Intra-Cranial Arterial Calcifications in Hemodialysis Patients
Abstract
:1. Introduction
Aim
2. Materials and Methods
2.1. Study Design
2.2. Calcification Scoring
2.3. Study Population and Data Collection
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drew, D.A.; Bhadelia, R.; Tighiouart, H.; Novak, V.; Scott, T.M.; Lou, K.V.; Shaffi, K.; Weiner, D.E.; Sarnak, M.J. Anatomic brain disease in hemodialysis patients: A cross-sectional study. Am. J. Kidney Dis. 2013, 61, 271–278. [Google Scholar] [CrossRef]
- Pi, H.-C.; Xu, Y.-F.; Xu, R.; Yang, Z.-K.; Qu, Z.; Chen, Y.-Q.; Liu, G.-L.; Dong, J. Cognitive Impairment and Structural Neuroimaging Abnormalities Among Patients with Chronic Kidney Disease. Kidney Blood Press. Res. 2016, 41, 986–996. [Google Scholar] [CrossRef]
- Savazzi, G.M.; Cusmano, F.; Musini, S. Cerebral imaging changes in patients with chronic renal failure treated conservatively or in hemodialysis. Nephron 2001, 89, 31–36. [Google Scholar] [CrossRef]
- Iwasa, Y.; Otsubo, S.; Nomoto, K.; Yashiro, N.; Yajima, A.; Kimata, N.; Akiba, T.; Nitta, K. Prevalence of intracranial artery calcification in hemodialysis patients—A case-control study. Int. Urol. Nephrol. 2012, 44, 1223–1228. [Google Scholar] [CrossRef]
- Bugnicourt, J.-M.; Chillon, J.-M.; Massy, Z.A.; Canaple, S.; Lamy, C.; Deramond, H.A.A.; Godefroy, O. High prevalence of intracranial artery calcification in stroke patients with CKD: A retrospective study. Clin. J. Am. Soc. Nephrol. 2009, 4, 284–290. [Google Scholar] [CrossRef]
- de Weert, T.; Cakir, H.; Rozie, S.; Cretier, S.; Meijering, E.; Dippel, D.; van der Lugt, A. Intracranial internal carotid artery calcifications: Association with vascular risk factors and ischemic cerebrovascular disease. Am. J. Neuroradiol. 2009, 30, 177–184. [Google Scholar] [CrossRef]
- Wu, X.H.; Chen, X.-Y.; Wang, L.J.; Wong, K.S. Intracranial Artery Calcification and Its Clinical Significance. J. Clin. Neurol. 2016, 12, 253–261. [Google Scholar] [CrossRef]
- Bugnicourt, J.-M.; Leclercq, C.; Chillon, J.-M.; Diouf, M.; Deramond, H.; Canaple, S.; Lamy, C.; Massy, Z.A.; Godefroy, O. Presence of intracranial artery calcification is associated with mortality and vascular events in patients with ischemic stroke after hospital discharge: A cohort study. Stroke 2011, 42, 3447–3453. [Google Scholar] [CrossRef]
- Power, A.; Chan, K.; Haydar, A.; Hamady, M.; Cairns, T.; Taube, D.; Duncan, N. Intracranial arterial calcification is highly prevalent in hemodialysis patients but does not associate with acute ischemic stroke. Hemodial. Int. 2011, 15, 256–263. [Google Scholar] [CrossRef]
- Jablonski, K.L.; Chonchol, M. Vascular calcification in end-stage renal disease. Hemodial. Int. 2013, 17 (Suppl. 1), S17–S21. [Google Scholar] [CrossRef]
- Babiarz, L.S.; Yousem, D.M.; Wasserman, B.A.; Wu, C.; Bilker, W.; Beauchamp, N., Jr. Cavernous carotid artery calcification and white matter ischemia. Am. J. Neuroradiol. 2003, 24, 872–877. [Google Scholar]
- Cohen-Hagai, K.; Fanadka, F.; Grumberg, T.; Topaz, G.; Nacasch, N.; Greenberg, M.; Zitman-Gal, T.; Benchetrit, S. Diastolic blood pressure is associated with brain atrophy in hemodialysis patients: A single center case–control study. Ther. Apher. Dial. 2022, 26, 94–102. [Google Scholar] [CrossRef]
- Fanadka, F.; Grumberg, T.; Topaz, G.; Benchetrit, S.; Zitman-Gal, T.; Wand, O.; Cohen-Hagai, K. Intracranial and heart valve calcifications in hemodialysis patients—Interrelationship and clinical impact. Hemodial. Int. 2022, 26, 527–532. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, Y.-K.; Cho, A.J.; Park, H.C.; Han, C.H.; Choi, M.-J.; Koo, J.-R.; Yoon, J.-W.; Noh, J.W. Malnutrition, inflammation, progression of vascular calcification and survival: Inter-relationships in hemodialysis patients. PLoS ONE 2019, 14, e0216415. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Towler, D.; Slatopolsky, E. Vascular calcification: The killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 1453–1464. [Google Scholar] [CrossRef]
- Grant, P.J.; Cosentino, F.; Marx, N. Diabetes and coronary artery disease: Not just a risk factor. Heart 2020, 106, 1357–1364. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Nigro, J.; Osman, N.; Dart, A.M.; Little, P.J. Insulin resistance and atherosclerosis. Endocr. Rev. 2006, 27, 242–259. [Google Scholar] [CrossRef]
- Chopra, S.; Peter, S. Screening for coronary artery disease in patients with type 2 diabetes mellitus: An evidence-based review. Indian. J. Endocrinol. Metab. 2012, 16, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Bloch, D.B.; Nazarian, R.M.; Vermeer, C.; Booth, S.L.; Xu, D.; Thadhani, R.I.; Malhotra, R. Vitamin K-Dependent Carboxylation of Matrix Gla Protein Influences the Risk of Calciphylaxis. J. Am. Soc. Nephrol. 2017, 28, 1717–1722. [Google Scholar] [CrossRef]
- Annweiler, G.; Labriffe, M.; Ménager, P.; Ferland, G.; Brangier, A.; Annweiler, C. Intracranial calcifications under vitamin K antagonists or direct oral anticoagulants: Results from the French VIKING study in older adults. Maturitas 2020, 132, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Topaz, G.; Yehezkel, E.; Benchetrit, S.; Korzets, Z.; Arnson, Y.; Kitay-Cohen, Y.; Pereg, D.; Cohen-Hagai, K. Non-coronary cardiac calcifications and outcomes in patients with heart failure. J. Cardiol. 2021, 77, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Anazodo, U.C.; Wong, D.Y.; Théberge, J.; Dacey, M.; Gomes, J.; Penny, J.D.; van Ginkel, M.; Poirier, S.E.; McIntyre, C.W. Hemodialysis-Related Acute Brain Injury Demonstrated by Application of Intradialytic Magnetic Resonance Imaging and Spectroscopy. J. Am. Soc. Nephrol. 2023, 34, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.C.; McNair, R.; Figg, N.; Skepper, J.N.; Schurgers, L.; Gupta, A.; Hiorns, M.; Donald, A.E.; Deanfield, J.; Rees, L.; et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008, 118, 1748–1757. [Google Scholar] [CrossRef]
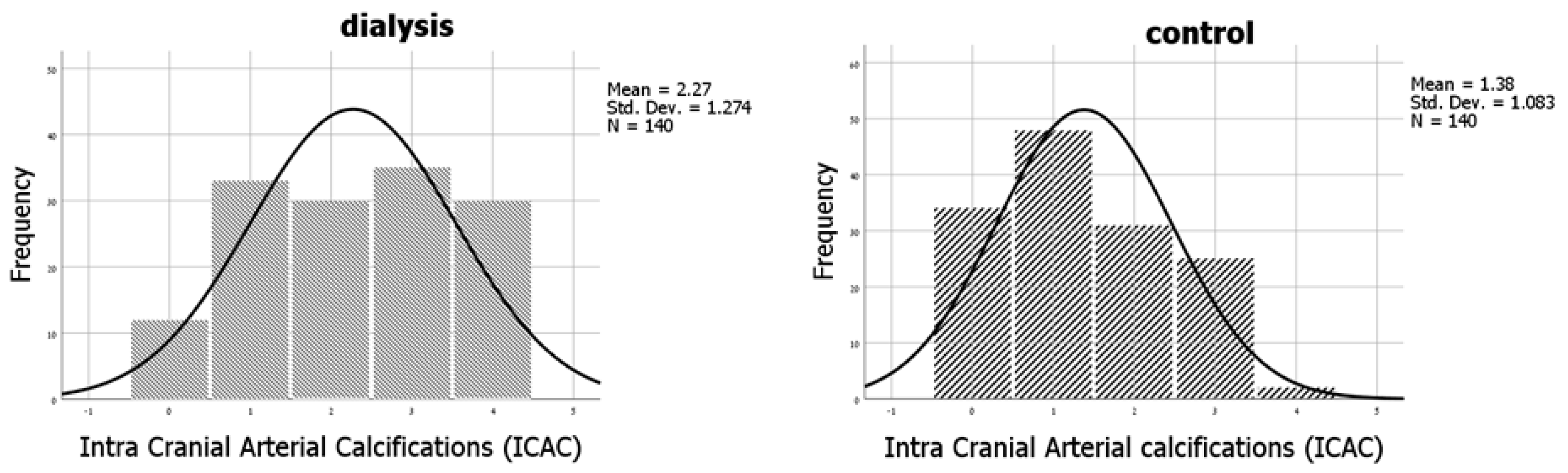
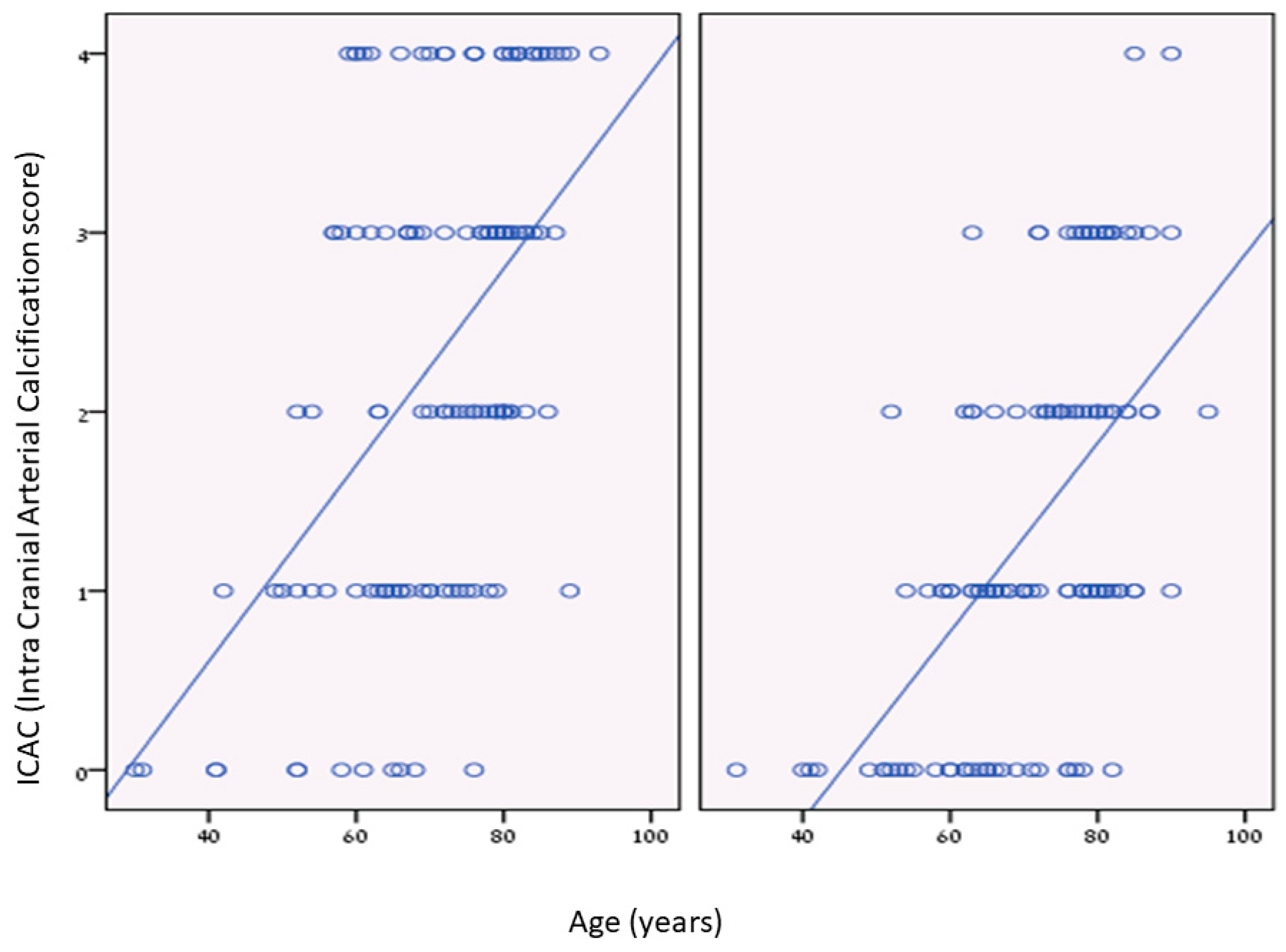
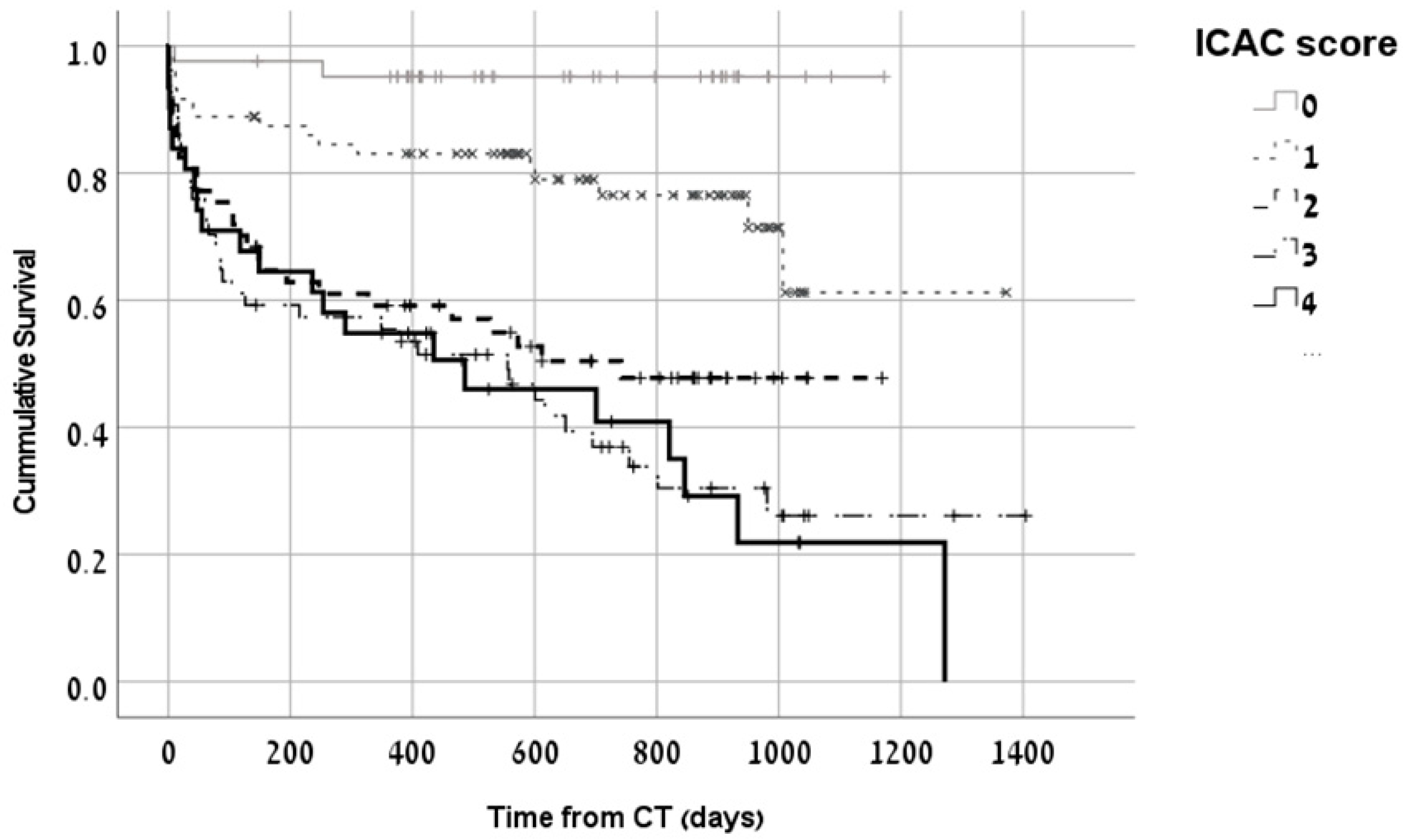
| Characteristic | ICAC Score 0 (n = 46) | ICAC Score 1,2 (n = 142) | ICAC Score 3,4 (n = 92) | p-Value * |
|---|---|---|---|---|
| Age, years | 57 ± 14 | 72 ± 10 | 77 ± 9 | <0.01 |
| Male sex | 27 (58.7) | 90 (63.4) | 53 (57.6) | 0.65 |
| Dialysis patients | 12 (26.1) | 63 (44.4) | 65 (70.7) | <0.01 |
| Dialysis vintage (months) | 30 ± 70 | 10 ± 13 | 24 ± 26 | 0.148 |
| Comorbidities | ||||
| Ischemic heart disease | 5 (10.9) | 37 (26.1) | 40 (44.4) | <0.01 |
| Heart failure | 3 (6.5) | 21 (14.8) | 32 (35.2) | <0.01 |
| Atrial Fibrillation | 3 (6.5) | 20 (14.1) | 23 (25.3) | 0.01 |
| Prior stroke | 10 (21.7) | 38 (26.8) | 31 (34.1) | 0.27 |
| Diabetes mellitus | 10 (21.7) | 58 (40.8) | 59 (64.8) | <0.01 |
| Peripheral vascular disease | 1 (2.2) | 10 (7.0) | 13 (14.3) | 0.04 |
| Hypertension | 22 (47.8) | 108 (76.1) | 76 (83.5) | <0.01 |
| Chronic medications | ||||
| Aspirin | 16 (34.8) | 72 (50.7) | 57 (63.3) | <0.01 |
| Warfarin | 1 (2.2) | 5 (3.5) | 9 (10.0) | 0.06 |
| Statins | 16 (34.8) | 66 (46.5) | 47 (52.2) | 0.16 |
| Calcium Carbonate | 6 (13.0) | 24 (16.9) | 20 (22.2) | 0.38 |
| Non-calcium-based phosphorus binders | 3 (6.5) | 10 (7.0) | 13 (14.1) | 0.15 |
| Beta Blockers | 10 (21.7) | 64 (45.1) | 48 (53.3) | <0.01 |
| ACEI/ARB | 8 (17.4) | 42 (29.6) | 32 (35.6) | 0.09 |
| Calcium channel blockers | 10 (21.7) | 40 (28.2) | 35 (38.9) | 0.08 |
| Laboratory | ||||
| Hemoglobin (g/dL) | 13.0 ± 1.8 | 12.0 ± 2.0 | 10.7 ± 2.0 | <0.01 |
| Albumin (gram/dL) | 3.3 ± 0.7 | 3.4 ± 0.6 | 3.4 ± 0.6 | 0.6 |
| Creatinine (mg/dL) | 3.8 ± 12 | 2.9 ± 2.5 | 4.1 ± 2.6 | 0.2 |
| Total calcium (mg/dL) | 8.8 ± 0.9 | 8.9 ± 0.9 | 8.5 ± 0.8 | 0.04 |
| Phosphorus (mg/dL) | 4.1 ± 1.7 | 4.5 ± 2 | 5.2 ± 1.6 | 0.005 |
| Calcium phosphorus product | 35 ± 12 | 39 ± 16 | 44 ± 13 | 0.006 |
| PTH (pg/mL) | 268± 251 | 308 ± 187 | 314 ± 178 | 0.8 |
| C reactive protein (mg/dL) | 6.3 ± 9 | 6.6 ± 10 | 4.8 ± 6.7 | 0.3 |
| Coefficients | Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| ICAC = 0 | Reference | |||
| ICAC = 1 | 3 | 0.6 | 14.7 | 0.2 |
| ICAC = 2 | 9.3 | 2 | 44 | 0.005 |
| ICAC = 3 | 8.4 | 1.8 | 40 | 0.007 |
| ICAC = 4 | 8.2 | 1.6 | 43 | 0.01 |
| Hypertension | 1.4 | 0.6 | 3.3 | 0.4 |
| Diabetes mellitus | 1.0 | 0.5 | 2 | 0.9 |
| Atrial fibrillation | 0.7 | 0.3 | 1.6 | 0.4 |
| Congestive heart failure | 2.4 | 1.2 | 4.9 | 0.02 |
| Ischemic heart disease | 2.4 | 1.3 | 4.5 | 0.006 |
| Peripheral vascular disease | 1.6 | 0.6 | 4.3 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanadka, F.; Rozenberg, I.; Nacasch, N.; Einbinder, Y.; Benchetrit, S.; Wand, O.; Hod, T.; Cohen-Hagai, K. Intra-Cranial Arterial Calcifications in Hemodialysis Patients. Medicina 2023, 59, 1706. https://doi.org/10.3390/medicina59101706
Fanadka F, Rozenberg I, Nacasch N, Einbinder Y, Benchetrit S, Wand O, Hod T, Cohen-Hagai K. Intra-Cranial Arterial Calcifications in Hemodialysis Patients. Medicina. 2023; 59(10):1706. https://doi.org/10.3390/medicina59101706
Chicago/Turabian StyleFanadka, Feda, Ilan Rozenberg, Naomi Nacasch, Yael Einbinder, Sydney Benchetrit, Ori Wand, Tammy Hod, and Keren Cohen-Hagai. 2023. "Intra-Cranial Arterial Calcifications in Hemodialysis Patients" Medicina 59, no. 10: 1706. https://doi.org/10.3390/medicina59101706
APA StyleFanadka, F., Rozenberg, I., Nacasch, N., Einbinder, Y., Benchetrit, S., Wand, O., Hod, T., & Cohen-Hagai, K. (2023). Intra-Cranial Arterial Calcifications in Hemodialysis Patients. Medicina, 59(10), 1706. https://doi.org/10.3390/medicina59101706






