Effect of Combined Electromagnetic Field and Plantar Flexion Resistance Exercise on Wound Healing in Patients with Venous Leg Ulcers: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Outcome Measures
2.3. Interventions
2.4. Sample Size and Statistical Analysis
3. Results
Demographic and Clinical Characteristics of the Patients
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Meara, S.; Al-Kurdi, D.; Ologun, Y.; Ovington, L.G.; Martyn-St James, M.; Richardson, R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst. Rev. 2014, CD003557. [Google Scholar] [CrossRef]
- Tripathi, R.K. Contemporary Management of Lower Extremity Venous Ulceration. Introduction. Semin. Vasc. Surg. 2015, 28, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Qiao, T.; Liu, C.; Ran, F. The impact of gastrocnemius muscle cell changes in chronic venous insufficiency. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 430–436. [Google Scholar] [CrossRef]
- Heinen, M.M.; van der Vleuten, C.; de Rooij, M.J.; Uden, C.J.; Evers, A.W.; van Achterberg, T. Physical activity and adherence to compression therapy in patients with venous leg ulcers. Arch. Dermatol. 2007, 143, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.; Kirsner, R.S.; Gailey, R.S.; Mandel, D.W.; Chen, S.C.; Tomic-Canic, M. Effect of physical therapy on wound healing and quality of life in patients with venous leg ulcers: A systematic review. JAMA Dermatol. 2015, 151, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Meissner, M.H.; Moneta, G.; Burnand, K.; Gloviczki, P.; Lohr, J.M.; Lurie, F.; Mattos, M.A.; McLafferty, R.B.; Mozes, G.; Rutherford, R.B. The hemodynamics and diagnosis of venous disease. J. Vasc. Surg. 2007, 46, S4–S24. [Google Scholar] [CrossRef]
- De Araujo, T.; Valencia, I.; Federman, D.G.; Kirsner, R.S. Managing the patient with venous ulcers. Ann. Intern. Med. 2003, 138, 326–334. [Google Scholar] [CrossRef]
- Franks, P.J.; Barker, J.; Collier, M.; Gethin, G.; Haesler, E.; Jawien, A.; Laeuchli, S.; Mosti, G.; Probst, S.; Weller, C. Management of Patients with Venous Leg Ulcers: Challenges and Current Best Practice. J. Wound Care 2016, 25 (Suppl. S6), S1–S67. [Google Scholar] [CrossRef]
- Alavi, A.; Sibbald, R.G.; Phillips, T.J.; Miller, O.F.; Margolis, D.J.; Marston, W.; Woo, K.; Romanelli, M.; Kirsner, R.S. What’s new: Management of venous leg ulcers: Treating venous leg ulcers. J. Am. Acad. Dermatol. 2016, 74, 643–664. [Google Scholar] [CrossRef]
- O’Meara, S.; Cullum, N.; Nelson, E.A.; Dumville, J.C. Compression for venous leg ulcers. Cochrane Database Syst. Rev. 2012, CD000265. [Google Scholar] [CrossRef]
- Kurd, S.K.; Hoffstad, O.J.; Bilker, W.B.; Margolis, D.J. Evaluation of the use of prognostic information for the care of individuals with venous leg ulcers or diabetic neuropathic foot ulcers. Wound Repair. Regen. 2009, 17, 318–325. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Percival, S.L. Biofilms in wounds: Management strategies. J. Wound Care 2008, 17, 502–508. [Google Scholar] [CrossRef]
- Tzaneva, V.; Mladenova, I.; Todorova, G.; Petkov, D. Antibiotic treatment and resistance in chronic wounds of vascular origin. Clujul Med. 2016, 89, 365. [Google Scholar] [CrossRef]
- Gottrup, F.; Jørgensen, B. Maggot debridement: An alternative method for debridement. Eplasty 2011, 11, e33. [Google Scholar]
- McLain, N.E.; Moore, Z.E.; Avsar, P. Wound cleansing for treating venous leg ulcers. Cochrane Database Syst. Rev. 2021, 3, CD011675. [Google Scholar] [CrossRef]
- Bradley, M.; Cullum, N.; Sheldon, T. The debridement of chronic wounds: A systematic review. In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews; University of York: York, UK, 2019. [Google Scholar]
- Bowler, P.; Davies, B.J. The Microbiology of Acute and Chronic Wounds. Wounds 1999, 11, 72–78. [Google Scholar]
- Radek, K.A.; Baer, L.A.; Eckhardt, J.; Di Pietro, L.A.; Wade, C.E. Mechanical unloading impairs keratinocyte migration and angiogenesis during cutaneous wound healing. J. Appl. Physiol. 2008, 104, 1295–1303. [Google Scholar] [CrossRef]
- Williams, K.J.; Ayekoloye, O.; Moore, H.M.; Davies, A.H. The calf muscle pump revisited. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 329–334. [Google Scholar] [CrossRef]
- Mulder, G.D. Treatment of open-skin wounds with electric stimulation. Arch. Phys. Med. Rehabil. 1991, 72, 375–377. [Google Scholar]
- Todd, D.; Heylings, D.; Allen, G.; McMillin, W. Treatment of chronic varicose ulcers with pulsed electromagnetic fields: A controlled pilot study. Ir. Med. J. 1991, 84, 54–55. [Google Scholar]
- Macklis, R.M. Magnetic healing, quackery, and the debate about the health effects of electromagnetic fields. Ann. Intern. Med. 1993, 118, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53, 2S–48S. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.K.; Rivera, M.A.; Matsagas, M.I.; Lazarides, M.K.; Robless, P.; Belcaro, G.; Geroulakos, G. Validation of the new venous severity scoring system in varicose vein surgery. J. Vasc. Surg. 2003, 38, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, R.T.; Raffetto, J.D. Chronic Venous Insufficiency. Circulation 2014, 130, 333–346. [Google Scholar] [CrossRef]
- Kloth, L.C. Electrical stimulation for wound healing: A review of evidence from in vitro studies, animal experiments, and clinical trials. Int. J. Low. Extrem. Wounds 2005, 4, 23–44. [Google Scholar] [CrossRef]
- Gschwandtner, M.E.; Ehringer, H. Microcirculation in chronic venous insufficiency. Vasc. Med. 2001, 6, 169–179. [Google Scholar] [CrossRef]
- Sopata, M.; Kucharzewski, M.; Tomaszewska, E. Antiseptic with modern wound dressings in the treatment of venous leg ulcers: Clinical and microbiological aspects. J. Wound Care 2016, 25, 419–426. [Google Scholar] [CrossRef]
- Hesham, S.E.D.A.; Shereen, M.A.; Sherief, E.R. Pulsed electromagnetic field (PEMF): Effective adjuvant therapy in venous and vasculitic leg ulcers. Mansoura Med. J. 2008, 37, 77–99. [Google Scholar]
- Tebbutt, N.; Robinson, L.; Todhunter, J.; Jonker, L. A plantar flexion device exercise programme for patients with peripheral arterial disease: A randomised prospective feasibility study. Physiotherapy 2011, 97, 244–249. [Google Scholar] [CrossRef]
- Ieran, M.; Zaffuto, S.; Bagnacani, M.; Annovi, M.; Moratti, A.; Cadossi, R. Effect of low frequency pulsing electromagnetic fields on skin ulcers of venous origin in humans: A double-blind study. J. Orthop. Res. 1990, 8, 276–282. [Google Scholar] [CrossRef]
- Stiller, M.; Pak, G.H.; Shupack, J.; Thaler, S.; Kenny, C.; Jondreau, L. A portable pulsed electromagnetic field (PEMF) device to enhance healing of recalcitrant venous ulcers: A double-blind, placebo-controlled clinical trial. Br. J. Dermatol. 1992, 127, 147–154. [Google Scholar] [CrossRef]
- Kenkre, J.; Hobbs, F.; Carter, Y.; Holder, R.; Holmes, E. A randomized controlled trial of electromagnetic therapy in the primary care management of venous leg ulceration. Fam. Pract. 1996, 13, 236–241. [Google Scholar] [CrossRef]
- Guerriero, F.; Botarelli, E.; Mele, G.; Polo, L.; Zoncu, D.; Renati, P.; Sgarlata, C.; Rollone, M.; Ricevuti, G.; Maurizi, N.; et al. Effectiveness of an Innovative Pulsed Electromagnetic Fields Stimulation in Healing of Untreatable Skin Ulcers in the Frail Elderly: Two Case Reports. Case Rep. Dermatol. Med. 2015, 2015, 576580. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; García-Cantú, R.; Barrera, R.; Méndez-Ramírez, I.; Navarro, V.c.H.; Serrano, G. Healing of chronic arterial and venous leg ulcers with systemic electromagnetic fields. Arch. Med. Res. 2002, 33, 281–289. [Google Scholar] [CrossRef]
- Keskin, Y.; Tastekin, N.; Kanter, M.; Top, H.; Ozdemir, F.; Erboga, M.; Taspinar, O.; Sut, N. The effect of magnetic field therapy and electric stimulation on experimental burn healing. Turk. J. Phys. Med. Rehabil. 2019, 65, 352–360. [Google Scholar] [CrossRef]
- Athanasiou, A.; Karkambounas, S.; Batistatou, A.; Lykoudis, E.; Katsaraki, A.; Kartsiouni, T.; Papalois, A.; Evangelou, A. The effect of pulsed electromagnetic fields on secondary skin wound healing: An experimental study. Bioelectromagnetics 2007, 28, 362–368. [Google Scholar] [CrossRef]
- Strauch, B.; Patel, M.K.; Navarro, J.A.; Berdichevsky, M.; Yu, H.L.; Pilla, A.A. Pulsed magnetic fields accelerate cutaneous wound healing in rats. Plast. Reconstr. Surg. 2007, 120, 425–430. [Google Scholar] [CrossRef]
- Costantini, E.; Sinjari, B.; D’Angelo, C.; Murmura, G.; Reale, M.; Caputi, S. Human gingival fibroblasts exposed to extremely low-frequency electromagnetic fields: In vitro model of wound-healing improvement. Int. J. Mol. Sci. 2019, 20, 2108. [Google Scholar] [CrossRef]
- Goodman, R.; Henderson, A.S. Some biological effects of electromagnetic fields. Bioelectrochem. Bioenerg. 1986, 15, 39–55. [Google Scholar] [CrossRef]
- Isakov, E.; Ring, H.; Mendelevich, I.; Boduragin, N.; Susak, Z.; Kupfert, Y.; Marchetti, N. Electromagnetic stimulation of stump wounds in diabetic amputees. J. Rehabil. Sci. 1996, 9, 46–48. [Google Scholar]
- Badea, M.; Vasilco, R.; Sandru, D.; Paslaru, L.; Jieanu, V.; Comorosan, S. The effect of pulsed electromagnetic field (Diapulse) on cellular systems. Rom. J. Physiol. Physiol. Sci. 1993, 30, 65–71. [Google Scholar]
- Granger, D.; Schmid-Shoenbein, G. Chronic venous ulceration: A role for leukocyte-mediated injury. In Physiology and Pathophysiology of Leukocyte Adhesion; Loosemore, T.M., Dormandy, J.A., Eds.; Oxford University Press: Oxford, UK, 1995; pp. 447–457. [Google Scholar]
- Ackerman, Z.; Seidenbaum, M.; Loewenthal, E.; Rubinow, A. Overload of iron in the skin of patients with varicose ulcers: Possible contributing role of iron accumulation in progression of the disease. Arch. Dermatol. 1988, 124, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Selvam, R.; Ganesan, K.; Raju, K.N.; Gangadharan, A.C.; Manohar, B.M.; Puvanakrishnan, R. Low frequency and low intensity pulsed electromagnetic field exerts its antiinflammatory effect through restoration of plasma membrane calcium ATPase activity. Life Sci. 2007, 80, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.M.; Moneta, G.L.; An International Consensus Committee on Chronic Venous Disease. Reporting standards in venous disease: An update. J. Vasc. Surg. 1995, 21, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Vandongen, Y.K.; Stacey, M.C. Changes in calf muscle function in chronic venous disease. Cardiovasc. Surg. 1999, 7, 451–456. [Google Scholar] [CrossRef]
- Kan, Y.M.; Delis, K.T. Hemodynamic effects of supervised calf muscle exercise in patients with venous leg ulceration: A prospective controlled study. Arch. Surg. 2001, 136, 1364–1369. [Google Scholar] [CrossRef]
- Jull, A.; Parag, V.; Walker, N.; Maddison, R.; Kerse, N.; Johns, T. The prepare pilot RCT of home-based progressive resistance exercises for venous leg ulcers. J. Wound Care 2009, 18, 497–503. [Google Scholar] [CrossRef]
- O’brien, J.A.; Edwards, H.E.; Finlayson, K.J.; Kerr, G. Understanding the relationships between the calf muscle pump, ankle range of motion and healing for adults with venous leg ulcers: A review of the literature. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2012, 20, 80–85. [Google Scholar]
- Jull, A.; Slark, J.; Parsons, J. Prescribed Exercise with Compression vs. Compression Alone in Treating Patients with Venous Leg Ulcers: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018, 154, 1304–1311. [Google Scholar] [CrossRef]
- Smith, D.; Lane, R.; McGinnes, R.; O’Brien, J.; Johnston, R.; Bugeja, L.; Team, V.; Weller, C. What is the effect of exercise on wound healing in patients with venous leg ulcers? A systematic review. Int. Wound J. 2018, 15, 441–453. [Google Scholar] [CrossRef]
- Qiu, Y.; Osadnik, C.R.; Team, V.; Weller, C.D. Effects of physical activity as an adjunct treatment on healing outcomes and recurrence of venous leg ulcers: A scoping review. Wound Repair. Regen. 2022, 30, 172–185. [Google Scholar] [CrossRef]
- Klonizakis, M.; Tew, G.; Gumber, A.; Crank, H.; King, B.; Middleton, G.; Michaels, J. Supervised exercise training as an adjunct therapy for venous leg ulcers: A randomized controlled feasibility trial. Br. J. Dermatol. 2018, 178, 1072–1082. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J.; Van Every, D.W.; Plotkin, D.L. Loading Recommendations for Muscle Strength, Hypertrophy, and Local Endurance: A Re-Examination of the Repetition Continuum. Sports 2021, 9, 32. [Google Scholar] [CrossRef]

| Variables | PEMF + PRE Group | PEMF Group | Control Group | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean (SD) | Min | Max | Mean (SD) | Min | Max | Mean (SD) | ||
| Age (years) | 46.02 | 58.10 | 52.06 (3.02) | 45.56 | 58.44 | 52 (3.22) | 45.47 | 58.79 | 52.13 (3.33) | 0.84 |
| Sex (male/female) | 9/6 | 6/9 | 7/8 | 0.56 | ||||||
| Weight (kg) | 52.20 | 91.80 | 72 (9.90) | 53.54 | 98.46 | 76 (11.23) | 54.08 | 95.92 | 75 (10.46) | 0.31 |
| Ulcer surface area (cm2) | 4.14 | 9.50 | 6.82 (1.34) | 4.44 | 9.24 | 6.84 (1.20) | 4.19 | 9.79 | 6.99 (1.40) | 0.92 |
| Ulcer volume (cm3) | 10.33 | 23.77 | 17.05 (3.36) | 11.07 | 23.15 | 17.11 (3.02) | 10.49 | 24.49 | 17.49 (3.50) | 0.97 |
| Characteristics | PEMF + PRE Group Mean (SD) | PEMF Group Mean (SD) | Control Group Mean (SD) | p-Value ** | |||
|---|---|---|---|---|---|---|---|
| PEMF + PRE Group Vs. PEMF Group | PEMF + PRE Vs. Control | PEMF Vs. Control | |||||
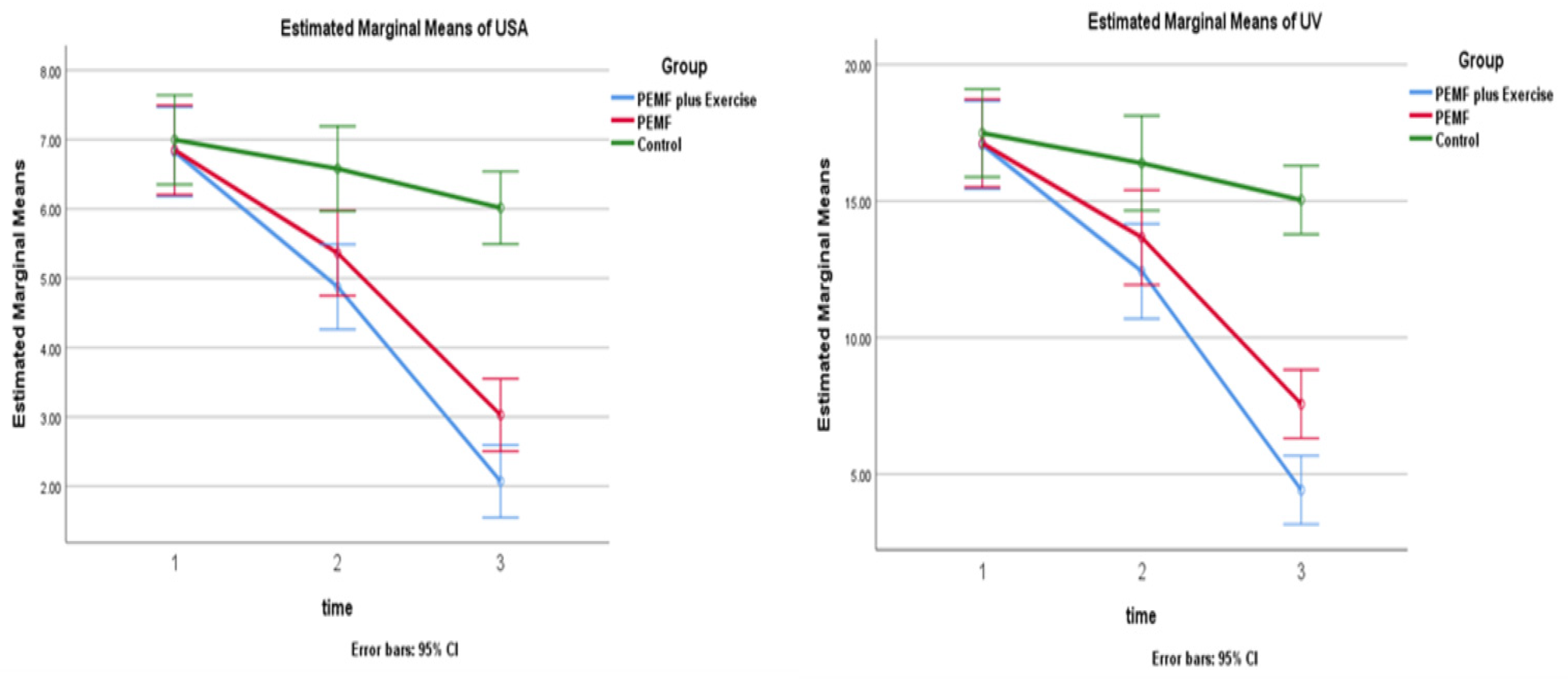
| Ulcer surface area (cm2) | Baseline | 6.82 (1.34) | 6.84 (1.20) | 6.99 (1.40) | 1.00 | 1.00 | 1.00 |
| 4 weeks | 4.87 (1.23) | 5.36 (1.44) | 6.57 (1.06) | 0.79 | 0.001 | 0.02 | |
| 12 weeks | 2.07 (1.15) | 3.02 (1.05) | 6.01 (0.99) | 0.037 | 0.001 | 0.001 | |
| Ulcer volume (cm3) | Baseline | 17.05 (3.36) | 17.11 (3.02) | 17.49 (3.50) | 1.00 | 1.00 | 1.00 |
| 4 weeks | 12.42 (4.05) | 13.66 (3.84) | 16.39 (2.60) | 0.94 | 0.006 | 0.01 | |
| 12 weeks | 4.41 (2.05) | 7.56 (3.08) | 15.04 (2.48) | 0.003 | 0.001 | 0.001 | |
| Characteristics | Baseline Vs. 4 Weeks | Baseline Vs. 12 Weeks | |||
|---|---|---|---|---|---|
| MD (95% CI) | p-Value ** | MD (95% CI) | p-Value ** | ||
| Ulcer surface area (cm2) | PEMF + PRE Group | −1.94 (−2.46, −1.42) | 0.0001 | −4.75 (−5.37, −4.12) | 0.0001 |
| PEMF Group | −1.48 (−2.00, −0.96) | 0.0001 | −3.82 (−4.44, −3.19) | 0.0001 | |
| Control Group | −0.41 (−0.93, −0.10) | 0.157 | −0.98 (−1.60, −0.35) | 0.001 | |
| Ulcer volume (cm3) | PEMF + PRE Group | −4.62 (−6.00, −3.24) | 0.0001 | −12.63 (−14.22, −11.05) | 0.0001 |
| PEMF Group | −3.45 (−4.83, −2.06) | 0.0001 | −9.55 (−11.13, −7.96) | 0.0001 | |
| Control Group | −1.10 (−2.48, −0.28) | 0.162 | −2.45 (−4.03, −0.86) | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamady, H.M.; Taha, M.M.; Aneis, Y.M.; Aldhahi, M.I.; Attalla, A.F. Effect of Combined Electromagnetic Field and Plantar Flexion Resistance Exercise on Wound Healing in Patients with Venous Leg Ulcers: A Randomized Controlled Trial. Medicina 2023, 59, 1157. https://doi.org/10.3390/medicina59061157
Mohamady HM, Taha MM, Aneis YM, Aldhahi MI, Attalla AF. Effect of Combined Electromagnetic Field and Plantar Flexion Resistance Exercise on Wound Healing in Patients with Venous Leg Ulcers: A Randomized Controlled Trial. Medicina. 2023; 59(6):1157. https://doi.org/10.3390/medicina59061157
Chicago/Turabian StyleMohamady, Heba Mohamed, Mona Mohamed Taha, Yasser M. Aneis, Monira I. Aldhahi, and Asmaa Fawzy Attalla. 2023. "Effect of Combined Electromagnetic Field and Plantar Flexion Resistance Exercise on Wound Healing in Patients with Venous Leg Ulcers: A Randomized Controlled Trial" Medicina 59, no. 6: 1157. https://doi.org/10.3390/medicina59061157
APA StyleMohamady, H. M., Taha, M. M., Aneis, Y. M., Aldhahi, M. I., & Attalla, A. F. (2023). Effect of Combined Electromagnetic Field and Plantar Flexion Resistance Exercise on Wound Healing in Patients with Venous Leg Ulcers: A Randomized Controlled Trial. Medicina, 59(6), 1157. https://doi.org/10.3390/medicina59061157






