Identification of Genes Associated with Prognosis and Immunotherapy Prediction in Triple-Negative Breast Cancer via M1/M2 Macrophage Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. TNBC Dataset and Pretreatment
2.2. Identification of M1/M2 Macrophage-Related Genes and Clustering
2.3. Inference of Immune Infiltration Microenvironment
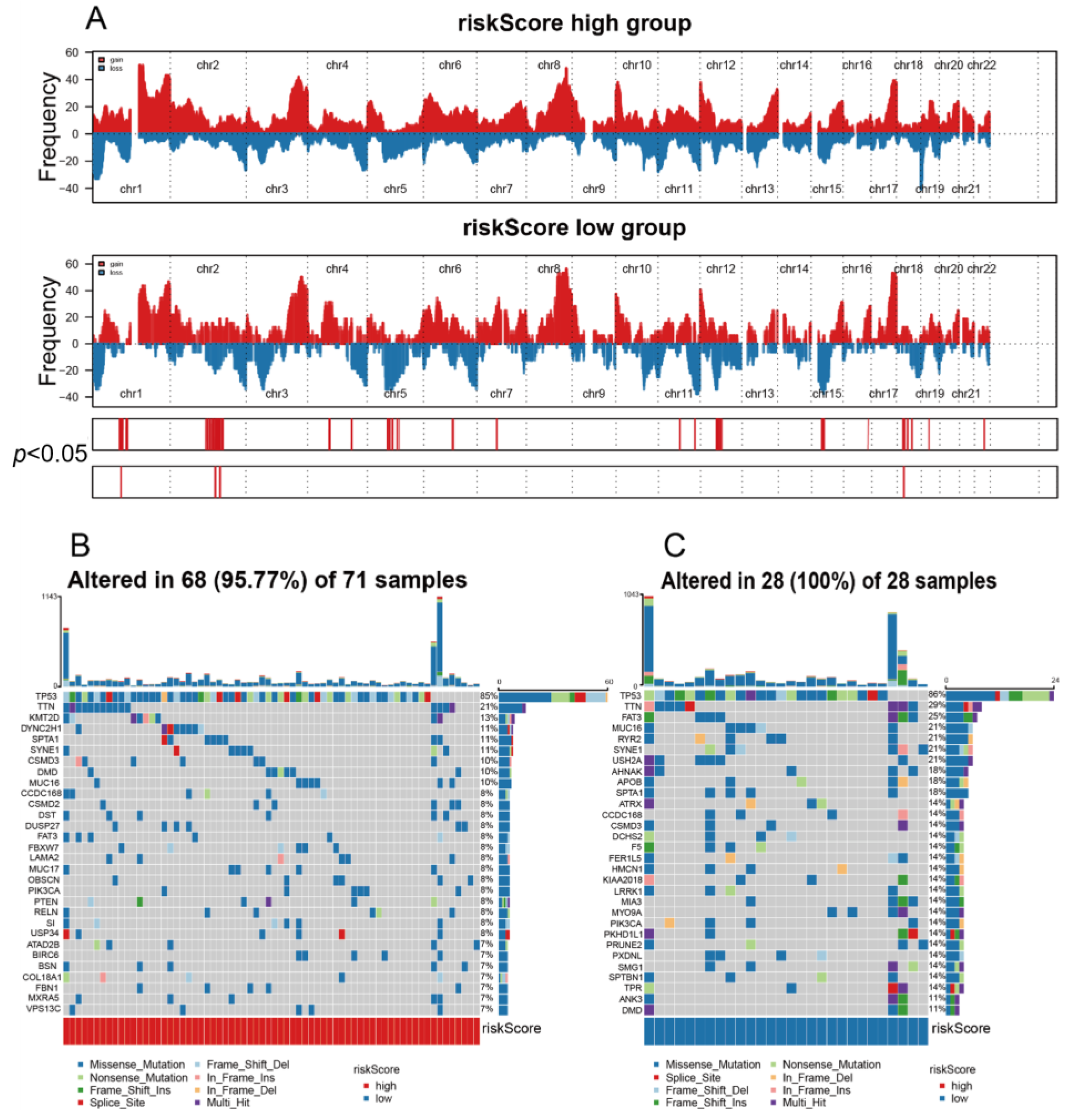
2.4. Genomic Alterations in Grouping
2.5. Functional and Pathway Enrichment Analysis
2.6. Prediction of Immunotherapy Response
2.7. Prediction of Immunotherapy Sensitivity
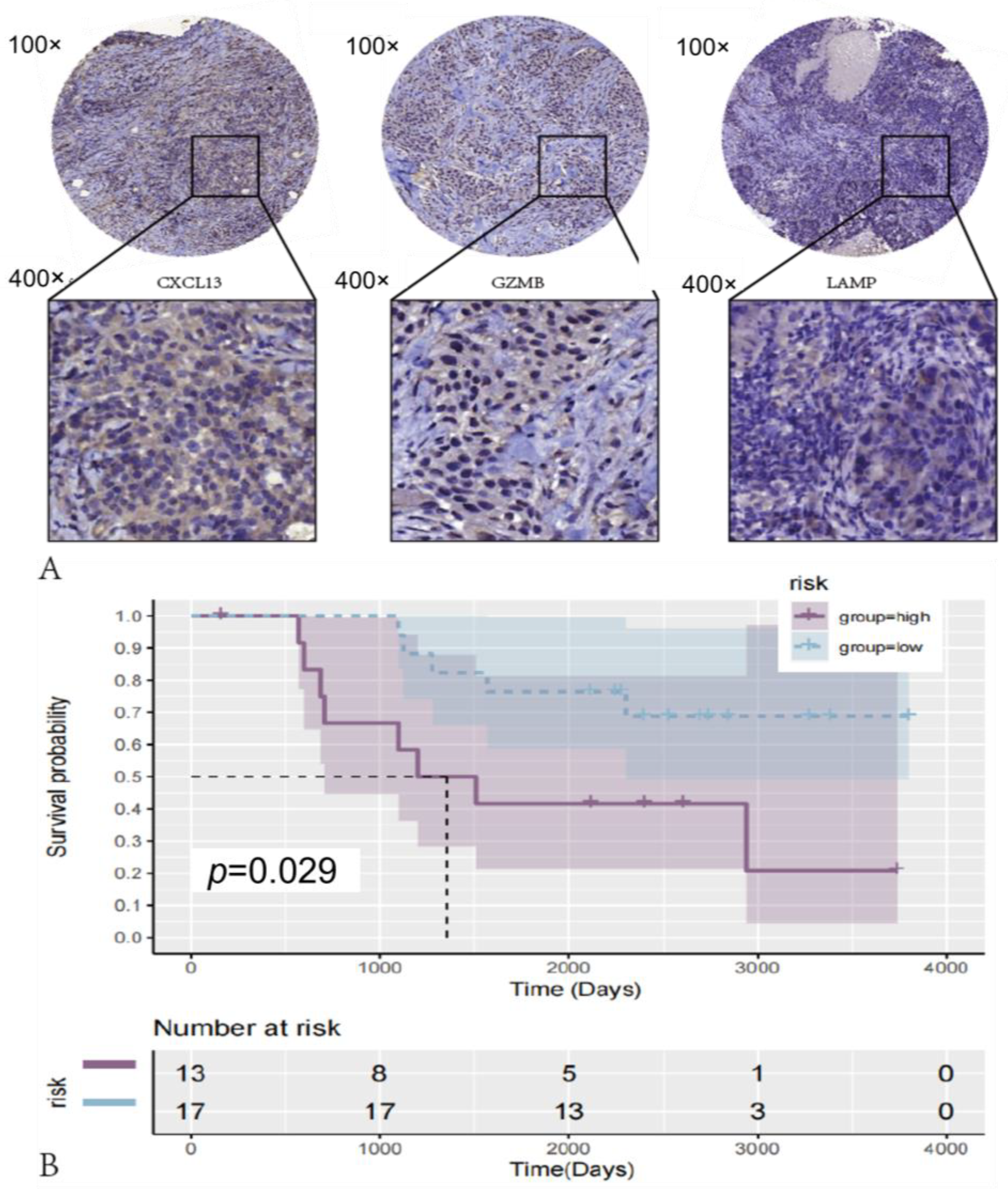
2.8. Immunohistochemistry
2.9. Survival Analysis
3. Results
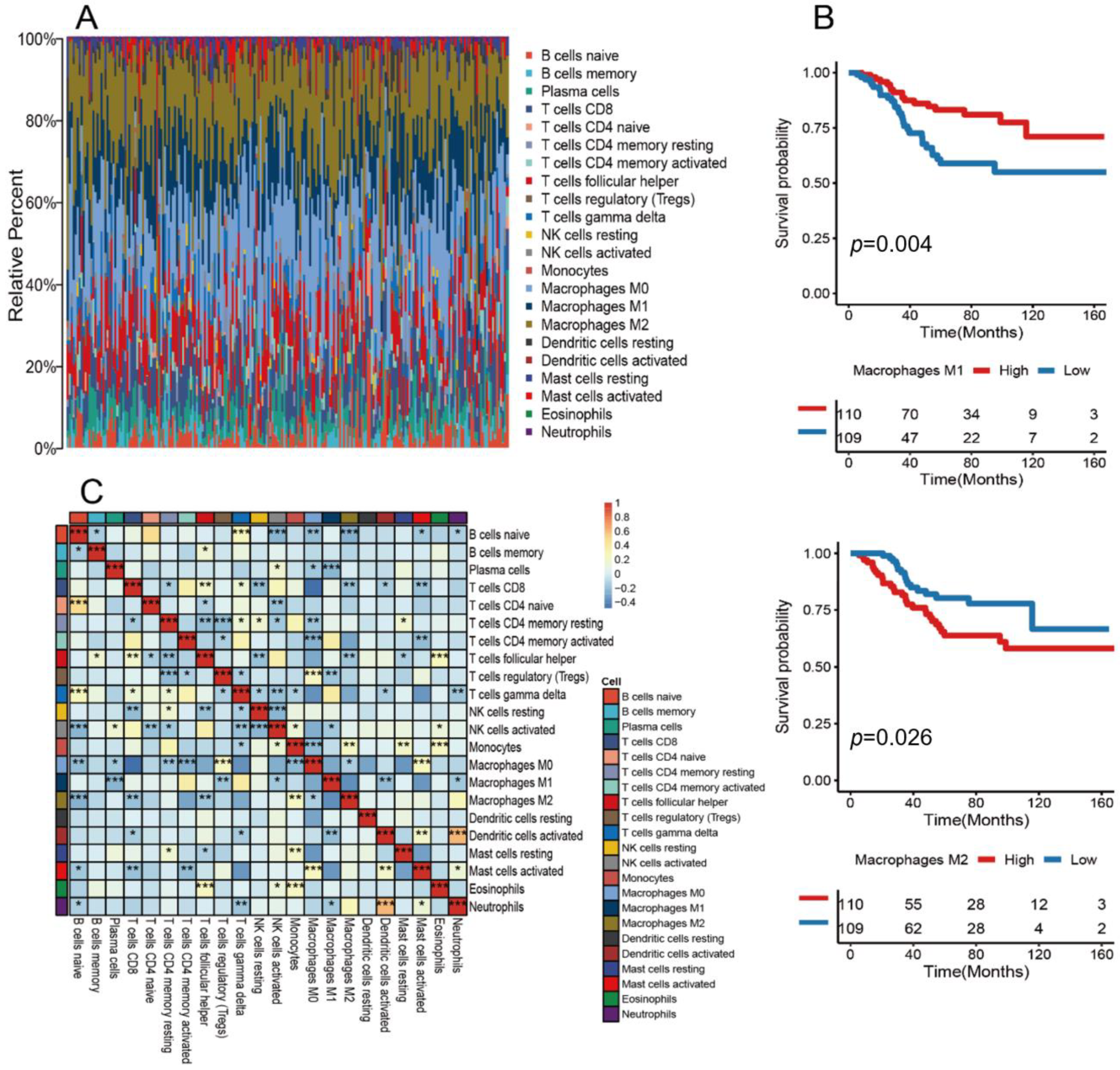
3.1. Landscape of the Tumor Immune Microenvironment in TNBC
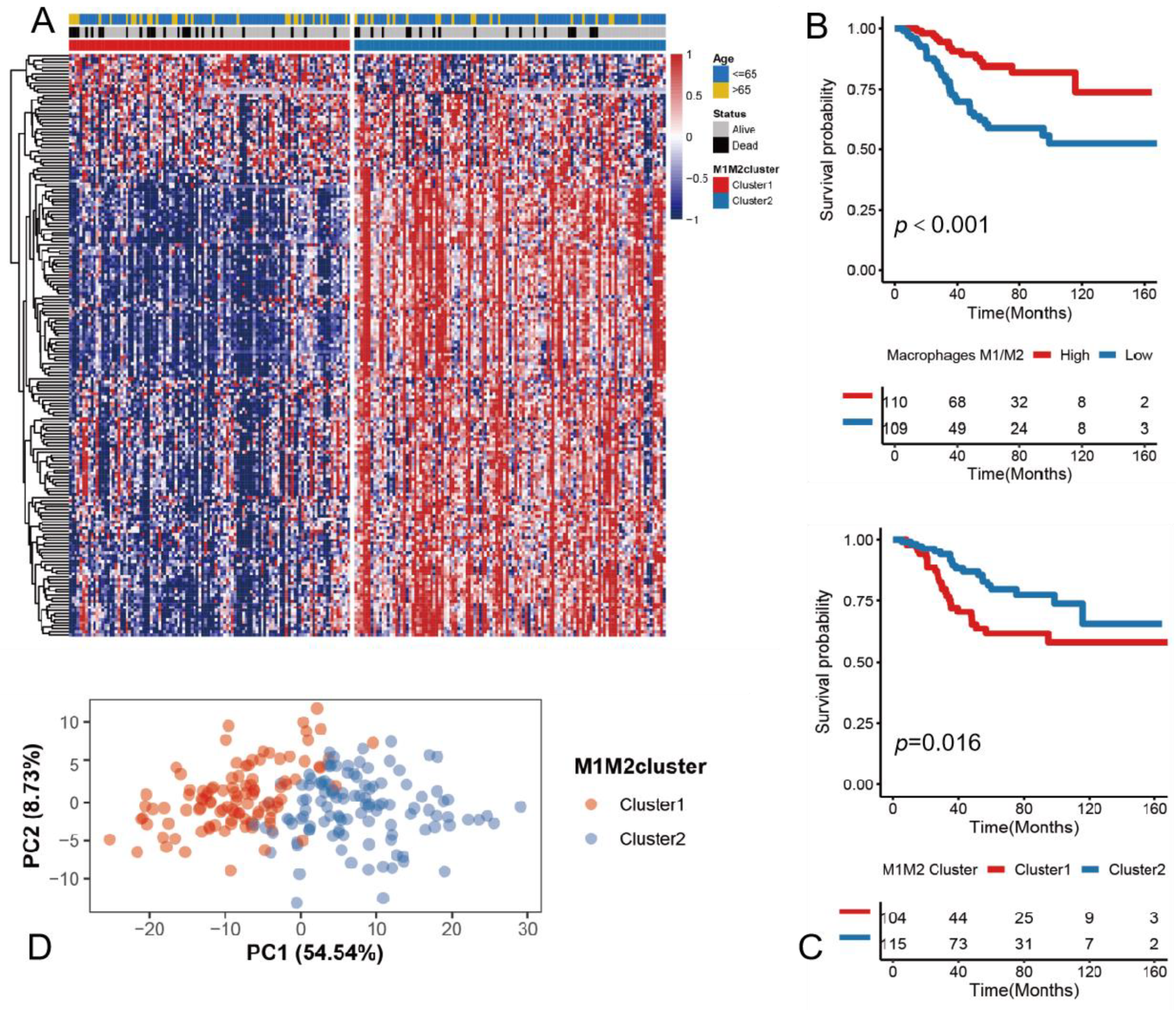
3.2. Determining the M1/M2 Macrophage Ratio as a Potential Prognostic Marker
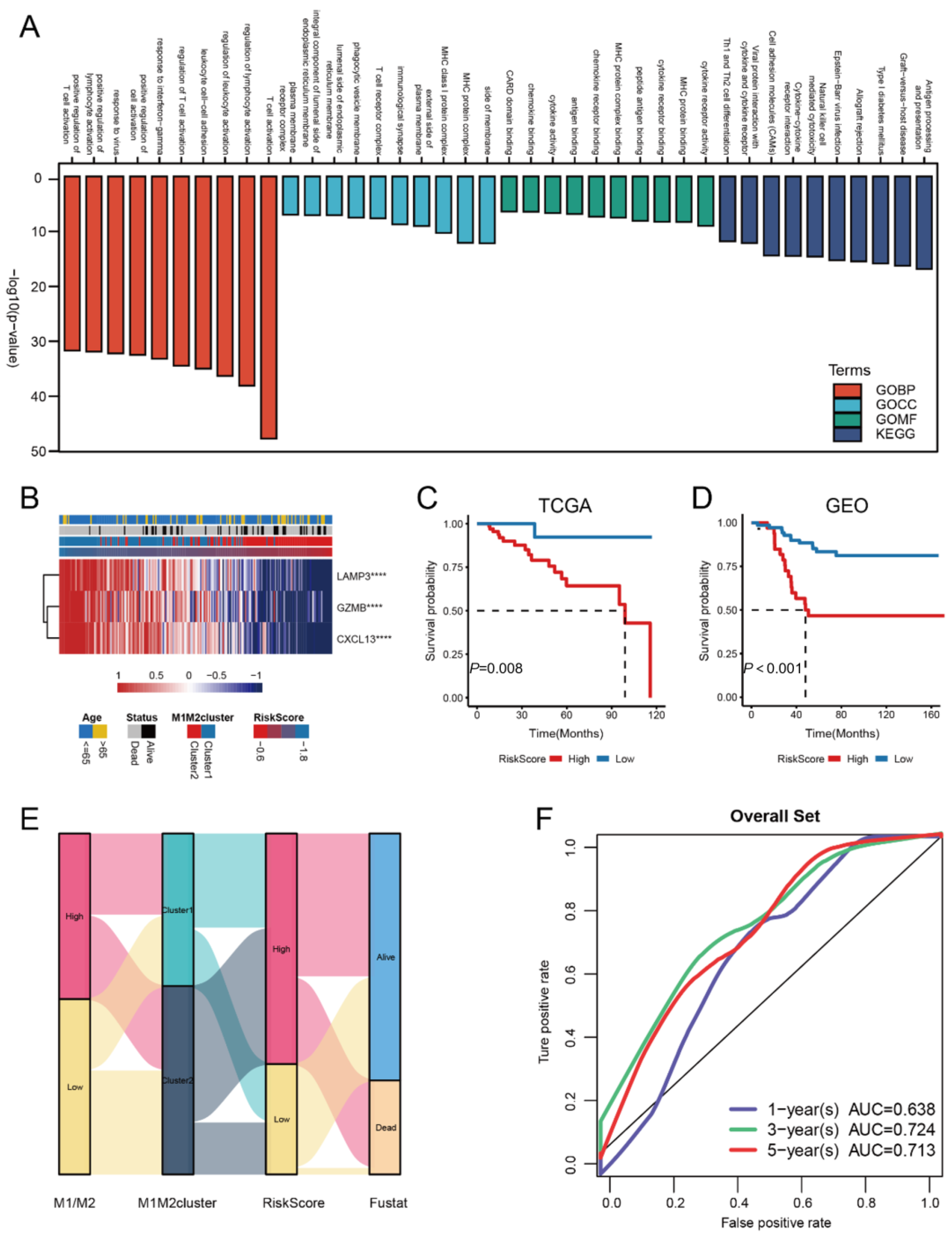
3.3. Construction of the riskScore Model and Its Functional Annotation
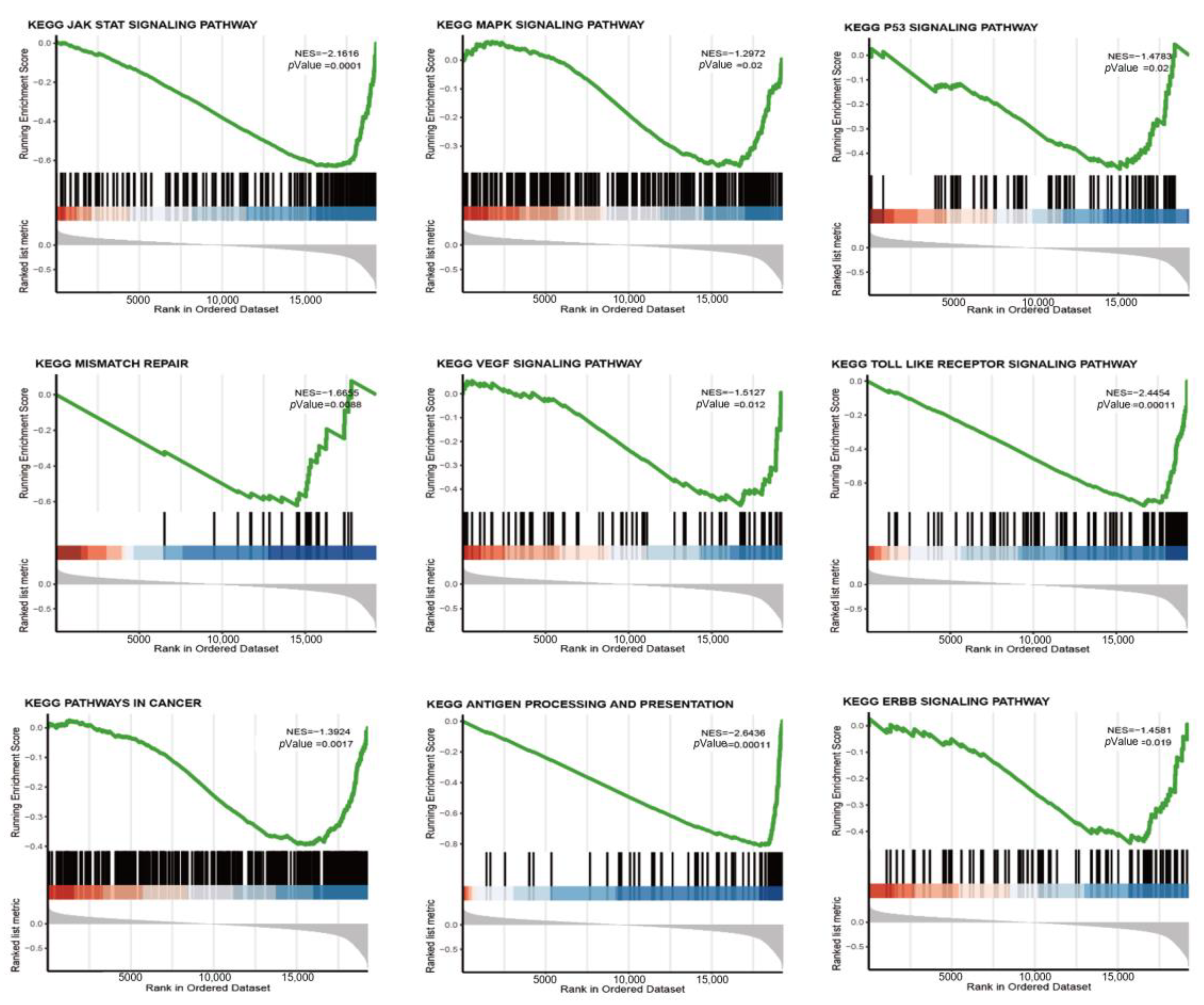
3.4. riskScore Model-Related GSEA Enrichment Pathways
3.5. Correlation Study of TME Characteristics, Clinical Characteristics, and riskScore
3.6. The High riskScore Group Showed More Malignant Genomic Features
3.7. riskScore Group Predicts Response to Immunotherapy
3.8. riskScore Model Validation in Clinical Sample Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| BC | Breast cancer |
| ChIP | Chromatin immunoprecipitation |
| CNV | Copy number variation |
| CR | Complete response |
| CSF1R | Colony-stimulating factor 1 receptor |
| CTCs | Circulating tumor cells |
| DCs | Dendritic cells |
| DEGs | Differentially expressed genes |
| ER | Estrogen receptor |
| G-CSF | Granulocyte colony-stimulating factor |
| HDAC2 | Histone deacetylase 2 |
| HER2 | Human epidermal growth factor receptor 2 |
| ICIs | Immune checkpoint inhibitors |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| IPS | ImmunophenotypeScore |
| IFN-γ | Interferon-γ |
| OS | Overall survival |
| PCA | Principal component analysis |
| PD | Progressive disease |
| PR | Partial response |
| PR | Progesterone receptor |
| ROC | Receiver operating characteristic |
| SD | Stable disease |
| SNPs | Single-nucleotide polymorphisms |
| SNVs | Single-nucleotide variants |
| TAMs | Tumor-associated macrophages |
| Th1 | Type 1 T helper cell |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| TNF | Tumor necrosis factor |
| Tregs | Regulatory T cells |
References
- Bergin, A.R.T.; Loi, S. Triple-negative breast cancer: Recent treatment advances. F1000Research 2019, 8, 1342. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Liu, L.; Gong, C.-Y.; Shi, H.-S.; Zeng, Y.-H.; Wang, X.-Z.; Zhao, Y.-W.; Wei, Y.-Q. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS ONE 2012, 7, e50946. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2017, 147, 181–187. [Google Scholar] [CrossRef]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. Theor. Den Biowiss. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Chan, C.-H.; Li, C.-F.; Yang, W.-L.; Gao, Y.; Lee, S.-W.; Feng, Z.; Huang, H.-Y.; Tsai, K.K.C.; Flores, L.G.; Shao, Y.; et al. The Skp2-SCF E3 Ligase Regulates Akt Ubiquitination, Glycolysis, Herceptin Sensitivity, and Tumorigenesis. Cell 2012, 151, 913–914. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Chen, X.; Zhang, Q.; Hong, J. Novel Immune-Related Gene Signature for Risk Stratification and Prognosis of Survival in Lower-Grade Glioma. Front. Genet. 2020, 11, 363. [Google Scholar] [CrossRef]
- Lin, J.; Li, M.; Wang, Z.; He, S.; Ma, X.; Li, D. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J. Lipid Res. 2010, 51, 1208–1217. [Google Scholar] [CrossRef]
- Jung, M.K.; Lee, J.S.; Kwak, J.-E.; Shin, E.-C. Tumor Necrosis Factor and Regulatory T Cells. Yonsei Med. J. 2019, 60, 126–131. [Google Scholar] [CrossRef]
- Aharinejad, S.; Paulus, P.; Sioud, M.; Hofmann, M.; Zins, K.; Schäfer, R.; Stanley, E.R.; Abraham, D. Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res. 2004, 64, 5378–5384. [Google Scholar] [CrossRef]
- Germano, G.; Frapolli, R.; Belgiovine, C.; Anselmo, A.; Pesce, S.; Liguori, M.; Erba, E.; Uboldi, S.; Zucchetti, M.; Pasqualini, F.; et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013, 23, 249–262. [Google Scholar] [CrossRef]
- Burr, M.L.; Sparbier, C.E.; Chan, Y.-C.; Williamson, J.C.; Woods, K.; Beavis, P.A.; Lam, E.Y.N.; Henderson, M.A.; Bell, C.C.; Stolzenburg, S.; et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 2017, 549, 101–105. [Google Scholar] [CrossRef]
- George, S.; Miao, D.; Demetri, G.D.; Adeegbe, D.; Rodig, S.J.; Shukla, S.; Lipschitz, M.; Amin-Mansour, A.; Raut, C.P.; Carter, S.L.; et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity 2017, 46, 197–204. [Google Scholar] [CrossRef]
- Wang, G.; Chow, R.D.; Zhu, L.; Bai, Z.; Ye, L.; Zhang, F.; Renauer, P.A.; Dong, M.B.; Dai, X.; Zhang, X.; et al. CRISPR-GEMM Pooled Mutagenic Screening Identifies KMT2D as a Major Modulator of Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1912–1933. [Google Scholar] [CrossRef]
- Klingen, T.A.; Chen, Y.; Aas, H.; Wik, E.; Akslen, L.A. Tumor-associated macrophages are strongly related to vascular invasion, non-luminal subtypes, and interval breast cancer. Hum. Pathol. 2017, 69, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–19: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Ren, X. Cancer immunology and immunotherapy. Cancer Biol. Med. 2021, 18, 931–933. [Google Scholar] [CrossRef]
- Baldominos, P.; Barbera-Mourelle, A.; Barreiro, O.; Huang, Y.; Wight, A.; Cho, J.-W.; Zhao, X.; Estivill, G.; Adam, I.; Sanchez, X.; et al. Quiescent cancer cells resist T cell attack by forming an immunosuppressive niche. Cell 2022, 185, 1694–1708.e19. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef]
- Ge, J.; Zuo, W.; Chen, Y.; Shao, Z.; Yu, K. The advance of adjuvant treatment for triple-negative breast cancer. Cancer Biol. Med. 2021, 19, 187–201. [Google Scholar] [CrossRef]
- Miyasato, Y.; Shiota, T.; Ohnishi, K.; Pan, C.; Yano, H.; Horlad, H.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Iwase, H.; Takeya, M.; et al. High density of CD204-positive macrophages predicts worse clinical prognosis in patients with breast cancer. Cancer Sci. 2017, 108, 1693–1700. [Google Scholar] [CrossRef]
- Guerriero, J.L.; Sotayo, A.; Ponichtera, H.E.; Castrillon, J.A.; Pourzia, A.L.; Schad, S.; Johnson, S.F.; Carrasco, R.D.; Lazo, S.; Bronson, R.T.; et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 2017, 543, 428–432. [Google Scholar] [CrossRef]
- Olson, O.C.; Kim, H.; Quail, D.F.; Foley, E.A.; Joyce, J.A. Tumor-Associated Macrophages Suppress the Cytotoxic Activity of Antimitotic Agents. Cell Rep. 2017, 19, 101–113. [Google Scholar] [CrossRef]
- Liang, S.; Chen, Z.; Jiang, G.; Zhou, Y.; Liu, Q.; Su, Q.; Wei, W.; Du, J.; Wang, H. Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-κB/IL-6 signals. Cancer Lett. 2017, 386, 12–23. [Google Scholar] [CrossRef]
- D’Esposito, V.; Liguoro, D.; Ambrosio, M.R.; Collina, F.; Cantile, M.; Spinelli, R.; Raciti, G.A.; Miele, C.; Valentino, R.; Campiglia, P.; et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget 2016, 7, 24495–24509. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Deng, Y.; Liu, Z.; Li, X.; Zhang, M.; Yu, X.; Liu, T.; Chen, K.; Li, Z. Identification of Genes Associated with Prognosis and Immunotherapy Prediction in Triple-Negative Breast Cancer via M1/M2 Macrophage Ratio. Medicina 2023, 59, 1285. https://doi.org/10.3390/medicina59071285
Liu J, Deng Y, Liu Z, Li X, Zhang M, Yu X, Liu T, Chen K, Li Z. Identification of Genes Associated with Prognosis and Immunotherapy Prediction in Triple-Negative Breast Cancer via M1/M2 Macrophage Ratio. Medicina. 2023; 59(7):1285. https://doi.org/10.3390/medicina59071285
Chicago/Turabian StyleLiu, Jianyu, Yuhan Deng, Zhuolin Liu, Xue Li, Mingxuan Zhang, Xin Yu, Tong Liu, Kexin Chen, and Zhigao Li. 2023. "Identification of Genes Associated with Prognosis and Immunotherapy Prediction in Triple-Negative Breast Cancer via M1/M2 Macrophage Ratio" Medicina 59, no. 7: 1285. https://doi.org/10.3390/medicina59071285
APA StyleLiu, J., Deng, Y., Liu, Z., Li, X., Zhang, M., Yu, X., Liu, T., Chen, K., & Li, Z. (2023). Identification of Genes Associated with Prognosis and Immunotherapy Prediction in Triple-Negative Breast Cancer via M1/M2 Macrophage Ratio. Medicina, 59(7), 1285. https://doi.org/10.3390/medicina59071285






