Sex-Related Differences in Short-Term Prognosis in Patients with Acute Myocardial Infarction-Related Cardiogenic Shock Receiving Impella Support in Japan: From the J-PVAD Registry
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Selection
2.2. Impella Device
2.3. Collected Data
2.4. Endpoints
2.5. Statistical Assessments
3. Results
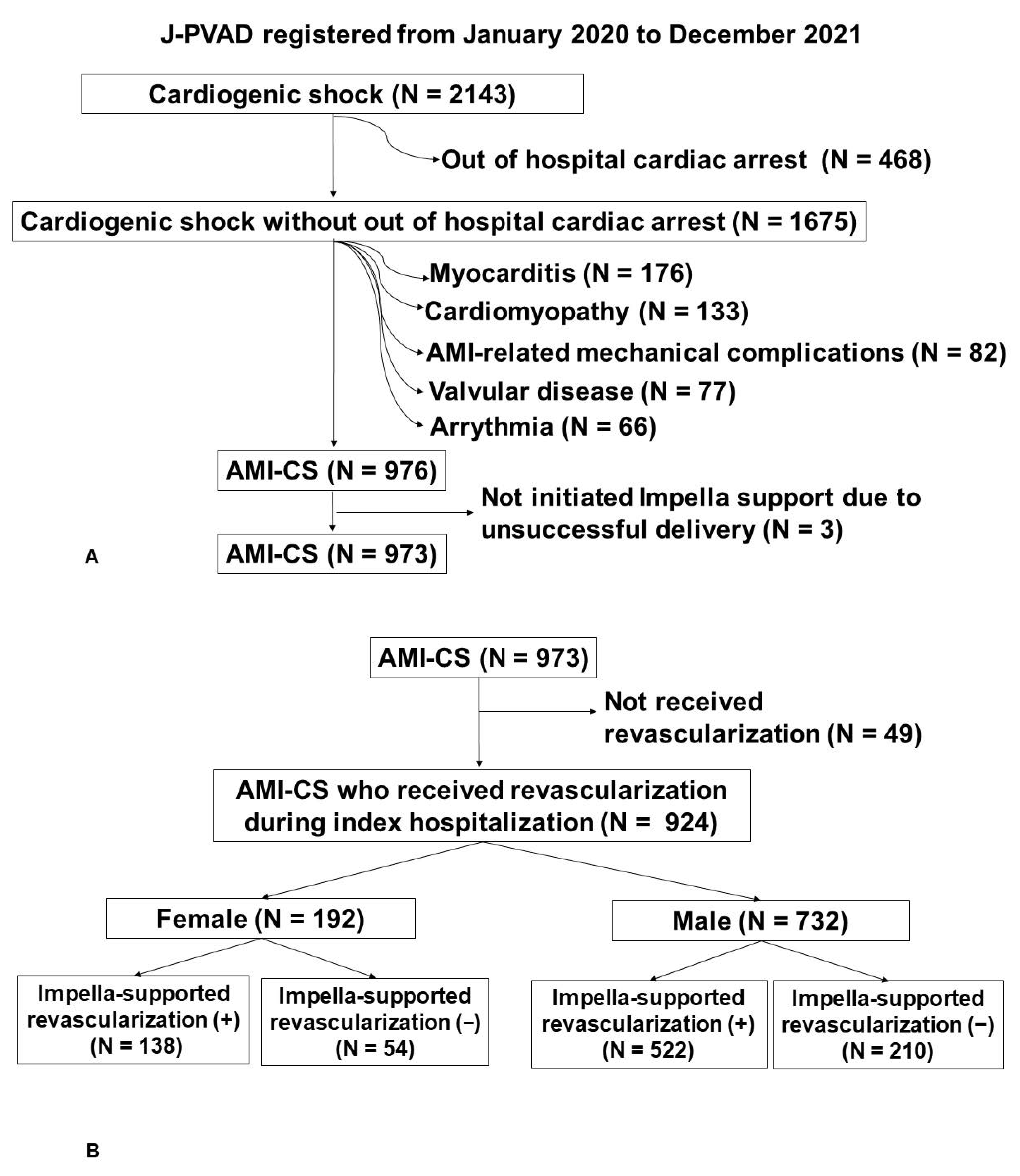
3.1. Patient Selection Flow
3.2. Baseline Characteristics
3.3. Impella Device and Concomitant Procedure
3.4. Impella Removal
3.5. Primary Outcome
3.6. Secondary Outcome
4. Discussion
4.1. Sex Differences in Patients with AHF
4.2. Sex Differences in Patients with AMI-CS
4.3. Impact of the Female Sex with MCS Therapy on the Short-Term Prognosis
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hochman, J.S.; Buller, C.E.; Sleeper, L.A.; Boland, J.; Dzavik, V.; Sanborn, T.A.; Godfrey, E.; White, H.D.; Lim, J.; LeJemtel, T. Cardiogenic shock complicating acute myocardial infarction--etiologies, management and outcome: A report from the SHOCK Trial Registry. Should we emergently revascularize Occluded Coronaries for cardiogenic shock? J. Am. Coll. Cardiol. 2000, 36, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic Shock after Acute Myocardial Infarction: A Review. JAMA 2021, 326, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.M.; Brisco, M.A.; Colvin, M.; Grady, K.L.; Walsh, M.N.; Cook, J.L. Women with Cardiogenic Shock Derive Greater Benefit from Early Mechanical Circulatory Support: An Update from the cVAD Registry. J. Interv. Cardiol. 2016, 29, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Vallabhajosyula, S.; Dunlay, S.M.; Hayes, S.N.; Best, P.J.M.; Brenes-Salazar, J.A.; Lerman, A.; Gersh, B.J.; Jaffe, A.S.; Bell, M.R.; et al. Sex and Gender Disparities in the Management and Outcomes of Acute Myocardial Infarction-Cardiogenic Shock in Older Adults. Mayo Clin. Proc. 2020, 95, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Alasnag, M.; Truesdell, A.G.; Williams, H.; Martinez, S.C.; Qadri, S.K.; Skendelas, J.P.; Jakobleff, W.A.; Alasnag, M. Mechanical Circulatory Support: A Comprehensive Review with a Focus on Women. Curr. Atheroscler. Rep. 2020, 22, 11. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Dunlay, S.M.; Barsness, G.W.; Miller, P.E.; Cheungpasitporn, W.; Stulak, J.M.; Rihal, C.S.; Holmes, D.R., Jr.; Bell, M.R.; Miller, V.M. Sex Disparities in the Use and Outcomes of Temporary Mechanical Circulatory Support for Acute Myocardial Infarction-Cardiogenic Shock. CJC Open 2020, 2, 462–472. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ako, J.; Toda, K.; Hirayama, A.; Kinugawa, K.; Kobayashi, Y.; Ono, M.; Nishimura, T.; Sato, N.; Shindo, T.; et al. Short-Term Outcomes of Impella Support in Japanese Patients with Cardiogenic Shock Due to Acute Myocardial Infarction—Japanese Registry for Percutaneous Ventricular Assist Device (J-PVAD). Circ. J. Off. J. Jpn. Circ. Soc. 2023, 87, 588–597. [Google Scholar] [CrossRef]
- Nakamura, M.; Imamura, T.; Ueno, H.; Kinugawa, K. Current indication and practical management of percutaneous left ventricular assist device support therapy in Japan. J. Cardiol. 2019, 75, 228–232. [Google Scholar] [CrossRef]
- Morray, B.H.; Dimas, V.V.; McElhinney, D.B.; Puri, K.; Qureshi, A.M. Patient size parameters to guide use of the Impella device in pediatric patients. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2019, 94, 618–624. [Google Scholar] [CrossRef]
- Toda, K.; Ako, J.; Hirayama, A.; Kinugawa, K.; Kobayashi, Y.; Ono, M.; Nishimura, T.; Sato, N.; Shindo, T.; Takayama, M.; et al. Three-year experience of catheter-based micro-axial left ventricular assist device, Impella, in Japanese patients: The first interim analysis of Japan registry for percutaneous ventricular assist device (J-PVAD). J. Artif. Organs Off. J. Jpn. Soc. Artif. Organs 2023, 26, 17–23. [Google Scholar] [CrossRef]
- Imamura, S.; Miyata, M.; Tagata, K.; Yokomine, T.; Ohmure, K.; Kawasoe, M.; Otsuji, H.; Chaen, H.; Oketani, N.; Ogawa, M.; et al. Prognostic predictors in patients with cardiopulmonary arrest: A novel equation for evaluating the 30-day mortality. J. Cardiol. 2023; in press. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; White, H.D.; Dzavik, V.; Wong, S.C.; Menon, V.; Webb, J.G.; Steingart, R.; Picard, M.H.; Menegus, M.A.; et al. One-year survival following early revascularization for cardiogenic shock. JAMA 2001, 285, 190–192. [Google Scholar] [CrossRef]
- Lala, A.; Tayal, U.; Hamo, C.E.; Youmans, Q.; Al-Khatib, S.M.; Bozkurt, B.; Davis, M.B.; Januzzi, J.; Mentz, R.; Sauer, A.; et al. Sex Differences in Heart Failure. J. Card. Fail. 2022, 28, 477–498. [Google Scholar] [CrossRef]
- Collado-Lledó, E.; de la Cuerda, F.; Ariza-Solé, A. Sex Differences in Acute Heart Failure Management: Is There a Gap in Treatment Quality? Curr. Heart Fail. Rep. 2023, 20, 121–128. [Google Scholar] [CrossRef]
- Espersen, C.; Campbell, R.T.; Claggett, B.; Lewis, E.F.; Groarke, J.D.; Docherty, K.F.; Lee, M.M.Y.; Lindner, M.; Biering-Sørensen, T.; Solomon, S.D.; et al. Sex differences in congestive markers in patients hospitalized for acute heart failure. ESC Heart Fail. 2021, 8, 1784–1795. [Google Scholar] [CrossRef]
- Valle, J.A.; Miyasaka, R.L.; Carroll, J.D. Acute Mitral Regurgitation Secondary to Papillary Muscle Tear: Is Transcatheter Edge-to-Edge Mitral Valve Repair a New Paradigm? Circ. Cardiovasc. Interv. 2017, 10, e005050. [Google Scholar] [CrossRef]
- Horstkotte, J.C.; Horstkotte, M.; Beucher, H.; Felderhoff, T.; Boekstegers, P. Percutaneous mitral valve repair as rescue procedure after post myocardial infarction papillary muscle rupture and acute cardiogenic shock. Clin. Res. Cardiol. 2015, 104, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Motiejūnaitė, J.; Akiyama, E.; Cohen-Solal, A.; Maggioni, A.P.; Mueller, C.; Choi, D.J.; Kavoliūnienė, A.; Čelutkienė, J.; Parenica, J.; Lassus, J.; et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur. Heart J. 2020, 41, 1357–1364. [Google Scholar] [CrossRef]
- Sambola, A.; Elola, F.J.; Buera, I.; Fernández, C.; Bernal, J.L.; Ariza, A.; Brindis, R.; Bueno, H.; Rodríguez-Padial, L.; Marín, F.; et al. Sex bias in admission to tertiary-care centres for acute myocardial infarction and cardiogenic shock. Eur. J. Clin. Investig. 2021, 51, e13526. [Google Scholar] [CrossRef] [PubMed]
- Yan, I.; Schrage, B.; Weimann, J.; Dabboura, S.; Hilal, R.; Beer, B.N.; Becher, P.M.; Seiffert, M.; Magnussen, C.; Schnabel, R.B.; et al. Sex differences in patients with cardiogenic shock. ESC Heart Fail. 2021, 8, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Collado-Lledó, E.; Llaó, I.; Rivas-Lasarte, M.; González-Fernández, V.; Noriega, F.J.; Hernández-Perez, F.J.; Alegre, O.; Sionis, A.; Lidón, R.M.; Viana-Tejedor, A.; et al. Clinical picture, management and risk stratification in patients with cardiogenic shock: Does gender matter? BMC Cardiovasc. Disord. 2020, 20, 189. [Google Scholar] [CrossRef] [PubMed]
- Rubini Gimenez, M.; Zeymer, U.; Desch, S.; de Waha-Thiele, S.; Ouarrak, T.; Poess, J.; Meyer-Saraei, R.; Schneider, S.; Fuernau, G.; Stepinska, J.; et al. Sex-Specific Management in Patients with Acute Myocardial Infarction and Cardiogenic Shock: A Substudy of the CULPRIT-SHOCK Trial. Circ. Cardiovasc. Interv. 2020, 13, e008537. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Nemeth, S.; Kurlansky, P.; Brodie, D.; Takayama, H.; Naka, Y.; Kaku, Y.; Fried, J.; Nir, U.; Takeda, K. Sex differences in patients with cardiogenic shock requiring extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2022, 164, 960–969.e966. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Singh, A.; Jauhar, R.; Meraj, P.M. Gender difference with the use of percutaneous left ventricular assist device in patients undergoing complex high-risk percutaneous coronary intervention: From pVAD Working Group. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 369–378. [Google Scholar] [CrossRef]
- Kuehnemund, L.; Lange, S.A.; Feld, J.; Padberg, J.S.; Fischer, A.J.; Makowski, L.; Engelbertz, C.; Dröge, P.; Ruhnke, T.; Guenster, C.; et al. Sex disparities in guideline-recommended therapies and outcomes after ST-elevation myocardial infarction in a contemporary nationwide cohort of patients over an eight-year period. Atherosclerosis 2023, 375, 30–37. [Google Scholar] [CrossRef]
- Alraies, M.C.; Kaki, A.; Kajy, M.; Blank, N.; Hasan, R.; Htun, W.W.; Glazier, J.J.; Elder, M.; O’Neill, W.W.; Grines, C.L.; et al. Sex-related difference in the use of percutaneous left ventricular assist device in patients undergoing complex high-risk percutaneous coronary intervention: Insight from the cVAD registry. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2020, 96, 536–544. [Google Scholar] [CrossRef]


| All Patients (n = 924) | Female (n = 192, 21%) | Male (n = 732, 79%) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 73 (64, 79) | 78 (72, 84) | 71 (62, 78) | <0.0001 * |
| Hypertension | 618 (66.9%) | 135 (70.3%) | 483 (66.0%) | 0.1036 |
| Diabetes mellitus | 407 (44.1%) | 84 (43.8%) | 323 (44.1%) | 0.9104 |
| Dyslipidemia | 462 (50.0%) | 92 (47.9%) | 370 (50.6%) | 0.3044 |
| Pulmonary hypertension | 23 (2.5) | 6 (3.1%) | 17 (2.3%) | 0.4967 |
| Cerebrovascular accident | 76 (8.2%) | 20 (10.4%) | 56 (7.7%) | 0.4007 |
| Smoking | 515 (55.7%) | 34 (17.7%) | 481 (65.7%) | <0.0001 * |
| Atrial fibrillation/tachycardia | 52 (5.7%) | 12 (6.3%) | 40 (5.5%) | 0.7247 |
| Ventricular tachycardia/fibrillation | 20 (2.2%) | 4 (2.1%) | 16 (2.2%) | 1.0000 |
| Body mass index (kg/m2) | 23.3 (20.7, 25.9) | 22.4 (19.6, 25.4) | 23.4 (21.0, 26.0) | 0.0045 * |
| Body surface area (m2) | 1.66 (1.52, 1.81) | 1.43 (1.33, 1.56) | 1.72 (1.59, 1.84) | <0.0001 * |
| Intravenous inotropes before Impella | 669 (72.4%) | 150 (78.1%) | 519 (70.9%) | 0.0465 * |
| Intra-aortic balloon pump | 96 (10.4%) | 17 (8.9%) | 79 (10.8%) | 0.5070 |
| Extracorporeal membrane oxygenation | 178 (19.4%) | 35 (18.3%) | 143 (19.6%) | 0.7578 |
| Systolic blood pressure (mmHg) | 89 (72, 107) | 91 (71, 110) | 89 (72, 106) | 0.5065 |
| Diastolic blood pressure (mmHg) | 59 (45, 72) | 58 (45, 73) | 59 (45, 72) | 0.5040 |
| Heart rate (bpm) | 91 (72, 110) | 90 (71, 106) | 92 (72, 110) | 0.2674 |
| Echocardiographic data | ||||
| LVEF before Impella insertion (%) | 30 (20, 40) | 35 (26, 44) | 30 (20, 35) | <0.0001 * |
| Laboratory data | ||||
| Lactate (mmol/L) | 4.4 (2.4, 7.8) | 5.0 (2.7, 8.5) | 4.2 (2.3, 7.5) | 0.0591 |
| Creatinine kinase (IU/L) | 250 (109, 869) | 369 (121, 948) | 234 (108, 853) | 0.2386 |
| Total bilirubin (mg/dL) | 0.7 (0.5, 1.0) | 0.6 (0.5, 1.0) | 0.7 (0.5, 1.0) | 0.4963 |
| Serum Creatinine (mg/dL) | 1.17 (0.92, 1.62) | 0.96 (0.78, 1.39) | 1.21 (0.97, 1.67) | <0.0001 * |
| C-reactive protein (mg/dL) | 0.40 (0.10, 3.10) | 0.61 (0.14, 3.47) | 0.35 (0.10, 2.90) | 0.0078 * |
| Lactate dehydrogenase (U/mL) | 342 (223, 637) | 391 (262, 711) | 323 (216, 604) | 0.0067 * |
| Albumin (mg/dL) | 3.6 (3.1, 4.0) | 3.5 (3.1, 3.8) | 3.7 (3.2, 4.0) | 0.0002 * |
| Aspartate Aminotransferase (IU/L) | 57 (30, 179) | 80 (36, 217) | 53 (30, 168) | 0.0102 * |
| Alanine Aminotransferase (IU/L) | 35 (20, 74) | 32 (18, 103) | 35 (21, 68) | 0.9314 |
| All Patients (n = 924) | Female (n = 192, 21%) | Male (n = 732, 79%) | p Value | |
|---|---|---|---|---|
| Device types of first Impella | ||||
| Impella 2.5 | 47 (5.1%) | 20 (10.4%) | 27 (3.7%) | 0.0006 * |
| Impella CP | 857 (92.8%) | 168 (87.5%) | 689 (94.1%) | 0.0028 * |
| Impella 5.0 | 20 (2.2%) | 4 (2.1%) | 16 (2.2%) | 1.0000 |
| Access site of first Impella | ||||
| Transfemoral approach | 898 (97.2%) | 187 (97.4%) | 711 (97.1%) | 1.0000 |
| Subclavian/axillary approach | 22 (2.4%) | 5 (2.6%) | 17 (2.3%) | 0.7920 |
| Use of pulmonary artery catheter | 570 (61.7%) | 114 (59.4%) | 456 (62.3%) | 0.0185 * |
| Cardioversion | 203 (22.0%) | 36 (18.8%) | 167 (22.8%) | 0.2412 |
| Non-pharmacological therapy during index hospitalization | ||||
| Percutaneous coronary intervention | 897 (97.1%) | 187 (97.4%) | 710 (97.0%) | 1.0000 |
| Coronary artery bypass grafting | 84 (9.1%) | 14 (7.3%) | 70 (9.6%) | 0.3978 |
| Valve surgery | 19 (2.1%) | 5 (2.6%) | 14 (1.9%) | 0.5678 |
| Impella-supported revascularization | 660 (71.4%) | 138 (71.9%) | 522 (71.3%) | 0.9286 |
| Other surgical operation | 37 (4.0%) | 10 (5.2%) | 27 (3.7%) | 0.4064 |
| Balloon aortic valvuloplasty | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Transcatheter SHD intervention | 9 (1.0%) | 5 (2.6%) | 4 (0.6%) | 0.0244 * |
| EP/ablation | 14 (1.5%) | 3 (1.6%) | 11 (1.5%) | 1.0000 |
| Additional MCS therapy during index hospitalization | ||||
| ECMO | 309 (33.4%) | 59 (30.7%) | 250 (34.2%) | 0.3911 |
| ECMO initiated during first Impella support | 101 (11.1%) | 21 (11.1%) | 80 (11.1%) | 1.0000 |
| Left ventricular assist device | 5 (0.5%) | 2 (1.0%) | 4 (0.4%) | 0.2786 |
| Second Impella | 57 (6.2%) | 10 (5.2%) | 47 (6.4%) | 0.6158 |
| Device type of second Impella | ||||
| Impella 2.5 | 1 (2.4%) | 1 (16.7%) | 0 (0%) | 0.1429 |
| Impella CP | 28 (50.9%) | 6 (66.7%) | 22 (47.8%) | 0.4688 |
| Impella 5.0 | 26 (47.3%) | 2 (22.2%) | 24 (52.2%) | 0.1485 |
| Support duration of first Impella (days) | 3.8 (1.8, 6.4) | 3.5 (1.1, 6.1) | 3.9 (1.9, 6.7) | 0.0145 * |
| Support duration of second Impella (days) | 6.9 (4.0, 11.6) | 2.2 (0.3, 6.0) | 8.8 (4.8, 12.7) | 0.0009 * |
| All Patients (n = 924) | Female (n = 192, 21%) | Male (n = 732, 79%) | p Value | |
|---|---|---|---|---|
| Intravenous inotropes at Impella removal | 647 (70.1%) | 129 (67.2%) | 518 (70.9%) | 0.3307 |
| Dobutamine | 469 (50.8%) | 90 (46.9%) | 379 (51.8%) | 0.2562 |
| Dopamine | 69 (7.5%) | 13 (6.8%) | 56 (7.7%) | 0.7594 |
| Noradrenaline | 404 (43.7%) | 77 (40.1%) | 327 (44.7%) | 0.2880 |
| PDEIII inhibitors | 57 (6.2%) | 11 (5.7%) | 46 (6.3%) | 0.8673 |
| Adrenaline | 46 (5.0%) | 9 (4.7%) | 37 (5.1%) | 1.0000 |
| Vasopressin | 29 (3.1%) | 6 (3.1%) | 23 (3.1%) | 1.0000 |
| Vital signs | ||||
| SBP at Impella removal (mmHg) | 99 (64, 114) | 95 (0, 114) | 100 (77, 114) | 0.1030 |
| DBP at Impella removal (mmHg) | 53 (32, 73) | 50 (0, 60) | 54 (40, 64) | 0.0024 * |
| Heart rate at Impella removal (bpm) | 80 (54, 93) | 76 (0, 94) | 81 (60, 93) | 0.0523 |
| Alive at Impella removal | 574 (62.1%) | 106 (55.2%) | 468 (63.9%) | 0.0760 |
| LVEF at Impella removal (%) | 40 (30, 48) | 42 (35, 52) | 40 (30, 48) | 0.0060 * |
| MCS therapy after Impella removal | ||||
| Upgrade to durable LVAD | 3 (0.3%) | 1 (0.5%) | 2 (0.3%) | 0.5041 |
| Addition of IABP | 49 (5.4%) | 8 (4.2%) | 41 (5.7%) | 0.4547 |
| Addition of ECMO | 17 (1.9%) | 5 (2.6%) | 12 (1.7%) | 0.3703 |
| Univariable Analyses | Multivariable Analyses | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Age (years old) | 1.015 (1.005–1.025) | 0.0033 * | 1.025 (1.013–1.038) | <0.0001 * |
| Female sex | 1.407 (1.094–1.809) | 0.0077 * | 1.365 (1.026–1.816) | 0.0324* |
| Smoking | 0.963 (0.753–1.233) | 0.7662 | ||
| Body mass index (kg/m2) | 1.055 (1.029–1.080) | <0.0001 * | 1.057 (1.030–1.085) | <0.0001 * |
| Impella 2.5 | 0.840 (0.472–1.435) | 0.5235 | ||
| Impella CP | 0.791 (0.544–1.152) | 0.2251 | ||
| PCI during index hospitalization | 0.616 (0.435–0.870) | 0.0060 * | ||
| CABG during index hospitalization | 0.598 (0.384–0.931) | 0.0277 * | ||
| Transcatheter SHD intervention | 0.271 (0.038–1.932) | 0.1929 | ||
| EP/ablation during index hospitalization | 0.563 (0.181–1.757) | 0.3230 | ||
| ECMO use before Impella placement | 2.218 (1.732–2.840) | <0.0001 * | 2.517 (1.967–3.221) | <0.0001 * |
| Pulmonary artery catheter use | 0.968 (0.767–1.222) | 0.7873 | ||
| LVEF at baseline (%) | 0.985 (0.973–0.998) | 0.0212 * | ||
| Serum Creatinine (mg/dL) | 1.009 (0.998–1.015) | 0.0970 | ||
| Total bilirubin (mg/dL) | 1.134 (1.064–1.193) | 0.0005 * | ||
| C-reactive protein (mg/dL) | 1.038 (1.021–1.054) | <0.0001 * | ||
| Creatinine kinase (IU/L) | 1.00004 (1.00002–1.00006) | 0.0001 * | ||
| Albumin (mg/dL) | 0.609 (0.517–0.720) | <0.0001 * | ||
| Impella-supported revascularization | 0.496 (0.398–0.617) | <0.0001 * | 0.605 (0.473–0.775) | <0.0001 * |
| All patients (n= 924) | Female (n = 192, 21%) | Male (n = 732, 79%) | p Value | |
|---|---|---|---|---|
| 30-day survival after Impella removal | 599 (64.8%) | 110 (57.3%) | 489 (66.8%) | 0.0173 |
| NYHA classification at 30 days after Impella removal | 0.0503 | |||
| Class I | 190 (24.0%) | 33 (20.8%) | 157 (24.8%) | |
| Class II | 206 (26.0%) | 36 (22.6%) | 170 (26.8%) | |
| Class III | 88 (11.1%) | 13 (8.2%) | 75 (11.8%) | |
| Class IV | 309 (49.0%) | 77 (48.4%) | 232 (36.6%) | |
| Survival discharge | 550 (59.5%) | 100 (52.1%) | 450 (61.5%) | 0.0514 |
| NYHA classification at discharge | 0.0047 | |||
| Class I | 200 (25.0%) | 43 (25.9%) | 157 (24.8%) | |
| Class II | 237 (29.7%) | 35 (21.1%) | 202 (31.9%) | |
| Class III | 54 (6.8%) | 7 (4.2%) | 47 (7.4%) | |
| Class IV | 308 (38.6%) | 81 (48.8%) | 227 (35.9%) | |
| All Patients (n = 924) | Female (n = 192, 21%) | Male (n = 732, 79%) | p Value | |
|---|---|---|---|---|
| Adverse events due to Impella device | 55 (6.0%) | 13 (6.8%) | 42 (5.8%) | 0.6070 |
| Adverse events during index hospitalization | 595 (64.4%) | 135 (70.3%) | 460 (62.8%) | 0.1191 |
| Bleeding (including hematoma) | 243(26.3%) | 53 (27.6%) | 190 (26.0%) | 0.646 |
| Sepsis and local infection | 73 (7.9%) | 19 (9.9%) | 54 (7.4%) | 0.2917 |
| Hemolysis | 156 (16.9%) | 29 (15.1%) | 127 (17.4%) | 0.5166 |
| Aortic valve regurgitation | 8 (0.9%) | 3 (1.6%) | 5 (0.7%) | 0.3737 |
| Cerebrovascular accident | 52 (5.6%) | 12 (6.3%) | 40 (5.5%) | 0.7247 |
| Renal failure | 93 (10.1%) | 18 (9.4%) | 75 (10.3%) | 0.7887 |
| Lower limb ischemia | 47 (5.1%) | 17 (8.9%) | 30 (4.1%) | 0.0148 |
| Vascular injury requiring intervention | 18 (2.0%) | 4 (2.1%) | 14 (1.9%) | 0.7761 |
| Thrombocytopenia | 68 (7.4%) | 10 (5.2%) | 58 (7.9%) | 0.2181 |
| Thrombotic complications without cerebrovascular accident | 12 (1.3%) | 4 (2.1%) | 8 (1.1%) | 0.2854 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Imamura, T.; Ueno, H.; Kinugawa, K.; Investigators, J.-P. Sex-Related Differences in Short-Term Prognosis in Patients with Acute Myocardial Infarction-Related Cardiogenic Shock Receiving Impella Support in Japan: From the J-PVAD Registry. Medicina 2023, 59, 1208. https://doi.org/10.3390/medicina59071208
Nakamura M, Imamura T, Ueno H, Kinugawa K, Investigators J-P. Sex-Related Differences in Short-Term Prognosis in Patients with Acute Myocardial Infarction-Related Cardiogenic Shock Receiving Impella Support in Japan: From the J-PVAD Registry. Medicina. 2023; 59(7):1208. https://doi.org/10.3390/medicina59071208
Chicago/Turabian StyleNakamura, Makiko, Teruhiko Imamura, Hiroshi Ueno, Koichiro Kinugawa, and J-PVAD Investigators. 2023. "Sex-Related Differences in Short-Term Prognosis in Patients with Acute Myocardial Infarction-Related Cardiogenic Shock Receiving Impella Support in Japan: From the J-PVAD Registry" Medicina 59, no. 7: 1208. https://doi.org/10.3390/medicina59071208
APA StyleNakamura, M., Imamura, T., Ueno, H., Kinugawa, K., & Investigators, J.-P. (2023). Sex-Related Differences in Short-Term Prognosis in Patients with Acute Myocardial Infarction-Related Cardiogenic Shock Receiving Impella Support in Japan: From the J-PVAD Registry. Medicina, 59(7), 1208. https://doi.org/10.3390/medicina59071208









