The Clinical Significance of LDL-Cholesterol on the Outcomes of Hemodialysis Patients with Acute Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Variables
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. LDL-C in the HD Group
3.2. 30-Day Mortality
3.3. One-Year Mortality
3.4. Predictors of Mortality after ACS in HD Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsushita, K.; Van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J.; Gansevoort, R.T.; Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar]
- Haim-Pinhas, H.; Yoskovitz, G.; Lishner, M.; Pereg, D.; Kitay-Cohen, Y.; Topaz, G.; Sela, Y.; Wand, O.; Rozenberg, I.; Benchetrit, S.; et al. Effect of aspirin on primary prevention of cardiovascular disease and mortality among patients with chronic kidney disease. Sci. Rep. 2022, 12, 17788. [Google Scholar] [CrossRef]
- US Renal Data System. USRDS 1999 annual data report. Am. J. Kidney Dis. 1999, 34 (Suppl. 1), S87–S94. [Google Scholar]
- Schamroth-Pravda, M.; Cohen-Hagai, K.; Topaz, G.; Schamroth-Pravda, N.; Makhoul, N.; Shuvy, M.; Benchetrit, S.; Assali, A.; Pereg, D. Assessment of the CHA2DS2-VASc Score in Predicting Mortality and Adverse Cardiovascular Outcomes of Patients on Hemodialysis. Am. J. Nephrol. 2020, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Hagai, K.; Nacasch, N.; Rozenberg, I.; Korzets, Z.; Einbinder, Y.; Zitman-Gal, T.; Benchetrit, S. Clinical outcomes of stroke in hemodialysis patients: A retrospective single-center study. Int. Urol. Nephrol. 2019, 51, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. Guideline on the management of blood cholesterol: Executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef] [PubMed]
- Prichard, S.S. Impact of dyslipidemia in end-stage renal disease. J. Am. Soc. Nephrol. 2003, 14 (Suppl. 4), S315–S320. [Google Scholar] [CrossRef] [PubMed]
- Avram, M.M.; Goldwasser, P.; Burrell, D.E.; Antignani, A.; Fein, P.A.; Mittman, N. The uremic dyslipidemia: A cross-sectional and longitudinal study. Am. J. Kidney Dis. 1992, 20, 324–335. [Google Scholar] [CrossRef]
- Elisof, M.; Mikhailidis, D.P.; Siampoulos, K.C. Dyslipidemia in patients with renal disease. J. Drug Dev. Clin. Pr. 1996, 17, 331–348. [Google Scholar]
- Kronenberg, F.; König, P.; Neyer, U.; Auinger, M.; Pribasnig, A.; Lang, U.; Reitinger, J.; Pinter, G.; Utermann, G.; Dieplinger, H. Multicenter study of lipoprotein(a) and apolipoprotein(a) phenotypes in patients with end-stage renal disease treated with hemodialysis or continuous ambulatory peritoneal dialysis. J. Am. Soc. Nephrol. 1995, 6, 110–120. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomized trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists (CTT) Collaboration. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomized trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Yamazato, M.; Tozawa, M.; Takishita, S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. 2002, 61, 1887–1893. [Google Scholar] [CrossRef]
- Seliger, S.L.; Weiss, N.S.; Gillen, D.L.; Kestenbaum, B.; Ball, A.; Sherrard, D.J.; Stehman-Breen, C.O. HMG-CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int. 2002, 61, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.A.; Bailie, G.R.; Satayathum, S.; Bragg-Gresham, J.L.; Akiba, T.; Akizawa, T.; Combe, C.; Rayner, H.C.; Saito, A.; Gillespie, B.W.; et al. HMGCoA reductase inhibitor use is associated with mortality reduction in hemodialysis patients. Am. J. Kidney Dis. 2005, 45, 119–126. [Google Scholar] [CrossRef]
- Amann, K.; Breitbach, M.; Ritz, E.; Mall, G. Myocyte/capillary mismatch in the heart of uremic patients. J. Am. Soc. Nephrol. 1998, 9, 1018–1022. [Google Scholar] [CrossRef]
- Einbinder, Y.; Shnaider, A.; Ghanayem, K.; Basok, A.; Rogachev, B.; Lior, Y.; Haviv, Y.S.; Cohen-Hagai, K.; Nacasch, N.; Rozenberg, I.; et al. Elevated Circulating Cell-Free DNA in Hemodialysis-Treated Patients Is Associated with Increased Mortality. Am. J. Nephrol. 2020, 51, 852–860. [Google Scholar] [CrossRef]
- Erez, D.; Fanadka, F.; Benchetrit, S.; Cohen-Hagai, K. The Combined Prognostic Significance of Alkaline Phosphatase and Intracranial Arterial Calcifications in Hemodialysis Patients. Am. J. Nephrol. 2021, 52, 763–770. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, Y.K.; Cho, A.J.; Park, H.C.; Han, C.H.; Choi, M.J.; Koo, J.R.; Yoon, J.W.; Noh, J.W. Malnutrition, inflammation, progression of vascular calcification and survival: Inter-relationships in hemodialysis patients. PLoS ONE 2019, 14, e0216415. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Chertow, G.M.; Adhikarla, R.; Young, B.; Ronco, C.; Levin, N.W. Inflammation and dietary protein intake exert competing effects on serum albumin and creatinine in hemodialys patients. Kidney Int. 2001, 60, 333–340. [Google Scholar] [CrossRef]
- Chiang, C.-K.; Ho, T.-I.; Hsu, S.-P.; Peng, Y.-S.; Pai, M.-F.; Yang, S.-Y.; Hung, K.-Y.; Tsai, T.-J. Low-density lipoprotein cholesterol: Association with mortality and hospitalization in hemodialysis patients. Blood Purif. 2005, 23, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Coresh, J.; Eustace, J.A.; Longenecker, J.C.; Jaar, B.; Fink, N.E.; Tracy, R.P.; Powe, N.R.; Klag, M.J. Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 2004, 291, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Martinez, J.; Campa, A.; Delgado-Enciso, I.; Hain, D.; George, F.; Huffman, F.; Baum, M. The relationship of blood lymphocute-to-neutrophil ratio with nutritional markers and health outcomes in hemodialysis patients. Int. Urol. Nephrol. 2019, 51, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Krane, V.; März, W.; Olschewski, M.; Mann, J.F.; Ruf, G.; Ritz, E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005, 353, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Baignet, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): A randomized placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef]
- Fellström, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.-W.; Chevaile, A.; Cobbe, S.M.; Grönhagen-Riska, C.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef]
- Sposito, A.C.; Chapman, M.J. Statin therapy in acute coronary syndromes, mechanistic insight into clinical benefit. Arter. Thromb. Vasc. Biol. 2002, 22, 1524–1534. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). New Equ. Estim. Glomerular Filtr. Rate. Ann. Intern. Med. 2009, 5, 604–612. [Google Scholar]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalizations. N. Eng. J. Med. 2004, 351, 1295–1305. [Google Scholar] [CrossRef]
- Krane, V.; Wanner, C. Statins, inflammation and kidney disease. Nat. Rev. Nephrol. 2011, 6, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L. Lipid disorders in uremia and dialysis. Contrib. Nephrol. 2012, 178, 100–105. [Google Scholar] [PubMed]
- Schiffrin, E.L.; Lipman, M.L.; Mann, J.F.E. Chronic kidney disease: Effects on the cardiovascular system. Circulation 2007, 116, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil HA, W.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; Fuller, J.H.; et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS) multicenter randomized placebo-controlled trial. Lancet 2004, 364, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Bohm, C.; Pandeya, S.; Gill, J.; Levin, A.; Kiberd, B.A. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am. J. Kidney Dis. 2001, 37, 484–489. [Google Scholar] [CrossRef]
- Nagata, I.; Ike, A.; Nishikawa, H.; Zhang, B.; Sugihara, M.; Mori, K.; Iwata, A.; Kawamura, A.; Shirai, K.; Uehara, Y.; et al. Associations beween lipid profile and MACE in hemodialysis patients with percutaneous coronary intervention: From the FU-Registry. J. Cardiol. 2015, 65, 105–111. [Google Scholar] [CrossRef]
- Natanzon, S.S.; Matetzky, S.; Beigel, R.; Iakobishvili, Z.; Goldenberg, I.; Shechter, M. Statin therapy among chronic kidney disease patients presenting with acute coronary syndrome. Atherosclerosis 2019, 286, 14–19. [Google Scholar] [CrossRef]
- Bologa, R.M.; Levine, D.M.; Parker, T.S.; Cheigh, J.S.; Serur, D.; Stenzel, K.H.; Rubin, A.L. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia and mortality in hemodialysis patients. Am. J. Kidney Dis. 1998, 32, 107–114. [Google Scholar] [CrossRef]
- Pekkanen, J.; Nissinen, A.; Vartiainen, E.; Salonen, J.T.; Punsar, S.; Karvonen, M.J. Changes in serum cholesterol level and mortality: A 30-year followup. The finish cohorts of the sevne countries study. Am. J. Epidemiol. 1994, 139, 155–165. [Google Scholar] [CrossRef]
- Krane, V.; Winkler, K.; Drechsler, C.; Lilienthal, J.; Marz, W.; Wanner, C. Association of LDL cholesterol and inflammation with cardiovascular events and mortality in patients with type 2 diabetes mellitus. Am. J. Kidney Dis. 2009, 54, 902–911. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Lindholm, B.; Stenvinkel, P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome. Nephrol. Dial. Transpl. 2002, 17, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Okyay, G.U.; İnal, S.; Öneç, K.; Er, R.E.; Paşaoğlu, Ö.; Paşaoğlu, H.; Derici, Ü.; Erten, Y. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren. Fail. 2013, 35, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K.; Guney, I.; Yerlikaya, F.H.; Tonbul, H.Z. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren. Fail. 2012, 34, 155–159. [Google Scholar] [CrossRef]
- Malhotra, R.; Marcelli, D.; von Gersdorff, G.; Grassmann, A.; Schaller, M.; Bayh, I.; Scatizzi, L.; Etter, M.; Guinsburg, A.; Barth, C.; et al. Relationship of neutrophil-to-lymphocyte ratio and serum albumin levels with C-reactive protein in hemodialysis patients: Results from two international cohort studies. Nephron 2015, 130, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, X.; Xiong, R.; Wang, S. High neutrophil-to-lymphocyte ratio predicts cardiovascular mortality in chronic hemodialysis patients. Mediat. Inflamm. 2017, 2017, 9327136. [Google Scholar] [CrossRef]
- Erdem, E.; Coşkun, K.A.; Karatas, A.; Dilek, M.; Akpolat, T. Neutrophil to lymphocyte ratio in predicting short-term mortality in hemodialysis patients. J. Exp. Clin. 2013, 30, 129–132. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, 529–555. [Google Scholar] [CrossRef]

| Variable | HD Group | Control Group (N = 103) | p-Value |
|---|---|---|---|
| (N = 246) | |||
| Age, years; mean ± SD | 72.4 ± 10.5 | 71.3 ± 11.8 | 0.68 |
| Male sex, n (%) | 168 (68.3) | 66 (64.1) | 0.45 |
| Diabetes, n (%) | 159 (64.9) | 40 (40.8) | <0.01 |
| Ischemic heart disease, n (%) | 175 (71.1) | 44 (44.9) | <0.01 |
| Heart failure, n (%) | 83 (33.7) | 12 (12.2) | <0.01 |
| Hypertension, n (%) | 231 (93.9) | 67 (68.4) | <0.01 |
| Previous stroke, n (%) | 53 (21.5) | 9 (9.2) | <0.01 |
| Atrial fibrillation, n (%) | 43 (17.6) | 8 (8.2) | 0.03 |
| Peripheral vascular disease, n (%) | 57 (23.2) | 6 (6.2) | <0.001 |
| Laboratory tests at admission for ACS hospitalization | |||
| WBC (K/microl) | 9.8 ± 7.9 | 10.4 ± 5.6 | 0.48 |
| Hemoglobin (g/dL) | 10.4 ± 1.7 | 12.7 ± 2.1 | <0.01 |
| Platelets (K/microl) | 234 ± 110 | 246 ± 87 | 0.34 |
| Neutrophil to lymphocyte ratio | 9 ± 10.6 | 7.4 ± 10.9 | 0.22 |
| C-reactive protein (mg/dL) | 7.9 ± 17.5 | 2.6 ± 4.7 | 0.01 |
| Serum creatinine (mg/dL) | 5.2 ± 11.8 | 1.1 ± 1 | <0.01 |
| Urea (mg/dL) | 118.9 ± 60.5 | 43.7 ± 18.1 | <0.01 |
| Albumin(g/dL) | 3.4 ± 2.3 | 3.7 ± 0.5 | 0.71 |
| Total cholesterol (mg/dL) | 153.1 ± 58.1 | 168 ± 55.2 | 0.03 |
| HDL-C (mg/dL) | 37.9 ± 14.3 | 40.6 ± 11.1 | 0.21 |
| Triglycerides (mg/dL) | 147.5 ± 80.2 | 157.2 ± 97.9 | 0.46 |
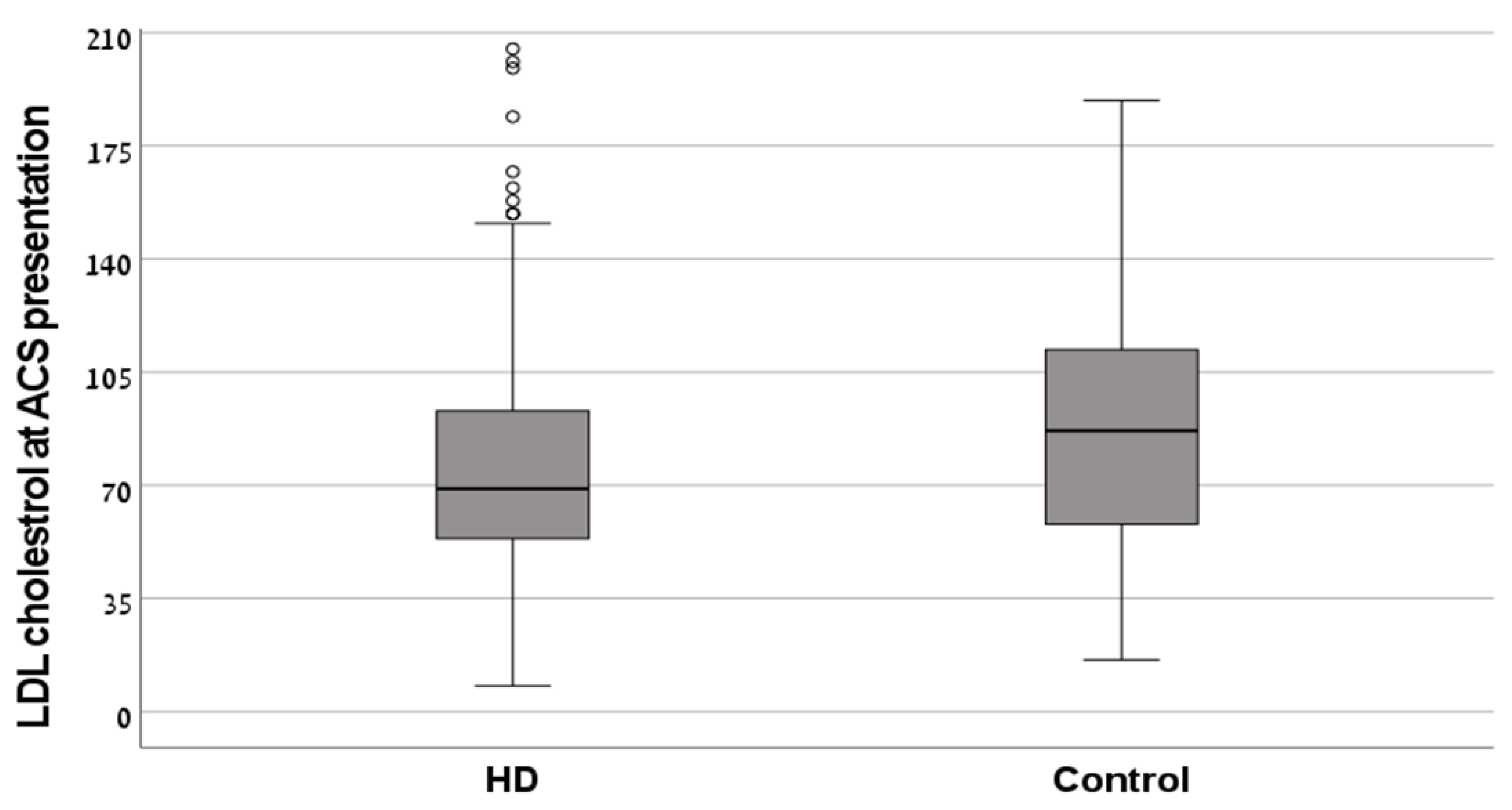
| LDL-C (mg/dL) | 77.2 ± 41.8 | 87.4 ± 36.1 | 0.11 |
| Variable | HD Group | Control Group | p-Value |
|---|---|---|---|
| N = 246 | N = 103 | ||
| LDL-C (mg/dL), mean ± SD | 77.2 ± 41.8 | 87.4 ± 36.1 | 0.11 |
| LDL-C < 70 mg/dL, N (%) | 80 (32.5) | 16 (15.5) | <0.01 |
| Statin treatment before, N (%) | 175 (71.1) | 61 (59.2) | 0.14 |
| Statin treatment after, N (%) | 212 (86.2) | 84 (81.6) | 0.92 |
| Coefficients | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| 30-day mortality | ||||
| Control group | Reference | |||
| HD group | 5.2 | 1.8 | 15 | <0.01 |
| 1-year mortality | ||||
| Control group | Reference | |||
| HD group | 3.4 | 1.9 | 6.1 | <0.01 |
| Coefficients | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Female sex | 0.907 | 0.489 | 1.681 | 0.756 |
| Diabetes | 0.783 | 0.436 | 1.406 | 0.413 |
| Ischemic heart disease | 1.633 | 0.865 | 3.083 | 0.13 |
| Heart failure | 2.765 | 1.468 | 5.208 | 0.002 |
| Previous stroke | 0.628 | 0.316 | 1.247 | 0.183 |
| Atrial fibrillation | 1.437 | 0.678 | 3.044 | 0.344 |
| Peripheral vascular disease | 0.748 | 0.38 | 1.469 | 0.399 |
| LDL-C < 70 (mg/dL) | 1.175 | 0.658 | 2.098 | 0.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen-Hagai, K.; Benchetrit, S.; Wand, O.; Grupper, A.; Shashar, M.; Solo, O.; Pereg, D.; Zitman-Gal, T.; Haskiah, F.; Erez, D. The Clinical Significance of LDL-Cholesterol on the Outcomes of Hemodialysis Patients with Acute Coronary Syndrome. Medicina 2023, 59, 1312. https://doi.org/10.3390/medicina59071312
Cohen-Hagai K, Benchetrit S, Wand O, Grupper A, Shashar M, Solo O, Pereg D, Zitman-Gal T, Haskiah F, Erez D. The Clinical Significance of LDL-Cholesterol on the Outcomes of Hemodialysis Patients with Acute Coronary Syndrome. Medicina. 2023; 59(7):1312. https://doi.org/10.3390/medicina59071312
Chicago/Turabian StyleCohen-Hagai, Keren, Sydney Benchetrit, Ori Wand, Ayelet Grupper, Moshe Shashar, Olga Solo, David Pereg, Tali Zitman-Gal, Feras Haskiah, and Daniel Erez. 2023. "The Clinical Significance of LDL-Cholesterol on the Outcomes of Hemodialysis Patients with Acute Coronary Syndrome" Medicina 59, no. 7: 1312. https://doi.org/10.3390/medicina59071312
APA StyleCohen-Hagai, K., Benchetrit, S., Wand, O., Grupper, A., Shashar, M., Solo, O., Pereg, D., Zitman-Gal, T., Haskiah, F., & Erez, D. (2023). The Clinical Significance of LDL-Cholesterol on the Outcomes of Hemodialysis Patients with Acute Coronary Syndrome. Medicina, 59(7), 1312. https://doi.org/10.3390/medicina59071312






