Immunolocalization of Enzymes/Membrane Transporters Related to Bone Mineralization in the Metaphyses of the Long Bones of Parathyroid-Hormone-Administered Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Histochemical Specimens
2.3. Immunostaining of TNALP, ENPP1, PHOSPHO1, FGF23, Osteopontin, and MEPE
2.4. Immunofluorescent Staining for the Detection of TNALP and ENPP1
2.5. Von Kossa Staining
2.6. Measurement of Serum Ca and Pi
2.7. Mineral Appositional Rate after PTH Administration
2.8. PCR and Real-Time PCR
2.9. Statistical Analysis
3. Results
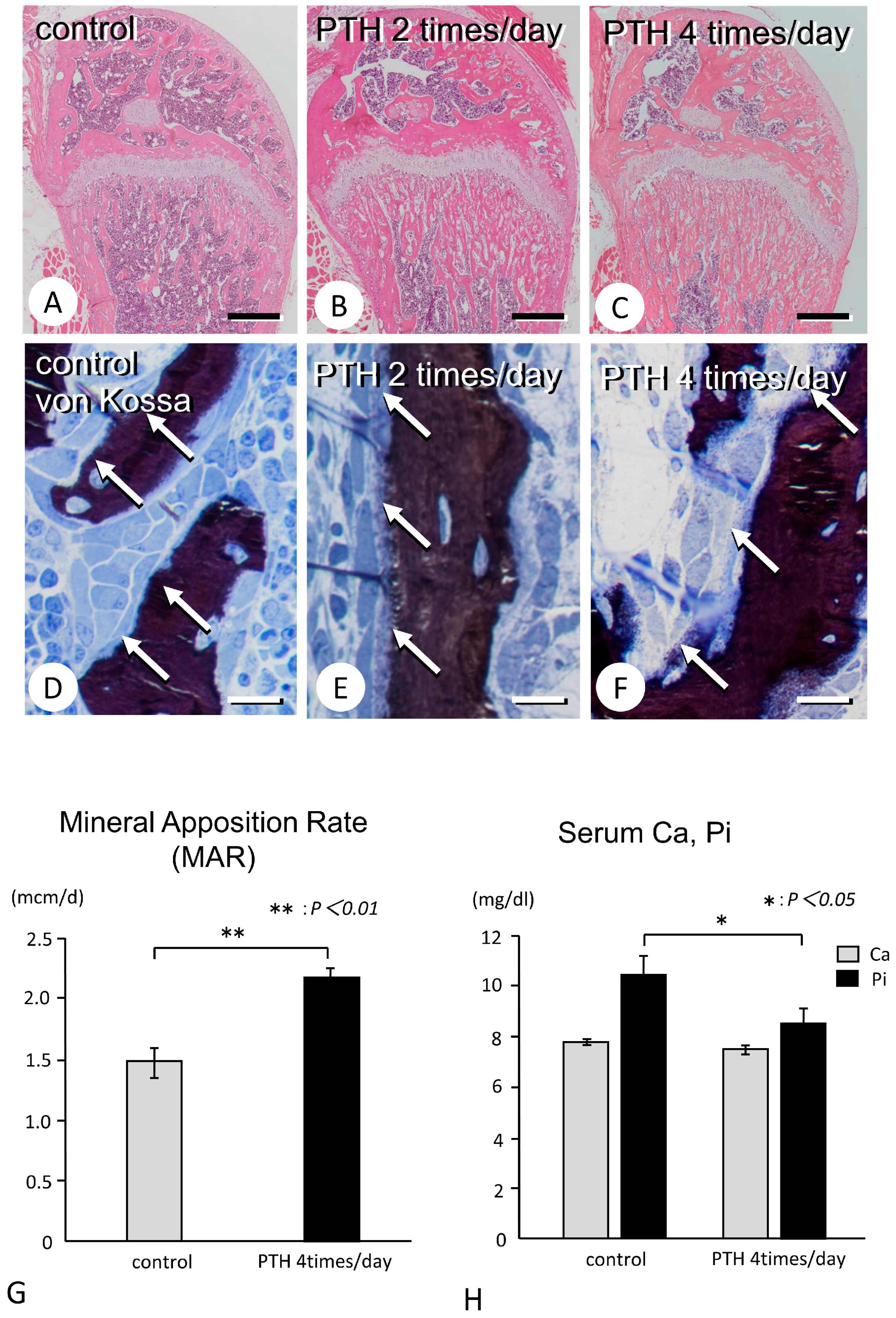
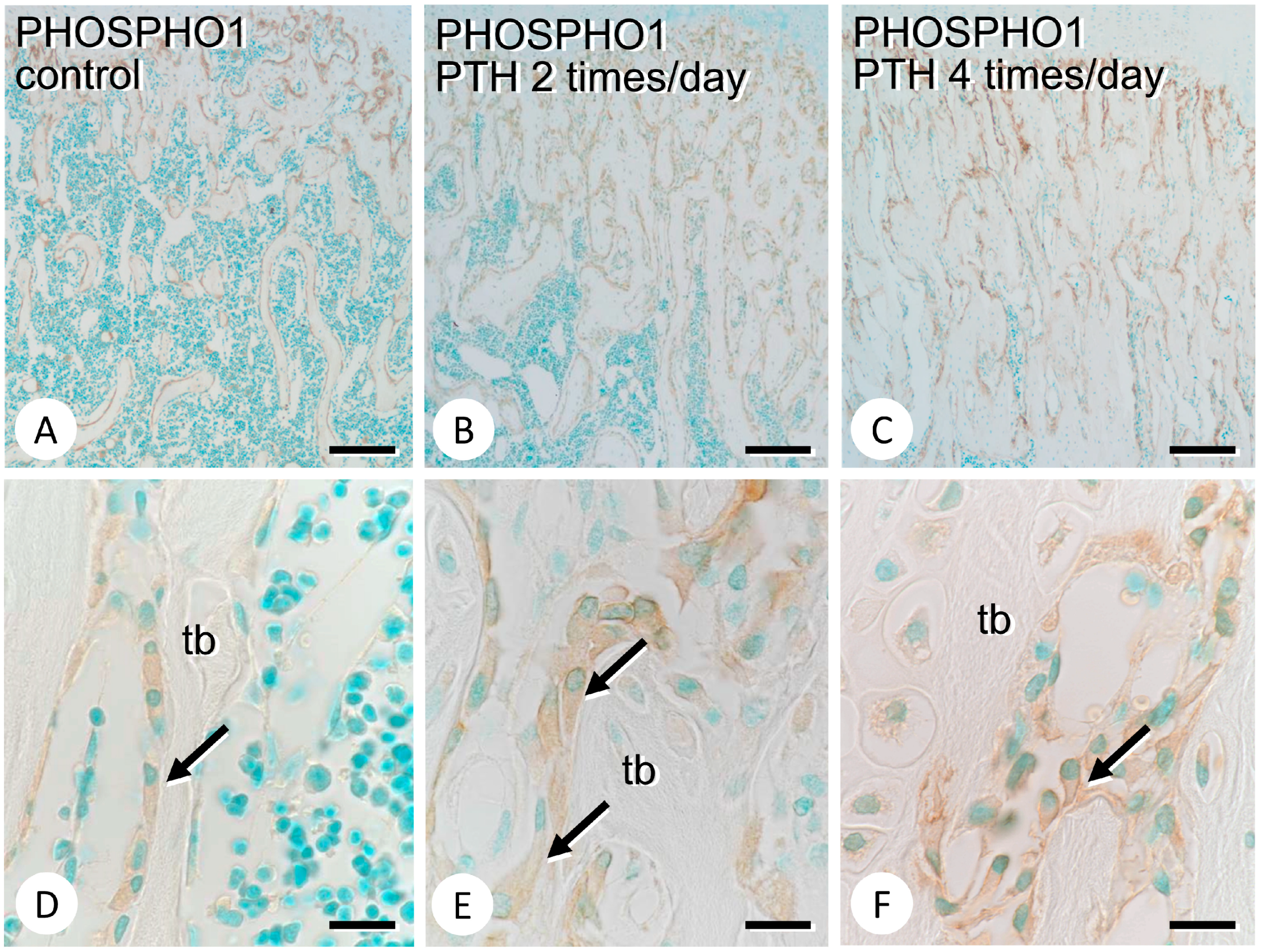
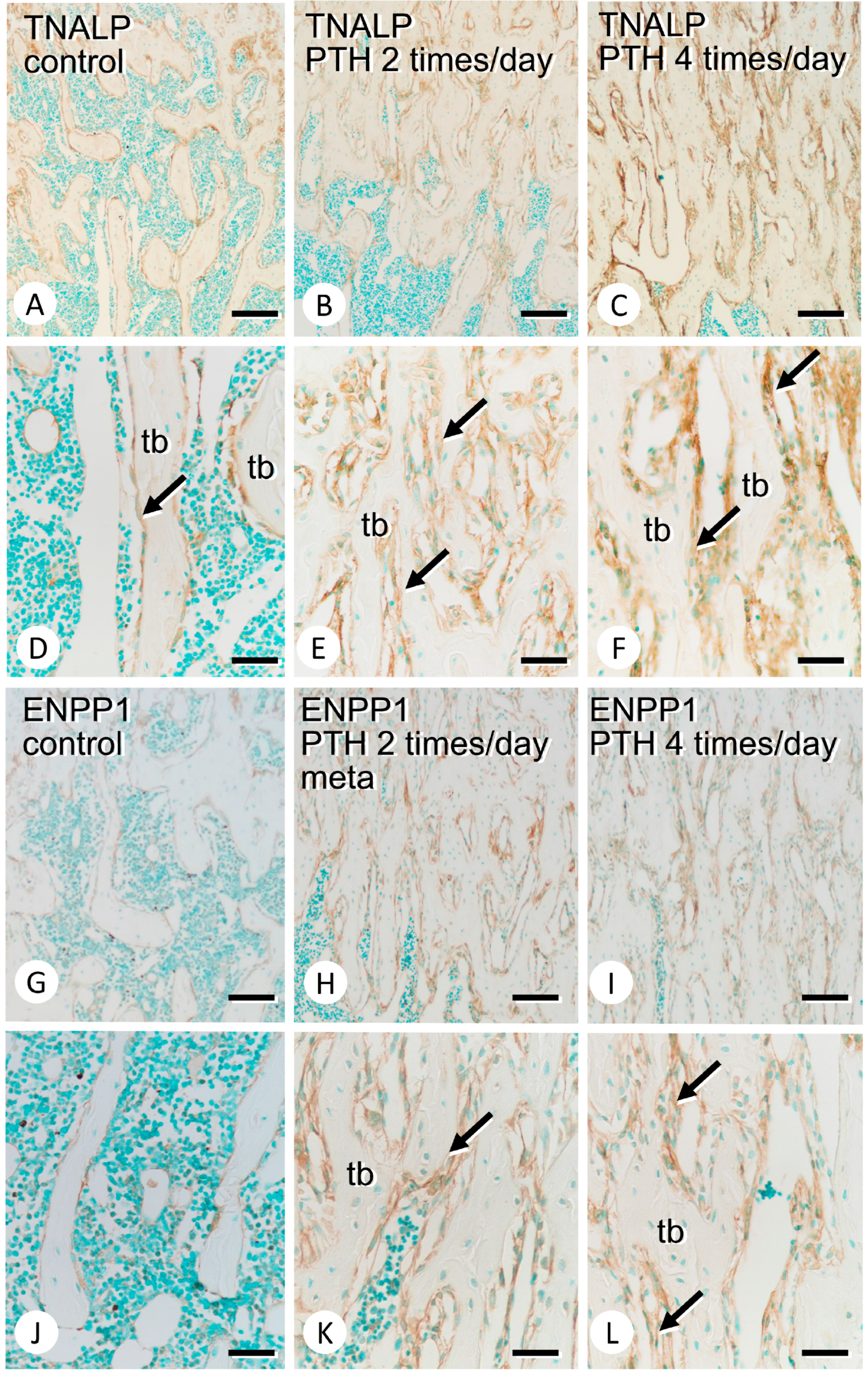
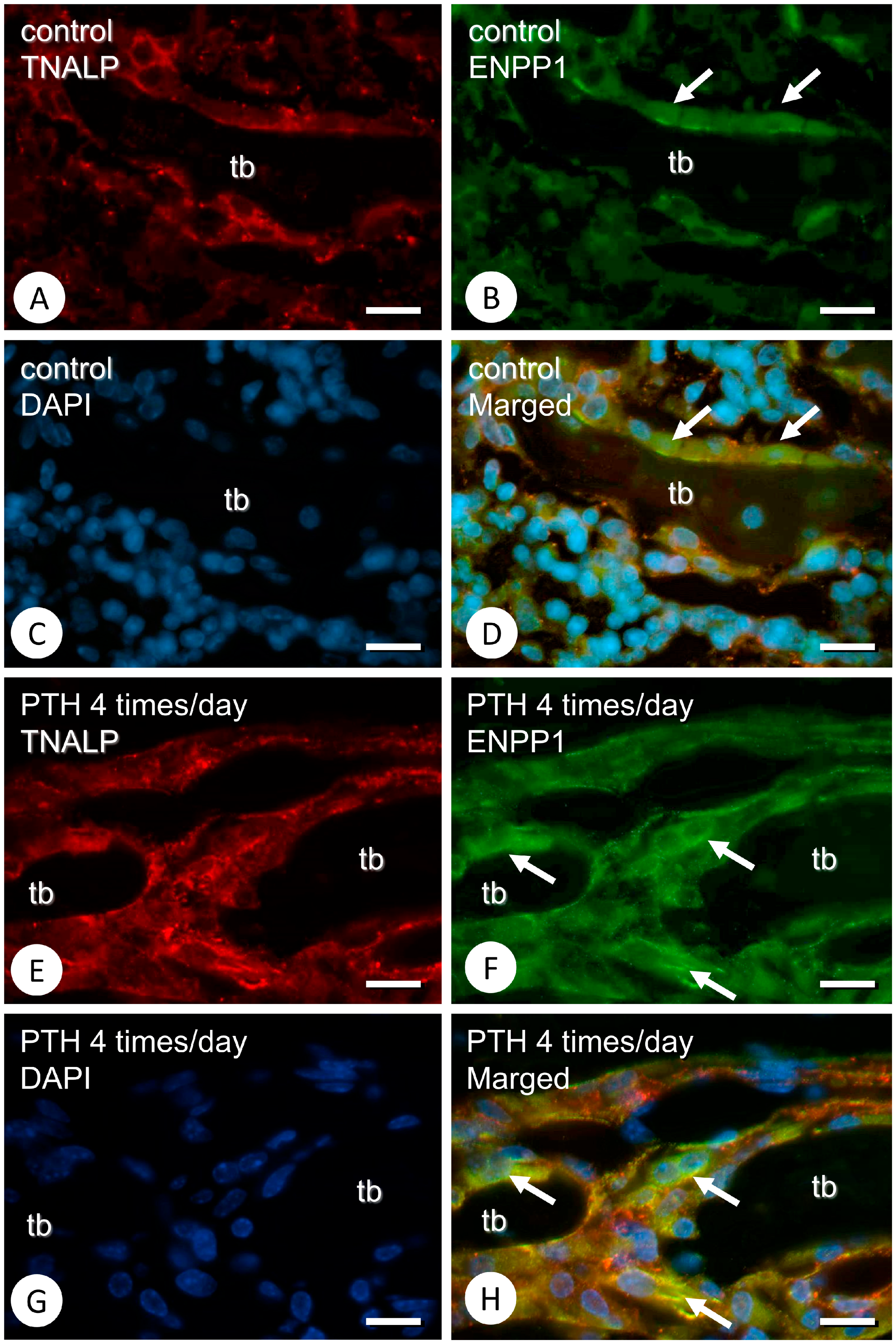
3.1. Metaphyseal Bone Mineralization and Immunolocalization of PHOSPHO1, TNALP, and ENPP1
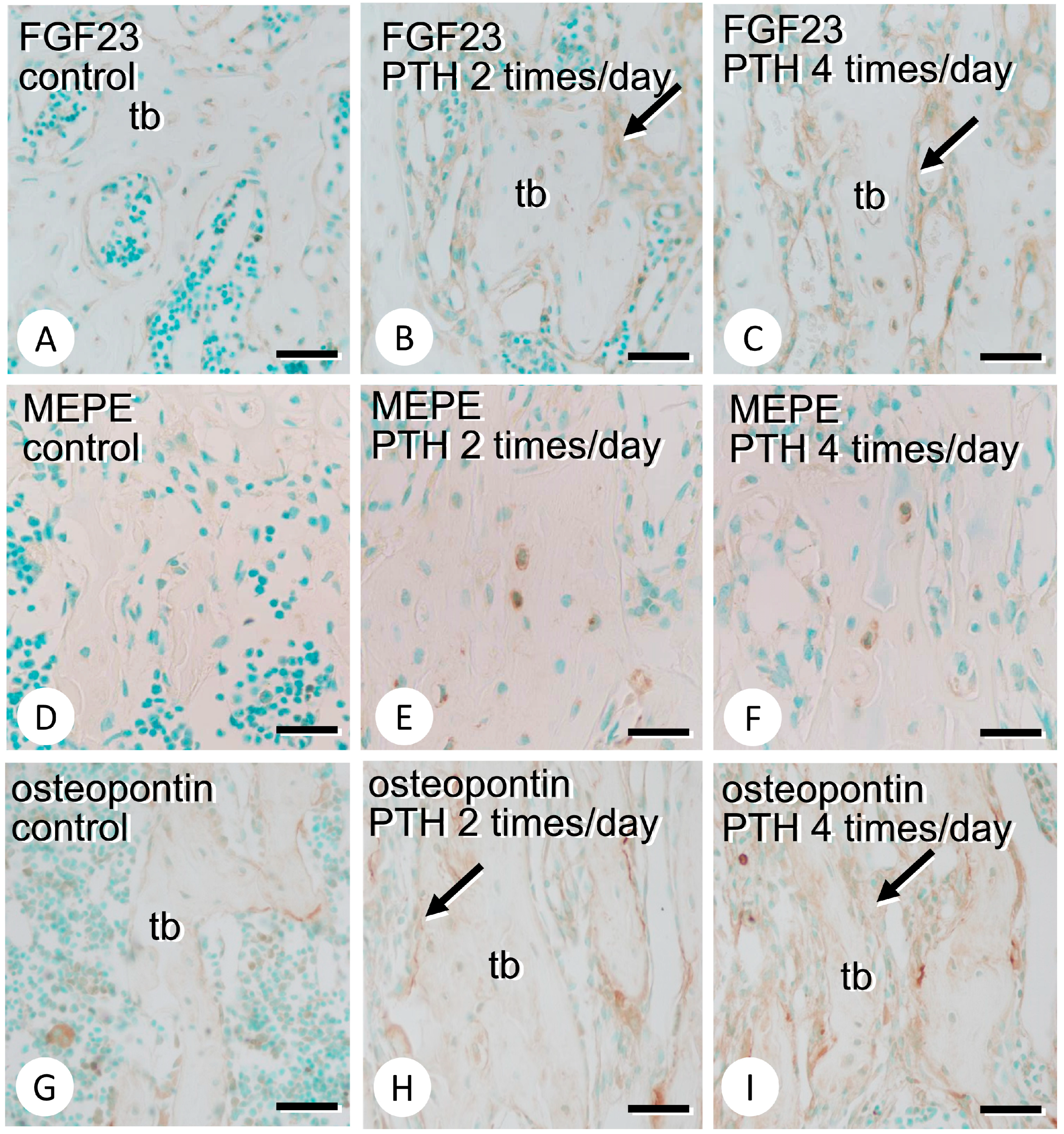
3.2. Immunolocalization of FGF23, MEPE, and Osteopontin in the Bone Matrix
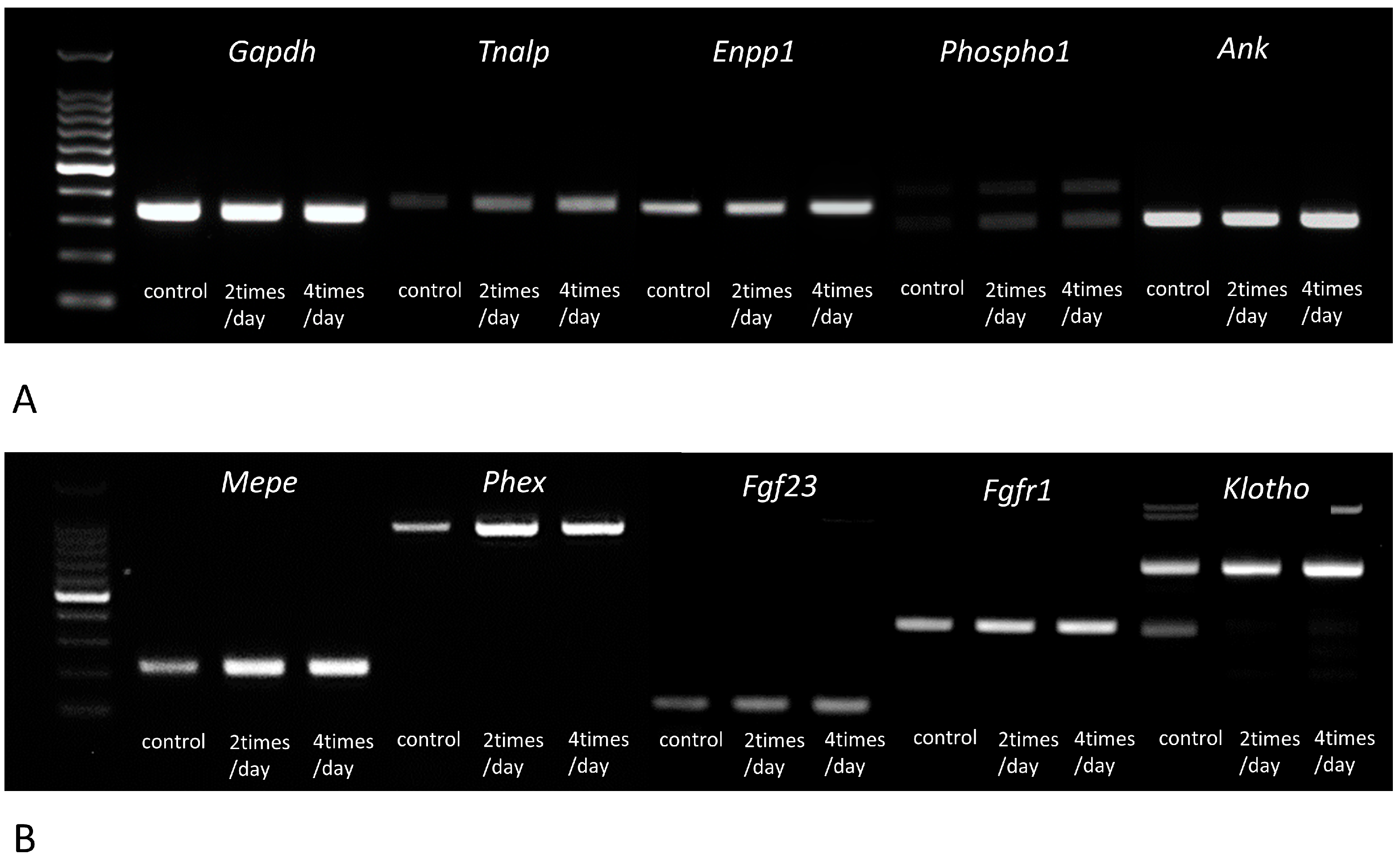
3.3. Gene Expressions of Tnalp, Enpp1, Phospho1, Ank, Mepe, Phex, Fgf23, Fgfr1, αKlotho, Osteopontin, Dmp1, and Cathepsin B
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozawa, H. Ultrastructural concepts on biological calcification; Focused on matrix vesicles. Jpn. J. Oral Biosci. 1985, 27, 751–774. [Google Scholar] [CrossRef]
- Ozawa, H.; Hoshi, K.; Amizuka, N. Current concepts of bone mineralization. Jpn. J. Oral Biosci. 2008, 50, 1–14. [Google Scholar] [CrossRef]
- Amizuka, N.; Hasegawa, T.; Oda, K.; Luiz de Freitas, P.H.; Hoshi, K.; Li, M.; Ozawa, H. Histology of epiphyseal cartilage calcification and endochondral ossification. Front. Biosci. 2012, 4, 2085–2100. [Google Scholar] [CrossRef]
- Hasegawa, T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem. Cell Biol. 2018, 149, 289–304. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hongo, H.; Yamamoto, T.; Abe, M.; Yoshino, H.; Haraguchi-Kitakamae, M.; Ishizu, H.; Shimizu, T.; Iwasaki, N.; Amizuka, N. Matrix vesicle-mediated mineralization and osteocytic regulation of bone mineralization. Int. J. Mol. Sci. 2022, 23, 9941. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Anderson, H.C. Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J. Histochem. Cytochem. 1971, 19, 801–808. [Google Scholar] [CrossRef] [PubMed]
- de Bernard, B.; Bianco, P.; Bonucci, E.; Costantini, M.; Lunazzi, G.C.; Martinuzzi, P.; Modricky, C.; Moro, L.; Panfili, E.; Pollesello, P. Biochemical and immunohistochemical evidence that in cartilage an alkaline phosphatase is a Ca2+-binding glycoprotein. J. Cell. Biol. 1986, 103, 1615–1623. [Google Scholar] [CrossRef]
- Hoshi, K.; Amizuka, N.; Oda, K.; Ikehara, Y.; Ozawa, H. Immunolocalization of tissue non-specific alkaline phosphatase in mice. Histochem. Cell. Biol. 1997, 107, 183–191. [Google Scholar] [CrossRef]
- Oda, K.; Amaya, Y.; Fukushi-Irie, M.; Kinameri, Y.; Ohsuye, K.; Kubota, I.; Fujimura, S.; Kobayashi, J. A general method for rapid purification of soluble versions of glycosylphosphatidylinositol-anchored proteins expressed in insect cells: An application for human tissue-nonspecific alkaline phosphatase. J. Biochem. 1999, 126, 694–699. [Google Scholar] [CrossRef]
- Nakano, Y.; Beertsen, W.; van den Bos, T.; Kawamoto, T.; Oda, K.; Takano, Y. Site-specific localization of two distinct phosphatases along the osteoblast plasma membrane: Tissue non-specific alkaline phosphatase and plasma membrane calcium ATPase. Bone 2004, 35, 1077–1085. [Google Scholar] [CrossRef]
- Houston, B.; Seawright, E.; Jefferies, D.; Hoogland, E.; Lester, D.; Whitehead, C.; Farquharson, C. Identification and cloning of a novel phosphatase expressed at high levels in differentiating growth plate chondrocytes. Biochim. Biophys. Acta 1999, 1448, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.; Stewart, A.J.; Farquharson, C. PHOSPHO1–A novel phosphatase specifically expressed at sites of mineralisation in bone and cartilage. Bone 2004, 34, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.J.; Stewart, A.J.; Sadler, P.J.; Farquharson, C. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem. J. 2004, 82, 59–65. [Google Scholar] [CrossRef]
- Roberts, S.; Narisawa, S.; Harmey, D.; Millán, J.L.; Farquharson, C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J. Bone Miner. Res. 2007, 22, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Terkeltaub, R.; Rosenbach, M.; Fong, F.; Goding, J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteoblasts with plasma cell membrane glycoprotein-1. Arthritis Rheum. 1994, 37, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Vaingankar, S.; Chen, Y.; Moffa, A.; Goldring, M.; Sano, K.; Jin-Hua, P.; Sali, A.; Goding, J.; Terkeltaub, R. Differential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondrocytes. Arthritis Rheum. 1999, 42, 1986–1997. [Google Scholar] [CrossRef]
- Johnson, K.; Moffa, A.; Chen, Y.; Pritzker, K.; Goding, J.; Terkeltaub, R. Matrix vesicle plasma membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J. Bone Miner. Res. 1999, 14, 883–892. [Google Scholar] [CrossRef]
- Andrilli, L.H.S.; Sebinelli, H.G.; Favarin, B.Z.; Cruz, M.A.E.; Ramos, A.P.; Bolean, M.; Millán, J.L.; Bottini, M.; Ciancaglini, P. NPP1 and TNAP hydrolyze ATP synergistically during biomineralization. Purinergic. Signal. 2022, in press. [Google Scholar] [CrossRef]
- Kato, K.; Nishimasu, H.; Okudaira, S.; Mihara, E.; Ishitani, R.; Takagi, J.; Aoki, J.; Nureki, O. Crystal structure of Enpp1, an extracellular glycoprotein involved in bone mineralization and insulin signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 16876–16881. [Google Scholar] [CrossRef]
- Ho, A.M.; Johnson, M.D.; Kingsley, D.M. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 2000, 289, 265–270. [Google Scholar] [CrossRef]
- Szeri, F.; Niaziorimi, F.; Donnelly, S.; Fariha, N.; Tertyshnaia, M.; Patel, D.; Lundkvist, S.; van de Wetering, K. The Mineralization Regulator ANKH Mediates Cellular Efflux of ATP, Not Pyrophosphate. J. Bone Miner. Res. 2022, 37, 1024–1031. [Google Scholar] [CrossRef]
- Lorenz-Depiereux, B.; Grabowski, M.; Meitinger, T.; Strom, T.M. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef]
- Kawai, M.; Kinoshita, S.; Shimba, S.; Ozono, K.; Michigami, T. Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm-dependent manner. J. Biol. Chem. 2014, 289, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Hasegawa, T.; Kudo, A.; Shen, Z.; Nagai, T.; Abe, M.; Yoshida, T.; Hongo, H.; Yamamoto, T.; Yamamoto, T.; et al. Chronological immunolocalization of sclerostin and FGF23 in the mouse metaphyseal trabecular and cortical bone. Biomed. Res. 2017, 38, 257–267. [Google Scholar] [CrossRef]
- Hasegawa, T.; Tokunaga, S.; Yamamoto, T.; Sakai, M.; Hongo, H.; Kawata, T.; Amizuka, N. Evocalcet rescues secondary hyperparathyroidism-driven cortical porosity in chronic kidney disease male rats. Endocrinology 2023, 164, bqad022. [Google Scholar] [CrossRef]
- Minamizaki, T.; Konishi, Y.; Sakurai, K.; Yoshioka, H.; Aubin, J.E.; Kozai, K.; Yoshiko, Y. Soluble Klotho causes hypomineralization in Klotho-deficient mice. J. Endocrinol. 2018, 237, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.T. The nature and functional significance of dentin extracellular matrix proteins. Int. J. Dev. Biol. 1995, 39, 169–179. [Google Scholar] [PubMed]
- Fisher, L.W. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 2001, 280, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Rowe, P.S. The wrickkened pathways of FGF23, MEPE and PHEX. Crit. Rev. Oral Biol. Med. 2004, 15, 264–281. [Google Scholar] [CrossRef]
- Bellahcène, A. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): Multifunctional proteins in cancer. Nat. Rev. Cancer 2008, 8, 212–226. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Martin, A.C.; Hedge, A.M.; Drezner, M.K.; Rowe, P.S. ASARM peptides: PHEX-dependent, independent regulation of serum phosphate. Am. J. Physiol. Renal Physiol. 2010, 300, F783–F791. [Google Scholar] [CrossRef] [PubMed]
- Staines, K.A.; MacRae, V.E.; Farquharson, C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 2012, 214, 241–255. [Google Scholar] [CrossRef]
- Luiz de Freitas, P.H.; Li, M.; Ninomiya, T.; Nakamura, M.; Ubaidus, S.; Oda, K.; Udagawa, N.; Maeda, T.; Takagi, R.; Amizuka, N. Intermittent PTH administration stimulates pre-osteoblastic proliferation without leading to enhanced bone formation in osteoclast-less c-fos(−/−) mice. J. Bone Miner. Res. 2009, 24, 1586–1597. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hasegawa, T.; Sasaki, M.; Hongo, H.; Tsuboi, K.; Shimizu, T.; Ohta, M.; Haraguchi, M.; Takahata, T.; Oda, K.; et al. Frequency of teriparatide administration affects the histological pattern of bone formation in young adult male mice. Endocrinology 2016, 157, 2604–2620. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Busse, B.; Ritchie, R.O. The fracture mechanics of human bone: Influence of disease and treatment. Bonekey Rep. 2015, 4, 743. [Google Scholar] [CrossRef]
- Buccino, F.; Colombo, C.; Vergani, L.M. A Review on Multiscale Bone Damage: From the Clinical to the Research Perspective. Materials 2021, 14, 1240. [Google Scholar] [CrossRef]
- Buccino, F.; Bagherifard, S.; D’Amico, L.; Zagra, L.; Banfi, G.; Tromba, G.; Vergani, L.M. Assessing the intimate mechanobiological link between human bone micro-scale trabecular architecture and micro-damages. Eng. Fract. Mech. 2022, 270, 108582. [Google Scholar] [CrossRef]
- Hasegawa, T.; Endo, T.; Tsuchiya, E.; Kudo, A.; Zhao, S.; Moritani, Y.; Abe, M.; Yamamoto, T.; Hongo, H.; Tsuboi, K.; et al. Biological application of focus ion beam-scanning electron microscopy (FIB-SEM) to the imaging of cartilaginous fibrils and osteoblastic cytoplasmic processes. J. Oral Biosci. 2017, 59, 55–62. [Google Scholar] [CrossRef]
- Hasegawa, T.; Amizuka, N.; Yamada, T.; Liu, Z.; Miyamoto, Y.; Yamamoto, T.; Sasaki, M.; Hongo, H.; Suzuki, R.; Freitas, P.H.L.; et al. Sclerostin is differently immunolocalized in metaphyseal trabecules and cortical bones of mouse tibiae. Biomed. Res. 2013, 34, 153–159. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamamoto, T.; Sakai, S.; Miyamoto, Y.; Hongo, H.; Qiu, Z.; Abe, M.; Takeda, S.; Oda, K.; Freitas, P.H.L.; et al. Histological effects of the combined administration of eldecalcitol and a parathyroid hormone in the metaphyseal trabeculae of ovariectomized rats. J. Histochem. Cytochem. 2019, 67, 169–184. [Google Scholar] [CrossRef]
- Hasegawa, T.; Li, M.; Hara, K.; Sasaki, M.; Tabata, C.; Freitas, P.H.L.; Hongo, H.; Suzuki, R.; Kobayashi, M.; Inoue, K.; et al. Morphological assessment of bone mineralization in tibial metaphyses of ascorbic acid-deficient ODS rats. Biomed. Res. 2011, 32, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.K.; Roschger, P.; Zeitz, U.; Klaushofer, K.; Andrukhova, O.; Erben, R.G. FGF23 regulates bone mineralization in a 1,25(OH)2 D3 and Klotho-independent manner. J. Bone Miner. Res. 2016, 31, 129–142. [Google Scholar] [CrossRef]
- Addison, W.N.; Nakano, Y.; Loisel, T.; Crine, P.; McKee, M.D. MEPE–ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: An inhibition regulated by PHEX cleavage of ASARM. J. Bone Miner. Res. 2008, 23, 1638–1649. [Google Scholar] [CrossRef]
- Martin, A.; David, V.; Laurence, J.S.; Schwarz, P.M.; Lafer, E.M.; Hedge, A.M.; Rowe, P.S. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology 2008, 149, 1757–1772. [Google Scholar] [CrossRef]
- Guo, R.; Rowe, P.S.; Liu, S.; Simpson, L.G.; Xiao, Z.S.; Quarles, L.D. Inhibition of MEPE cleavage by Phex. Biochem. Biophys. Res. Commun. 2002, 297, 38–45. [Google Scholar] [CrossRef]
- Han, S.L.; Wan, S.L. Effect of teriparatide on bone mineral density and fracture in postmenopausal osteoporosis: Meta-analysis of randomised controlled trials. Int. J. Clin. Pract. 2012, 66, 199–209. [Google Scholar] [CrossRef]
- Meier, C.; Lamy, O.; Krieg, M.A.; Mellinghoff, H.U.; Felder, M.; Ferrari, S.; Rizzoli, R. The role of teriparatide in sequential and combination therapy of osteoporosis. Eur. J. Med. Sci. 2014, 144, w13952. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Zhang, M.; Bockman, R. Bone mineral density response from teriparatide in patients with osteoporosis. Musculoskelet. J. Hosp. Spec. Surg. 2017, 13, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fenn, J.S.; Lorde, N.; Ward, J.M.; Borovickova, I. Hypophosphatasia. J. Clin. Pathol. 2021, 74, 635–640. [Google Scholar] [CrossRef]
- Rutsch, F.; Vaingankar, S.; Johnson, K.; Goldfine, I.; Maddux, B.; Schauerte, P.; Kalhoff, H.; Sano, K.; Boisvert, W.A.; Superti-Furga, A.; et al. PC-1 Nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am. J. Pathol. 2001, 158, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, F.; Ruf, N.; Vaingankar, S.; Toliat, M.R.; Suk, A.; Höhne, W.; Schauer, G.; Lehmann, M.; Roscioli, T.; Schnabel, D.; et al. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat. Genet. 2003, 34, 379–381. [Google Scholar] [CrossRef]
- Abhishek, A.; Doherty, M. Pathophysiology of articular chondrocalcinosis–role of ANKH. Nat. Rev. Rheumatol. 2011, 7, 96–104. [Google Scholar] [CrossRef]
- Francis, F.; Hennig, S.; Korn, B.; Reinhardt, R.; de Jong, P.; Poustka, A.; Lehrarch, H.; Rowe, P.S.N.; Goulding, J.N.; Summerfield, T.; et al. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 1995, 11, 130–136. [Google Scholar] [CrossRef]
| Gene Expression (Mean ± SE) | p-Value | Power (%) | ||
|---|---|---|---|---|
| Control | PTH 4 Times/Day | |||
| Tnalp (Alpl) | 1.00 ± 0.13 | 2.74 ± 0.19 | p < 0.01 | 100 |
| Enpp1 | 1.00 ± 0.14 | 1.76 ± 0.11 | p < 0.01 | 93 |
| Phosoho1 | 1.00 ± 0.13 | 2.79 ± 0.13 | p < 0.01 | 100 |
| Ank | 1.00 ± 0.02 | 1.28 ± 0.03 | p < 0.01 | 100 |
| Mepe | 1.00 ± 0.10 | 1.98 ± 0.17 | p < 0.01 | 100 |
| Phex | 1.00 ± 0.14 | 4.37 ± 0.45 | p < 0.01 | 100 |
| Fgf23 | 1.00 ± 0.03 | 1.38 ± 0.11 | p < 0.01 | 69 |
| Fgfr1 | 1.00 ± 0.02 | 2.18 ± 0.06 | p < 0.01 | 100 |
| Osteopontin | 1.00 ± 0.12 | 2.45 ± 0.28 | p < 0.01 | 98 |
| Dmp1 | 1.00 ± 0.10 | 2.65 ± 0.18 | p < 0.01 | 100 |
| Cathepsinb | 1.00 ± 0.01 | 0.92 ± 0.03 | p < 0.05 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mae, T.; Hasegawa, T.; Hongo, H.; Yamamoto, T.; Zhao, S.; Li, M.; Yamazaki, Y.; Amizuka, N. Immunolocalization of Enzymes/Membrane Transporters Related to Bone Mineralization in the Metaphyses of the Long Bones of Parathyroid-Hormone-Administered Mice. Medicina 2023, 59, 1179. https://doi.org/10.3390/medicina59061179
Mae T, Hasegawa T, Hongo H, Yamamoto T, Zhao S, Li M, Yamazaki Y, Amizuka N. Immunolocalization of Enzymes/Membrane Transporters Related to Bone Mineralization in the Metaphyses of the Long Bones of Parathyroid-Hormone-Administered Mice. Medicina. 2023; 59(6):1179. https://doi.org/10.3390/medicina59061179
Chicago/Turabian StyleMae, Takahito, Tomoka Hasegawa, Hiromi Hongo, Tomomaya Yamamoto, Shen Zhao, Minqi Li, Yutaka Yamazaki, and Norio Amizuka. 2023. "Immunolocalization of Enzymes/Membrane Transporters Related to Bone Mineralization in the Metaphyses of the Long Bones of Parathyroid-Hormone-Administered Mice" Medicina 59, no. 6: 1179. https://doi.org/10.3390/medicina59061179
APA StyleMae, T., Hasegawa, T., Hongo, H., Yamamoto, T., Zhao, S., Li, M., Yamazaki, Y., & Amizuka, N. (2023). Immunolocalization of Enzymes/Membrane Transporters Related to Bone Mineralization in the Metaphyses of the Long Bones of Parathyroid-Hormone-Administered Mice. Medicina, 59(6), 1179. https://doi.org/10.3390/medicina59061179







