment in moderating genetic influences on body mass. Am. J. Hum. Biol. 2020, 32, e23354. [Google Scholar] [CrossRef]
- Argyraki, M.; Damdimopoulou, P.; Chatzimeletiou, K.; Grimbizis, G.F.; Tarlatzis, B.C.; Syrrou, M.; Lambropoulos, A. In-utero stress and mode of conception: Impact on regulation of imprinted genes, fetal development and future health. Hum. Reprod. Update 2019, 5, 777–801. [Google Scholar] [CrossRef]
- Chatzimeletiou, K.; Sioga, A.; Petrogiannis, N.; Panagiotidis, Y.; Prapa, M.; Patrikiou, A.; Tarlatzis, B.C.; Grimbizis, G. Viability assessment using fluorescent markers and ultrastructure of human biopsied embryos vitrified in open and closed systems. RBMO 2021, 43, 833–842. [Google Scholar] [CrossRef]
- Chatzimeletiou, K.; Petrogiannis, N.; Sioga, A.; Emmanouil-Nikoloussi, E.N.; Panagiotidis, Y.; Prapa, M.; Patrikiou, A.; Filippa, M.; Zervakakou, G.; Papanikolaou, K.; et al. The human embryo following biopsy on day 5 vs day 3: Viability ultrastructure and spindle chromosome configurations. RBMO 2022, 45, 219–233. [Google Scholar]
- Newman, H.; Catt, S.; Vining, B.; Vollenhoven, B.; Horta, F. DNA repair and response to sperm DNA damage in oocytes and embryos, and the potential consequences in ART: A systematic review. Mol. Hum. Reprod. 2022, 28, gaab071. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Barres, R.; Kirchner, H.; Rasmussen, M.; Yan, J.; Kantor, F.R.; Krook, A.; Näslund, E.; Zierath, J.R. Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep. 2013, 25, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Ammar, O.; Tekeya, O.; Hannachi, I.; Sallem, A.; Haouas, Z.; Mehdi, M. Increased Sperm DNA Fragmentation in Infertile Men with Varicocele: Relationship with Apoptosis, Seminal Oxidative Stress, and Spermatic Parameters. Reprod. Sci. 2021, 28, 909–919. [Google Scholar] [CrossRef]
- Ammar, O.; Mehdi, M.; Muratori, M. Teratozoospermia: Its association with sperm DNA defects, apoptotic alterations, and oxidative stress. Andrology 2020, 8, 1095–1106. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Baldi, E. Sperm DNA Fragmentation: Mechanisms of Origin. Adv. Exp. Med. Biol. 2019, 1166, 75–85. [Google Scholar] [PubMed]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens. Health 2019, 37, 296–312. [Google Scholar] [CrossRef]
- Baldi, E.; Tamburrino, L.; Muratori, M.; Degl’Innocenti, S.; Marchiani, S. Adverse effects of in vitro manipulation of spermatozoa. Anim. Reprod. Sci. 2020, 220, 106314. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, L.; Barratt, C.L.R.; Mortimer, D.; Agarwal, A.; Aitken, R.J.; Alvarez, J.G.; Aneck-Hahn, N.; Arver, S.; Baldi, E.; Bassas, L.; et al. Standards in semen examination: Publishing reproducible and reliable data based on high-quality methodology. Hum. Reprod. 2022, 37, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P. Sperm Chromatin Structure Assay (SCSA®) for Fertility Assessment. Curr. Protoc. 2022, 2, e508. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).