Association of Non-Alcoholic Fatty Liver Disease and Metabolic-Associated Fatty Liver Disease with COVID-19-Related Intensive Care Unit Outcomes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods and Materials
2.1. Search and Selection
2.2. Data Extraction
- General information: author, title, DOI, and year of publication.
- Study characteristics: study site/country, study period, number of centers, journal, study design, and severe COVID-19 definition.
- Participant characteristics: number of MAFLD and non-MAFLD patients and the demographic characteristics and method of assessment of MAFLD.
- Outcomes: ICU admission, invasive mechanical ventilation, and severity of disease in MAFLD and non-MAFLD patients.
2.3. Statistical Analysis
2.4. Quality Assessment
3. Results
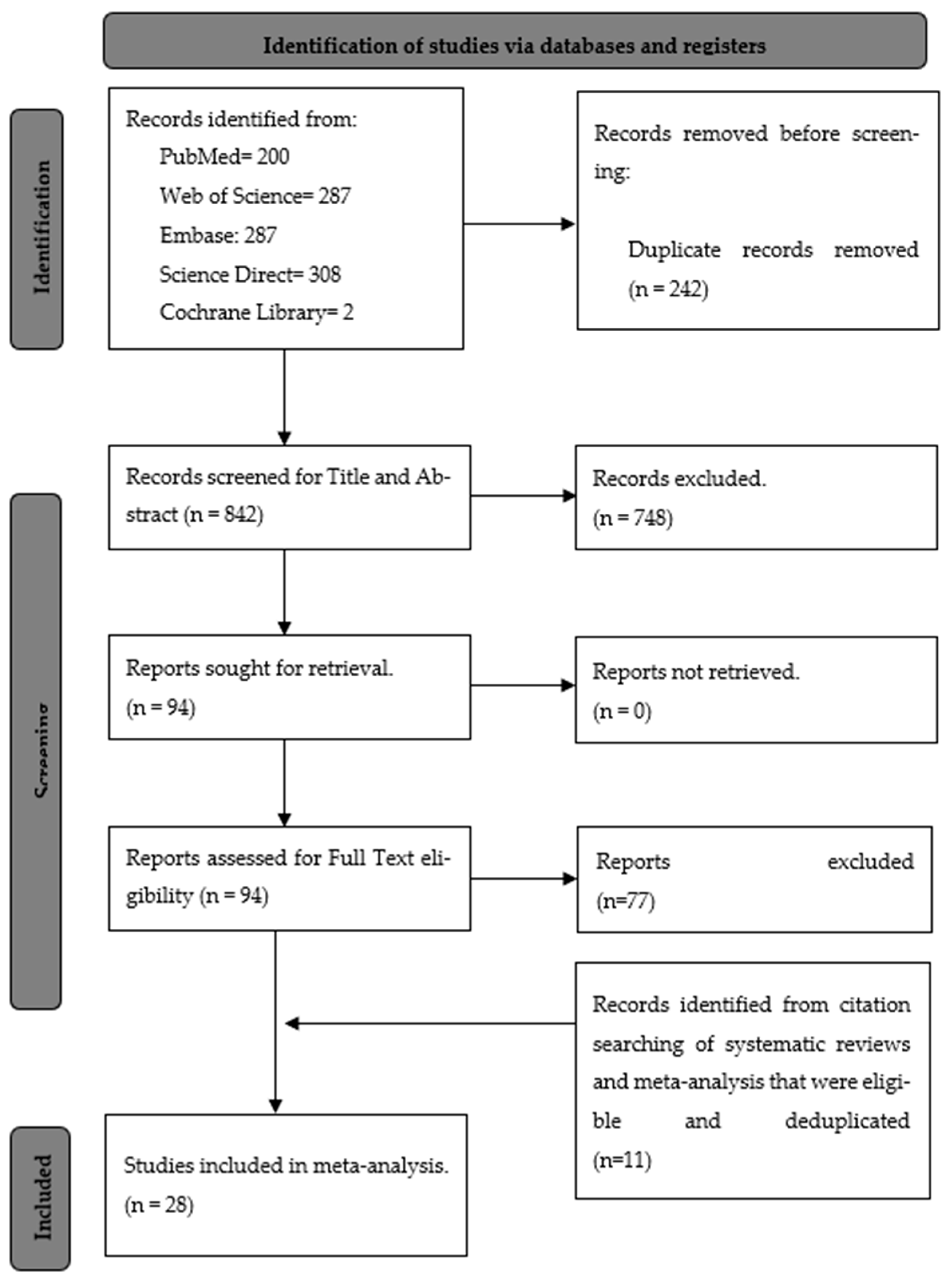
3.1. Search and Selection
3.2. Characteristics of the Included Studies
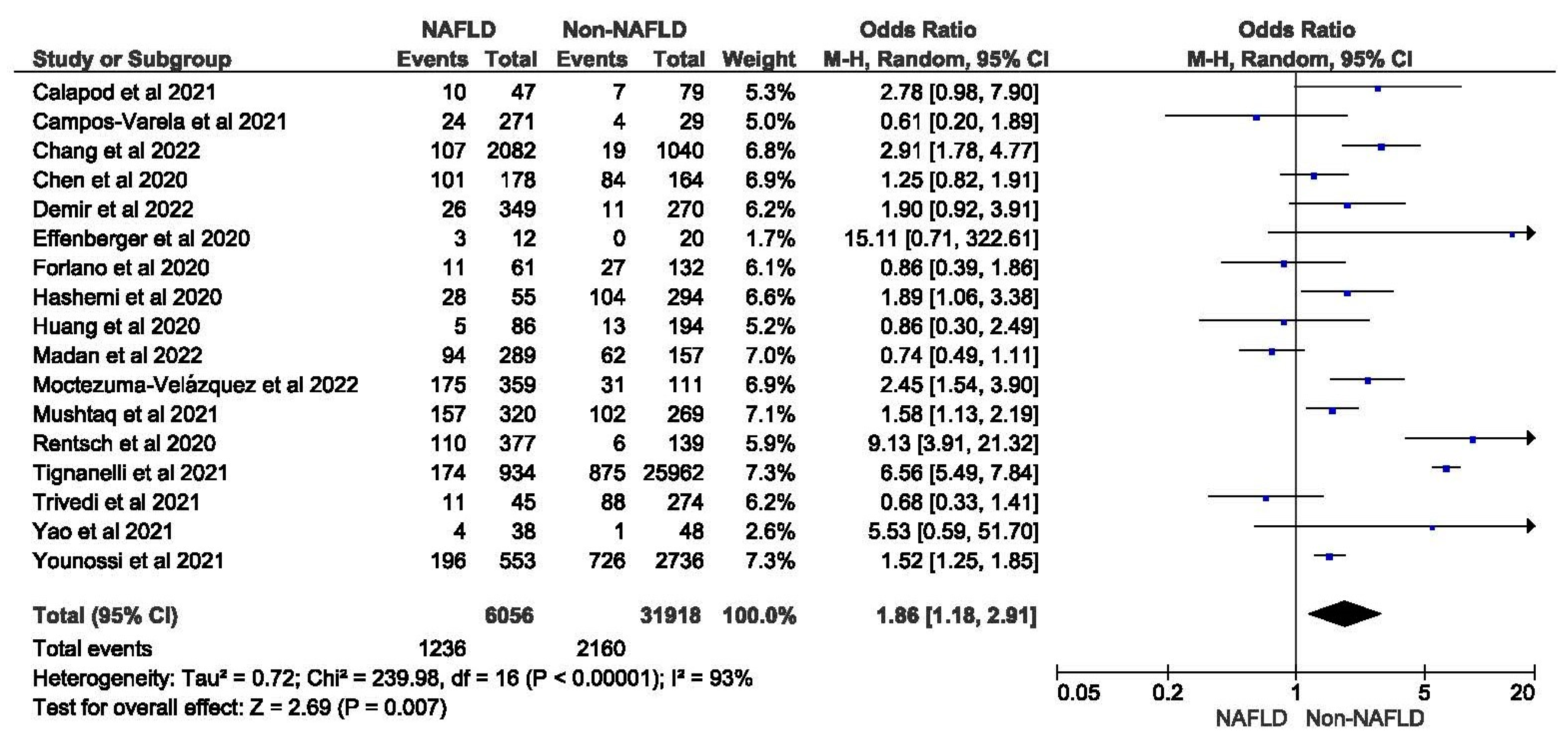
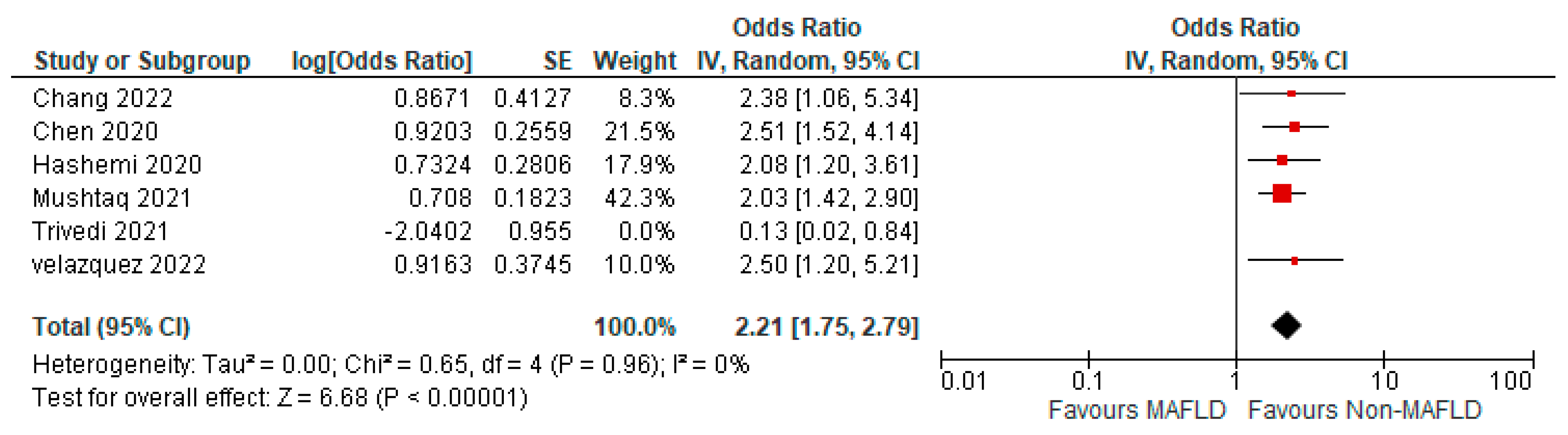
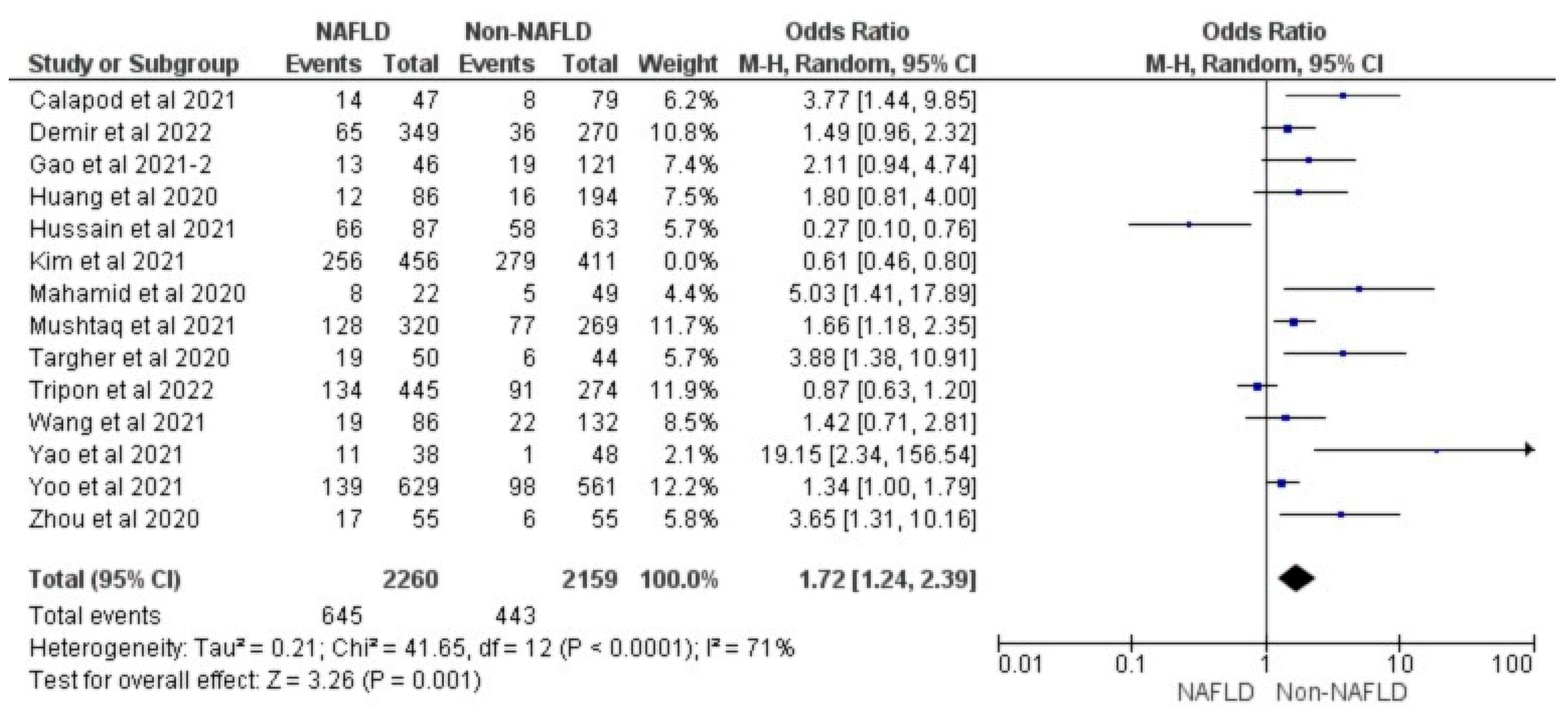
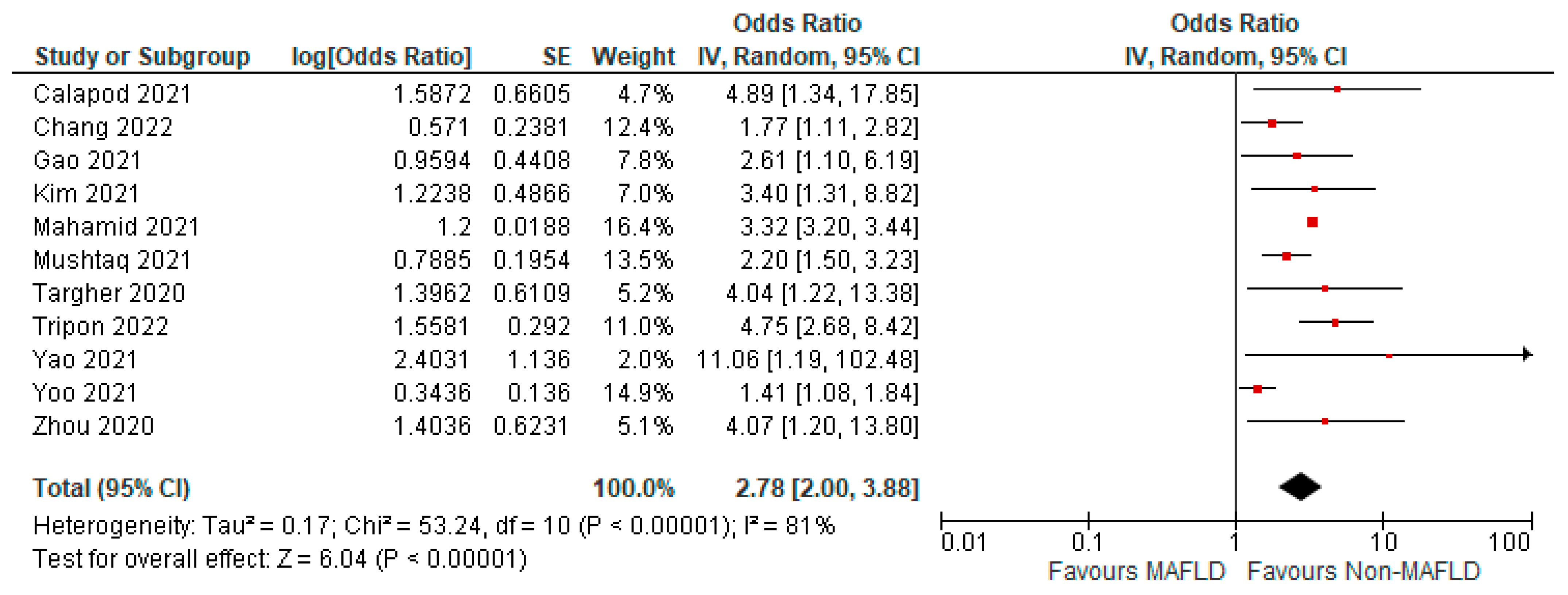
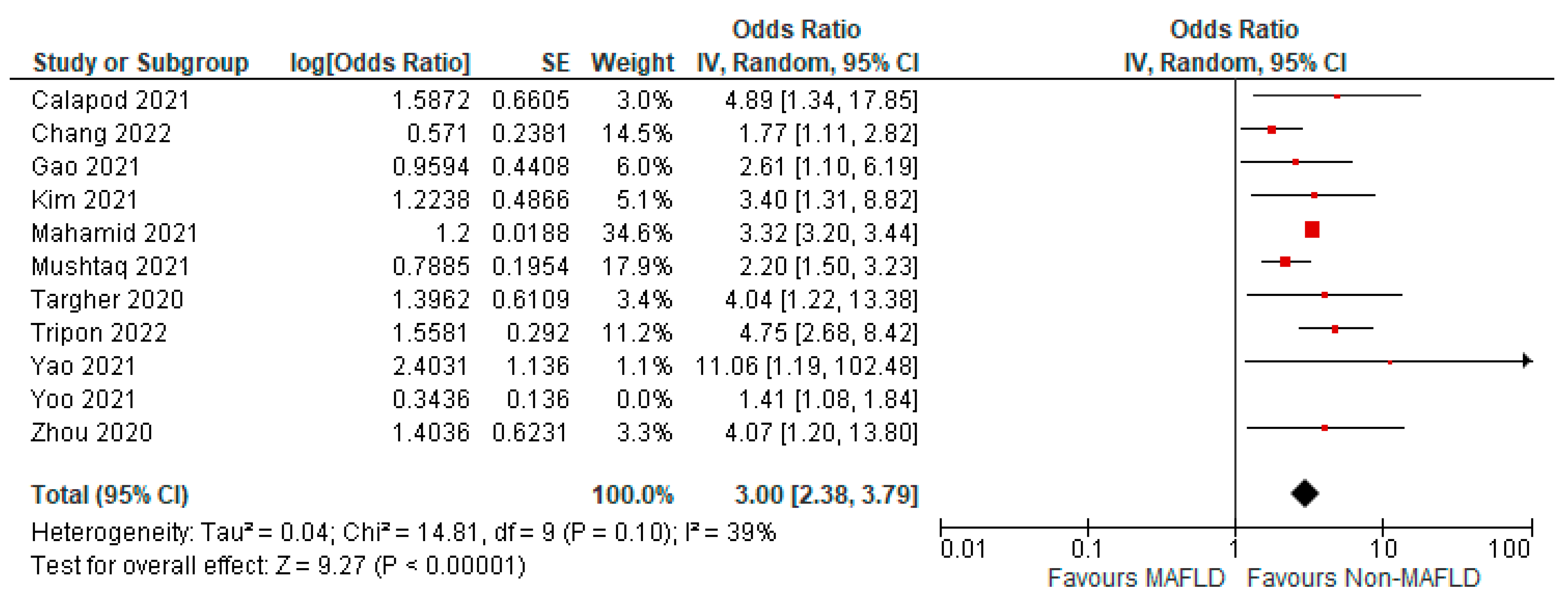
3.3. MAFLD and ICU Admission
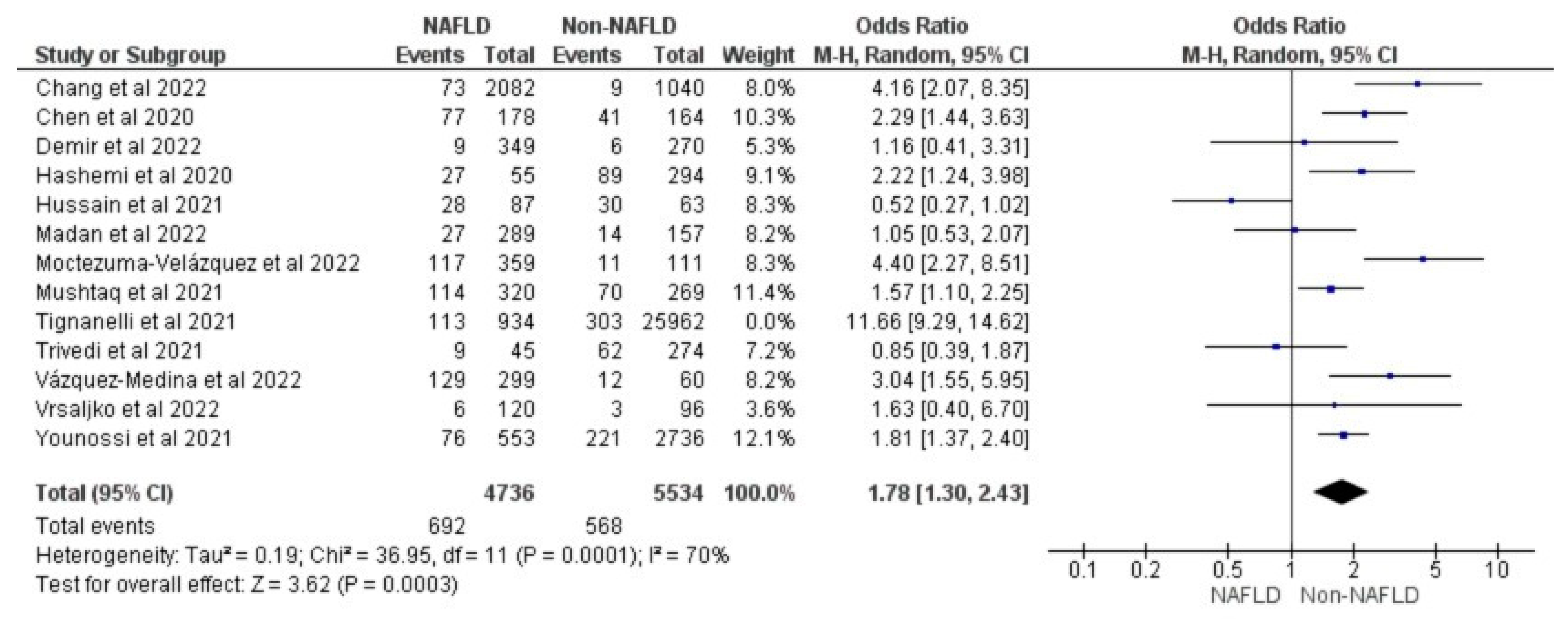
3.4. MAFLD and Invasive Mechanical Ventilation
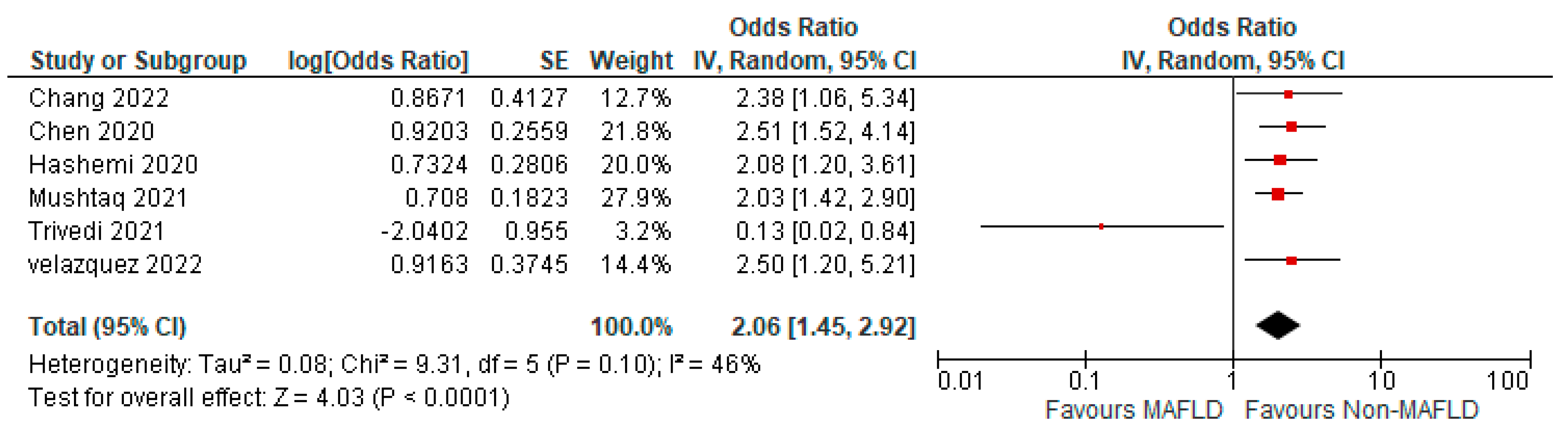
3.5. MAFLD and Severity of COVID-19
3.6. Quality Assessment
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 10 June 2023).
- Yip, T.C.-F.; Lui, G.C.-Y.; Wong, V.W.-S.; Chow, V.C.-Y.; Ho, T.H.-Y.; Li, T.C.-M.; Tse, Y.-K.; Hui, D.S.-C.; Chan, H.L.-Y.; Wong, G.L.-H. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut 2021, 70, 733–742. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Zhong, P.; Xu, J.; Yang, D.; Shen, Y.; Wang, L.; Feng, Y.; Du, C.; Song, Y.; Wu, C.; Hu, X.; et al. COVID-19-associated gastrointestinal and liver injury: Clinical features and potential mechanisms. Signal Transduct. Target. Ther. 2020, 5, 256. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Q.; Chi, J.; Dong, B.; Lv, W.; Shen, L.; Wang, Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 99, 47–56. [Google Scholar] [CrossRef]
- Petersen, A.; Bressem, K.; Albrecht, J.; Thieß, H.-M.; Vahldiek, J.; Hamm, B.; Makowski, M.R.; Niehues, A.; Niehues, S.M.; Adams, L.C. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism 2020, 110, 154317. [Google Scholar] [CrossRef]
- Földi, M.; Farkas, N.; Kiss, S.; Zádori, N.; Váncsa, S.; Szakó, L.; Dembrovszky, F.; Solymár, M.; Bartalis, E.; Szakács, Z.; et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13095. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, K.; Misra-Hebert, A.; Bena, J.; Kashyap, S.R. Impact of Metabolic Syndrome on Severity of COVID-19 Illness. Metab. Syndr. Relat. Disord. 2022, 20, 191–198. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Tilg, H.; Effenberger, M. From NAFLD to MAFLD: When pathophysiology succeeds. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 387–388. [Google Scholar] [CrossRef]
- Kim, C.H.; Younossi, Z.M. Nonalcoholic fatty liver disease: A manifestation of the metabolic syndrome. Clevel. Clin. J. Med. 2008, 75, 721. [Google Scholar] [CrossRef]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef]
- Liu, M.; Mei, K.; Tan, Z.; Huang, S.; Liu, F.; Deng, C.; Ma, J.; Yu, P.; Liu, X. Liver Fibrosis Scores and Hospitalization, Mechanical Ventilation, Severity, and Death in Patients with COVID-19: A Systematic Review and Dose-Response Meta-Analysis. Canadian J. Gastroenterol. Hepatol. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Pranata, R.; Yonas, E.; Huang, I.; Lim, M.A.; Nasution, S.A.; Kuswardhani, R.A.T. Fibrosis-4 index and mortality in coronavirus disease 2019: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2021, 33, e368–e374. [Google Scholar] [CrossRef]
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Zheng, K.I.; Wang, X.-B.; Yan, H.-D.; Sun, Q.-F.; Pan, K.-H.; Wang, T.-Y.; Ma, H.-L.; Chen, Y.-P.; George, J.; et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J. Hepatol. 2020, 73, 719–721. [Google Scholar] [CrossRef]
- Weber, S.; Hellmuth, J.C.; Scherer, C.; Muenchhoff, M.; Mayerle, J.; Gerbes, A.L. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: A prospective cohort study. Gut 2021, 70, 1925–1932. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Tang, X. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China. Glob. Health Amp; Med. 2020, 2, 66–72. [Google Scholar] [CrossRef]
- RevMan Web. Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 10 June 2023).
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. (Eds.) Chapter 10: Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; Cochrane: London, UK, 2022. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Boutron, I.; Page, M.J.; Higgins, J.P.T.; Altman, D.G.; Lundh, A.; Hróbjartsson, A. Chapter 7: Considering Bias and Conflicts of Interest among the Included Studies. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2022. [Google Scholar]
- Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 10 June 2023).
- Calapod, O.P.; Marin, A.M.; Onisai, M.; Tribus, L.C.; Pop, C.S.; Fierbinteanu-Braticevici, C. The Impact of Increased Fib-4 Score in Patients with Type II Diabetes Mellitus on COVID-19 Disease Prognosis. Medicina 2021, 57, 434. [Google Scholar] [CrossRef] [PubMed]
- Campos-Varela, I.; Villagrasa, A.; Simon-Talero, M.; Riveiro-Barciela, M.; Ventura-Cots, M.; Aguilera-Castro, L.; Alvarez-Lopez, P.; Nordahl, E.A.; Anton, A.; Bañares, J.; et al. The role of liver steatosis as measured with transient elastography and transaminases on hard clinical outcomes in patients with COVID-19. Ther. Adv. Gastroenterol. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jeon, J.; Song, T.J.; Kim, J. Association between the fatty liver index and the risk of severe complications in COVID-19 patients: A nationwide retrospective cohort study. BMC Infect. Dis. 2022, 22, 384. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.L.; Hawa, F.; Berinstein, J.A.; Reddy, C.A.; Kassab, I.; Platt, K.D.; Hsu, C.Y.; Steiner, C.A.; Louissaint, J.; Gunaratnam, N.T.; et al. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig. Dis. Sci. 2021, 66, 3192–3198. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Yüzbasıoglu, B.; Calhan, T.; Ozturk, S. Prevalence and Prognostic Importance of High Fibrosis-4 Index in COVID-19 Patients. Int. J. Clin. Pract. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Effenberger, M.; Grander, C.; Fritsche, G.; Bellmann-Weiler, R.; Hartig, F.; Wildner, S.; Seiwald, S.; Adolph, T.E.; Zoller, H.; Weiss, G.; et al. Liver stiffness by transient elastography accompanies illness severity in COVID-19. BMJ Open Gastroenterol. 2020, 7, e000445. [Google Scholar] [CrossRef]
- Forlano, R.; Mullish, B.; Mukherjee, S.; Nathwani, R.; Harlow, C.; Crook, P.; Judge, R.; Soubieres, A.; Middleton, P.; Daunt, A.; et al. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. Hepatology 2020, 72, 282A–283A. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, K.I.; Wang, X.B.; Yan, H.D.; Sun, Q.F.; Pan, K.H.; Wang, T.Y.; Chen, Y.P.; George, J.; Zheng, M.H. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J. Gastroenterol. Hepatol. 2021, 36, 204–207. [Google Scholar] [CrossRef]
- Hashemi, N.; Viveiros, K.; Redd, W.D.; Zhou, J.C.; McCarty, T.R.; Bazarbashi, A.N.; Hathorn, K.E.; Wong, D.; Njie, C.; Shen, L.; et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020, 40, 2515–2521. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, L.; Wang, J.; Xue, L.; Liu, L.; Yan, X.; Huang, S.; Li, Y.; Yan, X.; Zhang, B.; et al. Clinical Features of Patients With COVID-19 With Nonalcoholic Fatty Liver Disease. Hepatol. Commun. 2020, 4, 1758–1768. [Google Scholar] [CrossRef]
- Hussain, A.; Iftikhar, M.; Rizvi, A.; Latif, M.; Ahmed, M.J.; Zartash, S.; Laique, T. Outcomes of Nonalcoholic Fatty Liver Disease in COVID Positive Diabetic Patients: Cross Sectional Study. Pak. J. Med. Health Sci. 2021, 15, 3241–3243. [Google Scholar] [CrossRef]
- Kim, D.; Adeniji, N.; Latt, N.; Kumar, S.; Bloom, P.P.; Aby, E.S.; Perumalswami, P.; Roytman, M.; Li, M.; Vogel, A.; et al. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin. Gastroenterol. Hepatol. 2021, 19, 1469–1479.e1419. [Google Scholar] [CrossRef]
- Madan, K.; Rastogi, R.; Bhargava, R.; Dagar, V.; Singla, V.; Sahu, A.; Singh, P.; Garg, P.; Aggarwal, B.; Singh, R.K. Is Fatty Liver Associated with Increased Mortality and Morbidity in Coronavirus Disease 2019 (COVID-19) Pneumonia? J. Clin. Exp. Hepatol. 2022, 12, 1320–1327. [Google Scholar] [CrossRef]
- Mahamid, M.; Nseir, W.; Khoury, T.; Mahamid, B.; Nubania, A.; Sub-Laban, K.; Schifter, J.; Mari, A.; Sbeit, W.; Goldin, E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: A retrospective case-control study. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1578–1581. [Google Scholar]
- Mushtaq, K.; Khan, M.U.; Iqbal, F.; Alsoub, D.H.; Chaudhry, H.S.; Ata, F.; Iqbal, P.; Elfert, K.; Balaraju, G.; Almaslamani, M.; et al. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression—The debate continues. J. Hepatol. 2021, 74, 482–484. [Google Scholar] [CrossRef]
- Rentsch, C.T.; Kidwai-Khan, F.; Tate, J.P.; Park, L.S.; King, J.T.; Skanderson, M.; Hauser, R.G.; Schultze, A.; Jarvis, C.I.; Holodniy, M.; et al. COVID-19 Testing, Hospital Admission, and Intensive Care Among 2,026,227 United States Veterans Aged 54–75 Years. PLoS Med. 2020, 17, e1003379. [Google Scholar] [CrossRef]
- Targher, G.; Mantovani, A.; Byrne, C.D.; Wang, X.B.; Yan, H.D.; Sun, Q.F.; Pan, K.H.; Zheng, K.I.; Chen, Y.P.; Eslam, M.; et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut 2020, 69, 1545–1547. [Google Scholar] [CrossRef]
- Tignanelli, C.J.; Bramante, C.T.; Dutta, N.; Tamariz, L.; Usher, M.G.; Ikramuddin, S. Metabolic surgery may protect against admission for COVID-19 in persons with nonalcoholic fatty liver disease. Surg. Obes. Relat. Dis. 2021, 17, 1780–1786. [Google Scholar] [CrossRef]
- Tripon, S.; Bilbault, P.; Fabacher, T.; Lefebvre, N.; Lescuyer, S.; Andres, E.; Schmitt, E.; Garnier-Kep, K.S.; Borgne, P.L.; Muller, J.; et al. Abnormal liver tests and non-alcoholic fatty liver disease predict disease progression and outcome of patients with COVID-19. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101894. [Google Scholar] [CrossRef]
- Trivedi, H.D.; Wilechansky, R.; Goyes, D.; Barbosa, J.V.; Canakis, A.; Lai, M.; Long, M.T.; Fricker, Z. Radiographic Hepatic Steatosis Is Not Associated With Key Clinical Outcomes Among Patients Hospitalized with COVID-19. Gastroenterol. Res. 2021, 14, 179–183. [Google Scholar]
- Vázquez Medina, M.U.; Reyes, E.C.; De León, C.V. Association of nafld and mafld with mortality in patients hospitalized with COVID-19 infection. Hepatology 2021, 74, 311A. [Google Scholar]
- Moctezuma-Velázquez, P.; Miranda-Zazueta, G.; Ortiz-Brizuela, E.; Garay-Mora, J.A.; González-Lara, M.F.; Tamez-Torres, K.M.; Román-Montes, C.M.; Díaz-Mejía, B.A.; Pérez-García, E.; Villanueva-Reza, M.; et al. NAFLD determined by Dallas Steatosis Index is associated with poor outcomes in COVID-19 pneumonia: A cohort study. Intern. Emerg. Med. 2022, 17, 1355–1362. [Google Scholar] [CrossRef]
- Vrsaljko, N.; Samadan, L.; Viskovic, K.; Mehmedović, A.; Budimir, J.; Vince, A.; Papic, N. Association of Nonalcoholic Fatty Liver Disease With COVID-19 Severity and Pulmonary Thrombosis: CovidFAT, a Prospective, Observational Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac073. [Google Scholar] [CrossRef]
- Wang, G.; Wu, S.; Wu, C.; Zhang, Q.; Wu, F.; Yu, B.; Zhang, S.; Wu, C.; Wu, G.; Zhong, Y. Association between non-alcoholic fatty liver disease with the susceptibility and outcome of COVID-19: A retrospective study. J. Cell. Mol. Med. 2021, 25, 11212–11220. [Google Scholar] [CrossRef]
- Yao, R.; Zhu, L.; Wang, J.; Liu, J.; Xue, R.; Xue, L.; Liu, L.; Li, C.; Zhao, H.; Cheng, J.; et al. Risk of severe illness of COVID-19 patients with NAFLD and increased NAFLD fibrosis scores. J. Clin. Lab. Anal. 2021, 35, e23880. [Google Scholar] [CrossRef]
- Yoo, H.W.; Jin, H.Y.; Yon, D.K.; Effenberger, M.; Shin, Y.H.; Kim, S.Y.; Yang, J.M.; Kim, M.S.; Koyanagi, A.; Jacob, L.; et al. Non-alcoholic Fatty Liver Disease and COVID-19 Susceptibility and Outcomes: A Korean Nationwide Cohort. J. Korean Med. Sci. 2021, 36, e291. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Lam, B.; Cable, R.; Felix, S.; Jeffers, T.; Younossi, E.; Pham, H.; Srishord, M.; Austin, P.; et al. Independent Predictors of Mortality Among Patients With NAFLD Hospitalized With COVID-19 Infection. Hepatol. Commun. 2022, 6, 3062–3072. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Zheng, K.I.; Wang, X.-B.; Sun, Q.-F.; Pan, K.-H.; Wang, T.-Y.; Ma, H.-L.; Chen, Y.-P.; George, J.; Zheng, M.-H. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020, 40, 2160–2163. [Google Scholar] [CrossRef]
- Marjot, T.; Moon, A.M.; Cook, J.A.; Abd-Elsalam, S.; Aloman, C.; Armstrong, M.J.; Pose, E.; Brenner, E.J.; Cargill, T.; Catana, M.-A.; et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J. Hepatol. 2021, 74, 567–577. [Google Scholar] [CrossRef]
- Jagirdhar, G.S.K.; Qasba, R.K.; Pattnaik, H.; Rama, K.; Banga, A.; Reddy, S.T.; Flumignan Bucharles, A.C.; Kashyap, R.; Elmati, P.R.; Bansal, V.; et al. Association of non-alcoholic fatty liver and metabolic-associated fatty liver with COVID-19 outcomes: A systematic review and meta-analysis. World J. Gastroenterol. 2023, 29, 3362–3378. [Google Scholar] [CrossRef]
- Hayat, U.; Ashfaq, M.; Johnson, L.; Ford, R.; Wuthnow, C.; Kadado, K.; El Jurdi, K.; Okut, H.; Kilgore, W.; Assi, M.; et al. The Association of Metabolic-associated Fatty Liver Disease with Clinical Outcomes of COVID-19: A Systematic Review and Meta-Analysis. Kans. J. Med. 2022, 15, 241–246. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Lefere, S.; Tacke, F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Reports 2019, 1, 30–43. [Google Scholar] [CrossRef]
- Shao, J.; Liang, Y.; Li, Y.; Ding, R.; Zhu, M.; You, W.; Wang, Z.; Huang, B.; Wu, M.; Zhang, T.; et al. Implications of liver injury in risk-stratification and management of patients with COVID-19. Hepatol. Int. 2021, 15, 202–212. [Google Scholar] [CrossRef]
- Assante, G.; Williams, R.; Youngson, N.A. Is the increased risk for MAFLD patients to develop severe COVID-19 linked to perturbation of the gut-liver axis? J. Hepatol. 2021, 74, 487–488. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, A. Metabolic dysfunction associated fatty liver disease increases risk of severe COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 825–827. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, Y.; Kang, J.; Wang, D.; Bai, L.; Mao, Y.; Zha, G.; Tang, H.; Zhang, R. Not Only High Number and Specific Comorbidities but Also Age Are Closely Related to Progression and Poor Prognosis in Patients With COVID-19. Front. Med. 2021, 8, 736109. [Google Scholar] [CrossRef]
- Luzi, L.; Radaelli, M.G. Influenza and obesity: Its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020, 57, 759–764. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Moon, A.M. Surveillance Epidemiology of Coronavirus (COVID-19) under Research Exclusion. SECURE-Cirrhosis. Available online: https://covidcirrhosis.web.unc.edu/ (accessed on 10 June 2023).





| Serial No. | First Author | Country | Year of Publication | Study Design | MAFLD No. | Non-MAFLD No. | Mean Age | ±SD | Male (%) | Measure of FIB-4 | Measure of FIB-4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Calapod [26] | Romania | 2021 | Prospective descriptive study | 47 | 79 | 66.32 | 13.72 | 57.20% | Imaging evidence (ultrasound or computer tomography) | Biochemical enzymes (LFT) within the past 12 months |
| 2 | Campos [27] | Spain | 2021 | Prospective observational (cohort) study | 271 | 29 | 55.25 | 11.69 | 49% | Liver steatosis by hepatic steatosis index (HSI) | Transient elastography (TE) by controlled attenuation parameter (CAP) |
| 3 | Chang [28] | South Korea | 2022 | Retrospective cohort study | 2082 | 1040 | - | - | 30.72% | FLI index | |
| 4 | Chen [29] | USA | 2020 | Retrospective single-center cohort study | 178 | 164 | 62.6 | 15.6 | 53.50% | Liver steatosis | Imaging evidence of steatosis > 30 days before COVID-19 diagnosis, or hepatic steatosis index (HSI) |
| 5 | Demir [30] | Turkey | 2022 | Retrospective cohort study | 349 | 270 | 51.6 | 9.65 | 58.60% | FIB-4 index | |
| 6 | Effenberger [31] | Austria | 2020 | Prospective study | 12 | 20 | - | - | 40.62% | Liver stiffness measurements and CAP with a fibro scan | Liver and spleen sonography and elastography |
| 7 | Forlano [32] | UK | 2020 | Retrospective cohort study | 61 | 132 | - | - | 60% | Fibrosis-4 index (FIB-4) | Imaging (either ultrasound or computerized tomography) or past medical history |
| 8 | Gao [33] | China | 2021 | Cohort | 46 | 121 | 49 | 42.50% | Hepatic steatosis on CT scan | ||
| 9 | Hashemi [34] | USA | 2020 | Retrospective cohort | 55 | 294 | 63.4 | 16.5 | 55.4% | Hepatic steatosis on any prior imaging studies or liver histology | |
| 10 | Huang [35] | China | 2020 | Retrospective cohort study | 86 | 194 | 43.6 | 17.8 | 52.10% | Hepatic steatosis index (HSI) | |
| 11 | Hussain [36] | Pakistan | 2021 | Cross-sectional study | 87 | 63 | 59.73 | 11.35 | 56% | Clinical parameters, like hepatomegaly, and lab parameters, like AST, ALT | |
| 12 | Kim [37] | USA | 2021 | Observational cohort study | 456 | 411 | 56.9 | 14.5 | 54.70% | Fibrosis by magnetic resonance elastography | Fibro scan, fibrosis-4, or biopsy |
| 13 | Madan [38] | India | 2022 | Case-control study | 289 | 157 | - | - | 64.5% | Liver attenuation index (LAI) | |
| 14 | Mahamid [39] | Israel | 2021 | Retrospective, case-control study | 22 | 49 | 51 | 21.7 | 28.20% | CT scan | Medical records documentation |
| 15 | Mushtaq [40] | Qatar | 2021 | Prospective study | 320 | 269 | - | - | 84.71% | Hepatic steatosis index (HSI) | |
| 16 | Rentsch [41] | USA | 2020 | Retrospective cohort study | 377 | 139 | 65.8 | 7.8 | 95.4 | FIB-4 index | |
| 17 | Targher [42] | China | 2020 | Cohort study | 50 | 44 | - | - | 48% | Fibrosis-4 (FIB-4) | NAFLD fibrosis score (NFS) |
| 18 | Tignanelli [43] | USA | 2021 | Retrospective cohort study | 934 | 25,962 | 51 | 23.7 | 56% | Elevated Alanine Aminotransferase (ALT) Level On 3 Separate Dates | |
| 19 | Tripon [44] | France | 2022 | Retrospective study | 445 | 274 | 61.35 | 12.45 | 58.80% | Hepatic steatosis index | NAFLD fibrosis score (NFS) |
| 20 | Trivedi [45] | USA | 2021 | Retrospective Case-Control study | 45 | 274 | 65 (median) | - | 50% | Abdominal imaging (computed tomography (CT), magnetic resonance imaging (MRI), or ultrasound) | |
| 21 | Vazquez-Medina [46] | Mexico | 2022 | Retrospective case control study | 299 | 60 | 54.3 | 14.69 | 22.01% | FIB-4 index | |
| 22 | Moctezuma-Velazquez [47] | Mexico | 2022 | Retrospective cohort study | 359 | 111 | 51.6 | 14.8 | 63% | CT scans | |
| 23 | Vrsaljko [48] | Republic of Croatia | 2022 | Prospective observational (cohort) study | 120 | 96 | 59.3 | 12.6 | 63.43% | Ultrasound | Difference between liver and spleen computed tomography (CT) attenuation |
| 24 | Wang [49] | China | 2021 | Retrospective cohort study | 86 | 132 | - | - | 50.40% | Ultrasound parameters | |
| 25 | Yao [50] | China | 2021 | Retrospective Cohort study | 38 | 48 | 43.2 | 15.45 | 58.10% | Hepatic steatosis index (HSI) | NAFLD fibrosis score (NFS) |
| 26 | Yoo [51] | South Korea | 2021 | Retrospective Cohort study | 629 | 561 | - | - | - | HSI, FLI, claims based on NAFLD | |
| 27 | Younossi [52] | USA | 2021 | Observational cohort study | 553 | 2736 | - | - | 49.55% | Abdominal imaging, magnetic resonance imaging, computer tomography, ultrasound | |
| 28 | Zhou [53] | China | 2020 | Cohort study | 55 | 55 | 42.1 | 11.4 | 74.50% | Computed tomography (CT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagirdhar, G.S.K.; Pattnaik, H.; Banga, A.; Qasba, R.K.; Rama, K.; Reddy, S.T.; Bucharles, A.C.F.; Kashyap, R.; Elmati, P.R.; Bansal, V.; et al. Association of Non-Alcoholic Fatty Liver Disease and Metabolic-Associated Fatty Liver Disease with COVID-19-Related Intensive Care Unit Outcomes: A Systematic Review and Meta-Analysis. Medicina 2023, 59, 1239. https://doi.org/10.3390/medicina59071239
Jagirdhar GSK, Pattnaik H, Banga A, Qasba RK, Rama K, Reddy ST, Bucharles ACF, Kashyap R, Elmati PR, Bansal V, et al. Association of Non-Alcoholic Fatty Liver Disease and Metabolic-Associated Fatty Liver Disease with COVID-19-Related Intensive Care Unit Outcomes: A Systematic Review and Meta-Analysis. Medicina. 2023; 59(7):1239. https://doi.org/10.3390/medicina59071239
Chicago/Turabian StyleJagirdhar, Gowthami Sai Kogilathota, Harsha Pattnaik, Akshat Banga, Rakhtan K. Qasba, Kaanthi Rama, Shiva Teja Reddy, Anna Carolina Flumignan Bucharles, Rahul Kashyap, Praveen Reddy Elmati, Vikas Bansal, and et al. 2023. "Association of Non-Alcoholic Fatty Liver Disease and Metabolic-Associated Fatty Liver Disease with COVID-19-Related Intensive Care Unit Outcomes: A Systematic Review and Meta-Analysis" Medicina 59, no. 7: 1239. https://doi.org/10.3390/medicina59071239
APA StyleJagirdhar, G. S. K., Pattnaik, H., Banga, A., Qasba, R. K., Rama, K., Reddy, S. T., Bucharles, A. C. F., Kashyap, R., Elmati, P. R., Bansal, V., Bains, Y., DaCosta, T., & Surani, S. (2023). Association of Non-Alcoholic Fatty Liver Disease and Metabolic-Associated Fatty Liver Disease with COVID-19-Related Intensive Care Unit Outcomes: A Systematic Review and Meta-Analysis. Medicina, 59(7), 1239. https://doi.org/10.3390/medicina59071239










