Abstract
Background and Objectives: This study aimed to investigate the prevalence of sarcopenia in patients undergoing total knee arthroplasty (TKA) for advanced knee osteoarthritis (OA), and to assess whether sarcopenia accompanying OA affects patient-reported outcome measures (PROMs) after TKA. We evaluated which predisposing factors could influence the development of sarcopenia in patients with advanced knee OA. Material and Methods: A total of 445 patients whose body composition, muscle strength, and physical performance could be measured before primary TKA were enrolled. Sarcopenia was defined according to the Asian Working Group for Sarcopenia 2019 criteria. Patients were categorized into sarcopenia (S, n = 42) and non-sarcopenia groups (NS, n = 403). PROMs were investigated using the Knee Injury and Osteoarthritis Outcome Score and Western Ontario and McMaster Universities Osteoarthritis Index. Additionally, postoperative complications and predisposing factors for sarcopenia were evaluated. Results: The incidence of sarcopenia in the entire sample was 9.4%; the prevalence was higher in men (15.4%) than in women (8.7%), and significantly increased with advancing age (p < 0.001). At the six–month follow-up, PROMs in group S were significantly inferior to those in group NS, except for the pain score; however, at the 12-month follow-up, no significant difference was observed between the groups. Multivariate logistic regression indicated that age, body mass index (BMI), and a higher modified Charlson Comorbidity Index (mCCI) were predisposing factors for sarcopenia. Conclusions: A higher prevalence of sarcopenia was observed in men with progressive knee OA. Up to six months after primary TKA, PROMs in group S were inferior to those in group NS, except for the pain score; however, no significant difference was observed between the groups at 12 months. Age, BMI, and higher mCCI were predisposing factors for sarcopenia in patients with OA.
1. Introduction
Osteoarthritis (OA) is a destructive joint disease that cause cartilage degeneration, osteophyte formation, and changes in the periarticular bones, resulting in disability []. Total knee arthroplasty (TKA) is an effective treatment for end-stage OA of the knee joint [,], and its use has increased globally over the past few decades [,,,].
The role of muscles in maintaining functional performance to prevent falls and related fragility fractures has been emphasized [,]. Skeletal muscles naturally degenerate with age, and this loss accelerates after the age of 65 years, with a risk of adverse outcomes such as physical disability, poor quality of life, and death [,]. This condition is called sarcopenia, and is characterized by decreased muscle mass and strength and impaired muscle function [].
Sarcopenia and OA, which are clinically important and common pathological states, have become increasingly significant with the increase in the population of older adults [,]. In skeletal tissues, muscles and joints interact both mechanically and functionally. Therefore, aging and various pathological states simultaneously influence the muscles and joints [,]. Sarcopenia is an indicator of frailty and loss of independence in elderly individuals. Furthermore, it is associated with increased physical disability, resulting in an increased risk of falls. Patients with end-stage OA necessitating TKA have significant impairments in walking and daily living due to pain. Therefore, weakness in the muscles of the lower extremities, including the quadriceps, is inevitable [,]. Furthermore, sarcopenia caused by OA may affect the outcomes after TKA. However, there is a paucity of literature on the impact of sarcopenia on the outcomes of TKA.
This study aimed to investigate the prevalence of sarcopenia in patients undergoing TKA for end-stage knee OA, and whether sarcopenia accompanying OA affects patient-reported outcome measures (PROMs) after TKA. We attempted to answer the following questions:
- What is the incidence of sarcopenia in patients undergoing TKA for advanced knee OA?
- Are the PROMs in patients with sarcopenia associated with knee OA inferior to those in patients without sarcopenia?
- If yes, what are the predisposing factors for sarcopenia in patients with advanced knee OA?
2. Materials and Methods
2.1. Participants
The medical records of 471 consecutive patients (665 knees) who underwent primary TKA between May 2020 and August 2021 were screened. Inclusion criteria for this study were as follows: (1) patients with symptomatic advanced OA (Kellgren—Lawrence [K—L] grade ≥ 3), (2) age > 60 years, (3) having a minimum follow-up period of 12 months, and (4) whose body composition, muscle strength, and physical performance could be measured before the index operation. All procedures were performed by two experienced surgeons using the same cemented and posterior-stabilized implants. Patients with other types of diagnosis (rheumatoid arthritis, n = 7; post-traumatic OA, n = 4), non-independent ambulation within the previous one year due to other comorbid medical conditions (n = 5), metal implants in the appendicular body parts that could potentially affect the accuracy of appendicular skeletal muscle mass measurement (n = 6), or severe obesity (body mass index [BMI] > 35 kg/m2; n = 4) were excluded. Finally, 445 patients, including 190 who underwent bilateral primary TKA, were included in the analysis (Figure 1). This retrospective study was approved by the institutional review board of our hospital prior to gathering patient data.
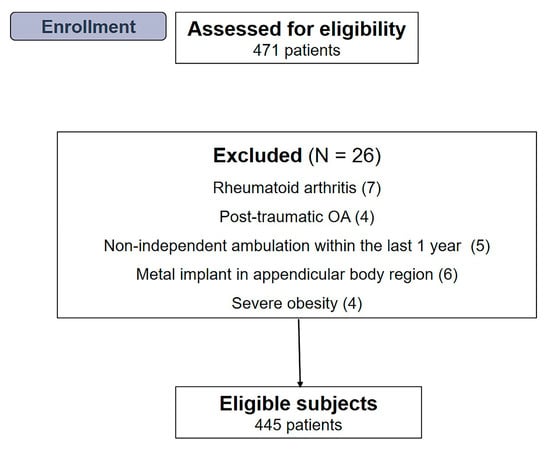
Figure 1.
Flow diagram illustrating patient enrollment. Overall, 445 patients were enrolled in our study.
2.2. Anthropometric Measurements
Body composition was measured using whole-body dual-energy X-ray absorptiometry (DXA) (Horizon, Hologic, Bedford, MA, USA) to calculate the skeletal muscle mass index (SMI). As absolute muscle mass correlates with height, SMI was calculated by correcting muscle mass with height (lean mass/height2 [kg/m2]). The total appendicular SMI (ASMI) was calculated as the sum of the arm SMI (arm lean mass/height2 [kg/m2]) and leg SMI (leg lean mass/height2 [kg/m2]) [].
2.3. Definition of Sarcopenia
Sarcopenia was defined based on the Asian Working Group for Sarcopenia (AWGS) 2019 diagnostic criteria []. Individuals with low muscle mass, muscle strength, and/or physical performance were classified as sarcopenia. Low muscle mass was defined as an ASMI < 5.4 kg/m2 for women and <7.0 kg/m2 for men [] (Figure 2).
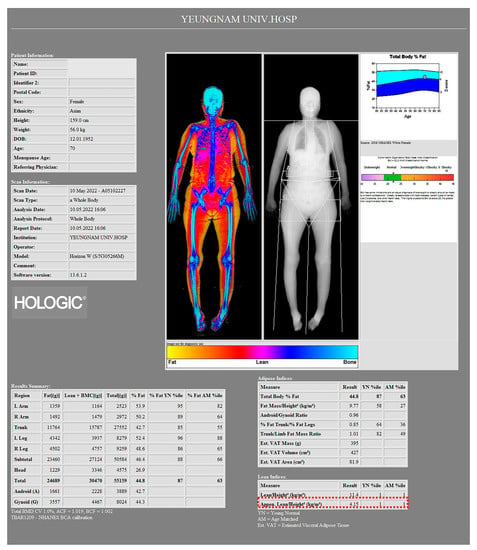
Figure 2.
Image of a 70-year-old female patient from a body composition measurement made by a whole-body dual-energy X-ray absorptiometry (DXA) scanner. According to the Asian Working Group for Sarcopenia (AWGS) 2019 diagnostic criteria, ASMI of this patient was 4.15 kg/m2 (red dotted box), which can be considered as a low muscle mass.
Muscle strength was assessed with a handgrip strength test using a handgrip dynamometer (Jamar, Bolingbrook, IL, USA) by applying the Southampton protocol []. Low muscle strength was defined as a handgrip strength <28 kg for men and <18 kg for women []. Physical performance was assessed using the six-meter walking speed test. Low physical performance was defined as a gait speed <1.0 m/s for both men and women []. Based on these criteria, the incidence of sarcopenia in patients with advanced OA was determined, and patients were classified to the sarcopenia group (group S) or the non-sarcopenia group (group NS).
2.4. Outcome Assessments
Demographic characteristics (including age, sex, BMI, follow-up period, and modified Charlson Comorbidity Index [mCCI] []) and laboratory data (including hemoglobin [Hb, g/dL] and total protein [g/dL] levels) were investigated before the index surgery. mCCI is a valuable preoperative risk assessment tool for patients undergoing surgery []. It was calculated by summing the weighted scores for the associated comorbidities (Table 1).

Table 1.
Scoring system for the modified Charlson Comorbidity Index (mCCI).
The prevalence of sarcopenia according to age was evaluated to identify age-related characteristics.
PROMs were assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS) [] and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) []. The range of motion (ROM) of the knee joint (including flexion contracture and further flexion) was measured using a standardized manual goniometer with a 30 cm plastic movable long arm []. Patients were regularly followed up postoperatively at six weeks and three, six, and 12 months. All clinical evaluations were compared between the two groups.
Further, the incidence of postoperative complications was compared between the groups. In addition to surgery-related complications such as postoperative blood transfusion or periprosthetic joint infection (PJI), systemic complications were investigated. Blood transfusion was performed in patients whose Hb levels dropped to <7.0 g/dL within 2 weeks of the index surgery []. PJI was diagnosed according to the latest evidence-based criteria from the International Consensus Meeting []. Systemic complications were defined as the exacerbation of underlying systemic comorbidities or the development of new medical problems [].
To identify the predisposing factors for sarcopenia in patients with end-stage knee OA, all variables were assessed using univariate and multivariate logistic regression analyses.
2.5. Postoperative Protocols
All patients underwent the same rehabilitation protocol. The drain was removed 24 h after surgery. A perioperative pain-control protocol was performed, including a multimodal drug regimen, postoperative patient-controlled analgesia, and intraoperative periarticular injections. Active and passive postoperative ROM exercises were initiated on the day of surgery. If the acute pain was tolerable, partial weightbearing with a crutch was allowed on the first postoperative day. Full weight-bearing was permitted 3 weeks after surgery.
2.6. Statistical Analysis
Statistical evaluation was performed using SPSS software version 28 (IBM Corp, Armonk, NY, USA), and continuous data are expressed as means ± standard deviation with ranges. All dependent variables were tested for normality of distribution and equality of variance using the Kolmogorov–Smirnov test. A two-sided Pearson’s χ2 test or Fisher’s exact test were used to compare the ratios between the groups. An independent samples t-test was used to determine significant differences between the two groups. Univariate and binary logistic regression analysis were performed on categorical and continuous variables to determine the predisposing factors for sarcopenia in patients with end-stage knee OA. All variables, including demographic data, preoperative laboratory data, and clinical outcomes, were analyzed individually using the univariate regression analysis. A multivariate binary logistic regression analysis was subsequently performed to identify the predisposing factors for sarcopenia while accounting for potential confounding variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using binary logistic regression analyses. Statistical significance was set at p < 0.05.
3. Results
A total of 445 patients (393 women, 52 men) were included in this study. The average age at operation was 71.8 years (range, 60–88 years), and the average follow-up period was 14.5 months (range, 12.0–26.0 months). According to the AWGS 2019 diagnostic criteria, the overall prevalence of sarcopenia in this cohort was 9.4% (34/363), and the prevalence was higher in men (8/52, 15.4%) than in women (34/393, 8.7%). Baseline characteristics and sarcopenia parameters are summarized in Table 2.

Table 2.
Baseline characteristics according to the presence or absence of sarcopenia.
The prevalence of sarcopenia increased significantly with age (p < 0.001) (Table 3).

Table 3.
Prevalence of sarcopenia according to age group 1.
At the six-month follow-up, group S showed significantly worse PROMs than group NS, except for the pain score; however, at the 12-month follow-up, there was no significant difference between the groups (Table 4).

Table 4.
Comparison of clinical outcomes between the groups.
(A) KOOS 1
More patients in group S received postoperative blood transfusions (p < 0.001). PJI occurred significantly more frequently in group S (acute postoperative infection, one; acute hematogenous infection, one) (p = 0.009). There was no significant difference in systemic complications between the two groups (Table 5).

Table 5.
The incidence of systemic and specific complications 1.
The results of the logistic regression analysis are presented in Table 6. The univariate binary logistic regression showed that age (OR, 1.5; 95% CI, 0.8–1.7; p < 0.001), BMI (OR, 0.8; 95% CI, 0.6–0.9; p < 0.001), preoperative levels of Hb (OR, 0.8; 95% CI, 0.6–0.9; p = 0.021) and total protein (OR, 0.7; 95% CI, 0.5–0.8; p = 0.037), and higher mCCI (OR, 1.1; 95% CI, 0.8–1.4; p < 0.001) were associated with sarcopenia. The multivariate binary logistic regression analysis to determine each variable’s independent contribution to the sarcopenic status after accounting for confounding factors showed that age (OR, 1.4; 95% CI, 0.9–1.8; p < 0.001), BMI (OR, 0.7; 95% CI, 0.6–0.9; p = 0.019), and higher mCCI (OR, 1.2; 95% CI, 0.8–1.5; p = 0.039) were risk factors for sarcopenia.

Table 6.
Univariate and multivariate logistic regression analyses for the presence of sarcopenia 1.
4. Discussion
The most notable findings of the present study were that a higher prevalence of sarcopenia was observed in men with progressive knee OA. Although PROMs in group S were inferior to those in group NS up to six months after primary TKA, no significant difference was observed between the groups postoperatively at 12 months. In addition, age, BMI, and higher mCCI were predisposing factors for sarcopenia in patients with progressive knee OA.
To date, few studies have reported the prevalence of sarcopenia in patients with knee OA [,]. However, there is a paucity of literature regarding the prevalence of sarcopenia in patients undergoing TKA for advanced OA. According to the diagnostic criteria and age, the incidence of sarcopenia in community-dwelling elderly individuals has been reported to vary from 5 to 50% [] and 0.1 to 23.6% []. The most important finding of the present study was that the overall prevalence of sarcopenia was 9.4%, which did not differ significantly from the prevalence in the general community-dwelling elderly population. Therefore, considering that sarcopenia often occurs in patients undergoing TKA for advanced arthritis, clinical outcomes can be predicted by diagnosing sarcopenia through preoperative anthropometric measurements and handgrip strength. Furthermore, interventions to improve sarcopenia as a modifiable factor should be considered.
In the present study, group S showed significantly inferior PROMs up to six months after TKA, and there was no significant difference between the two groups at 12 months postoperatively. This indicates that sarcopenic OA may have adversely affected early clinical outcomes after TKA. This is the first study to report that sarcopenia affects the outcome of TKA. Although a recent study reported the prevalence of sarcopenia and its effect on clinical outcomes in patients who underwent TKA for end-stage OA [], it only suggested that TKA could lead to significant improvements in clinical outcomes, even in patients with sarcopenic OA and did not compare clinical outcomes after TKA according to the presence of sarcopenia. Moreover, despite similar demographic characteristics compared to the present study, the reported incidence of sarcopenia in that study was 32.8% (19/58), which may be attributed to the relatively small sample size.
In terms of postoperative complications, patients in group S received postoperative blood transfusion more frequently. This is consistent with the results of a recent study that diagnosed sarcopenia based on bioelectrical impedance analysis and reported that patients with sarcopenia who underwent TKA for sarcopenic OA were more likely to require postoperative blood transfusion. This may be due to the lower preoperative Hb and total protein levels in group S in that study.
In addition, PJI was reported in two patients in group S. In a study that diagnosed sarcopenia using the psoas-lumber vertebral index, sarcopenia was reported to be a risk factor for PJI after TKA []. Further studies on sarcopenia as an optimizable predisposing factor for PJI after TKA are required.
Previous studies have reported that age, low BMI, and lower activity levels influence the progression of sarcopenia-related OA [,,]. Severe knee pain caused by advanced OA may impair physical function and increase the risk of sarcopenia. The increase in the prevalence of sarcopenia with age in the present study supports this finding. In addition, the prevalence of sarcopenia was higher in men than in women (15.4% vs. 8.7%), which may be attributed to a significant decrease in physical activity owing due to advanced knee OA in men, who were usually more active than women.
A lower BMI is a risk factor for sarcopenia [,]. As ASMI is positively correlated with BMI and it is well known that obesity or sarcopenic obesity increases the risk of arthritis, the relationship between BMI and sarcopenia is contentious []. In the present study, lower BMI was associated with an increased risk of sarcopenia. However, BMI is a modifiable factor that can be improved through dietary interventions. Therefore, further studies on improving the BMI in patients with advanced OA-related sarcopenia are required.
Despite these informative results, this study had some limitations. First, this was a retrospective study with a short-term follow-up. Although the recovery of muscle mass or muscle strength after TKA could not been confirmed, clinical outcomes continuously improved after TKA and were maintained for 12 months after surgery. A follow-up period of approximately 12 months can be considered reasonable for the recovery of muscle mass or strength. Second, it was not possible to evaluate the degree of objective recovery after surgery because additional DXA and handgrip strength evaluations were not performed for muscle mass and muscle strength after TKA, respectively. Although there may be additional medical costs or insurance issues, future research should objectively evaluate data on recovery using additional anthropometric measurements. Third, the cohort in the present study was not representative of all grades of knee OA. Sarcopenic parameters and clinical outcomes may vary in patients with mild arthritis. This study provides meaningful information on the effects of muscle mass or strength on the outcomes in patients who underwent TKA for advanced knee OA. Finally, women were predominant in the present study. Because the prevalence of knee OA in women is much higher in Asia, women usually undergo TKAs more frequently than men []. Moreover, since the prevalence of sarcopenia and knee OA differed by gender, several factors leading to the progression of OA independently of sarcopenia may need to be considered. Particularly, the relationship between obesity and sarcopenia is controversial, and the variables may have different effects depending on ethnicity [,]. Therefore, the results of this study may not be generalizable to other ethnic groups.
Nevertheless, our study had important strengths. This study assessed the incidence of sarcopenia in patients with progressive knee OA and presented the differences according to sex and age group. Furthermore, the predisposing factors for sarcopenia in these patients were investigated.
5. Conclusions
A higher prevalence of sarcopenia was observed in men with progressive knee OA. Up to six months after primary TKA, PROMs in group S were inferior to those in group NS, except for the pain score; however, no significant difference was observed between the groups at 12 months. Age, BMI, and higher mCCI were predisposing factors for sarcopenia in patients with OA.
Author Contributions
Conceptualization, G.B.K.; methodology, G.B.K. and O.-J.S.; formal analysis, G.B.K. and S.J.C.; resources, G.B.K.; data curation, G.B.K. and S.J.C.; writing—original draft preparation, G.B.K.; writing—review and editing, G.B.K. and O.-J.S.; visualization, G.B.K.; supervision, O.-J.S.; project administration, G.B.K.; funding acquisition, G.B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A1A03040177).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the Yeungnam University Medical Center (Protocol No. 2022-10-022), the approved date is 24 October 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data supporting the reported findings are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Stenquist, D.S.; Elman, S.A.; Davis, A.M.; Bogart, L.M.; Brownlee, S.A.; Sanchez, E.S.; Santiago, A.; Ghazinouri, R.; Katz, J.N. Physical activity and experience of total knee replacement in patients one to four years postsurgery in the dominican republic: A qualitative study. Arthritis Care Res. 2015, 67, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Canovas, F.; Dagneaux, L. Quality of life after total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2018, 104, S41–S46. [Google Scholar] [CrossRef]
- Chang, M.J.; Kim, S.H.; Kang, Y.G.; Chang, C.B.; Kim, T.K. Activity levels and participation in physical activities by korean patients following total knee arthroplasty. BMC Musculoskelet. Disord. 2014, 15, 240. [Google Scholar] [CrossRef]
- Inacio, M.; Paxton, E.; Graves, S.; Namba, R.; Nemes, S. Projected increase in total knee arthroplasty in the united states—An alternative projection model. Osteoarthr. Cart. 2017, 25, 1797–1803. [Google Scholar] [CrossRef]
- Kim, T.W.; Kang, S.-B.; Chang, C.B.; Moon, S.-Y.; Lee, Y.-K.; Koo, K.-H. Current trends and projected burden of primary and revision total knee arthroplasty in korea between 2010 and 2030. J. Arthroplast. 2021, 36, 93–101. [Google Scholar] [CrossRef]
- Koh, I.J.; Kim, T.K.; Chang, C.B.; Cho, H.J.; In, Y. Trends in use of total knee arthroplasty in korea from 2001 to 2010. Clin. Orthop. Relat. Res. 2013, 471, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M. Sarcopenia: European consensus on definition and diagnosisreport of the european working group on sarcopenia in older peoplea. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Bokshan, S.L.; Han, A.L.; DePasse, J.M.; Eltorai, A.E.; Marcaccio, S.E.; Palumbo, M.A.; Daniels, A.H. Effect of sarcopenia on postoperative morbidity and mortality after thoracolumbar spine surgery. Orthopedics 2016, 39, e1159–e1164. [Google Scholar] [CrossRef]
- Evans, W.J. Sarcopenia should reflect the contribution of age-associated changes in skeletal muscle to risk of morbidity and mortality in elderly people. J. Am. Med. Dir. Assoc. 2015, 16, 546–547. [Google Scholar] [CrossRef]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised european consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Wrigley, T.V.; Hunt, M.A.; Lim, B.-W.; Hinman, R.S. Update on the role of muscle in the genesis and management of knee osteoarthritis. Rheum. Dis. Clin. North. Am. 2013, 39, 145–176. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.; Mills, P.M.; Barrett, R.S. Muscle weakness in hip osteoarthritis: A systematic review. Arthritis Care Res. 2013, 65, 340–352. [Google Scholar] [CrossRef]
- Baker, K.R.; Xu, L.; Zhang, Y.; Nevitt, M.; Niu, J.; Aliabadi, P.; Yu, W.; Felson, D. Quadriceps weakness and its relationship to tibiofemoral and patellofemoral knee osteoarthritis in chinese: The beijing osteoarthritis study. Arthritis Rheum. 2004, 50, 1815–1821. [Google Scholar] [CrossRef]
- Zhai, G.; Blizzard, L.; Srikanth, V.; Ding, C.; Cooley, H.; Cicuttini, F.; Jones, G. Correlates of knee pain in older adults: Tasmanian older adult cohort study. Arthritis Care Res. 2006, 55, 264–271. [Google Scholar] [CrossRef]
- Ho, K.K.-W.; Lau, L.C.-M.; Chau, W.-W.; Poon, Q.; Chung, K.-Y.; Wong, R.M.-Y. End-stage knee osteoarthritis with and without sarcopenia and the effect of knee arthroplasty—A prospective cohort study. BMC Geriatr. 2021, 21, 2. [Google Scholar] [CrossRef]
- McIsaac, D.; Beaule, P.; Bryson, G.; Van Walraven, C. The impact of frailty on outcomes and healthcare resource usage after total joint arthroplasty: A population-based cohort study. Bone Jt. J. 2016, 98, 799–805. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Smith, R.; Aulet, M.; Bensen, B.; Lichtman, S.; Wang, J.; Pierson, R., Jr. Appendicular skeletal muscle mass: Measurement by dual-photon absorptiometry. Am. J. Clin. Nutr. 1990, 52, 214–218. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Lakomkin, N.; Zuckerman, S.L.; Stannard, B.; Montejo, J.; Sussman, E.S.; Virojanapa, J.; Kuzmik, G.; Goz, V.; Hadjipanayis, C.G.; Cheng, J.S. Preoperative risk stratification in spine tumor surgery: A comparison of the modified charlson index, frailty index, and asa score. Spine 2019, 44, E782–E787. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Lohmander, L.S. The knee injury and osteoarthritis outcome score (koos): From joint injury to osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of womac: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Norkin, C.C.; White, D.J. Measurement of Joint Motion: A Guide to Goniometry; FA Davis: Philadelphia, PA, USA, 2016. [Google Scholar]
- Jo, C.; Ko, S.; Shin, W.C.; Han, H.-S.; Lee, M.C.; Ko, T.; Ro, D.H. Transfusion after total knee arthroplasty can be predicted using the machine learning algorithm. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 1757–1764. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 definition of periprosthetic hip and knee infection: An evidence-based and validated criteria. J. Arthroplast. 2018, 33, 1309–1314. [Google Scholar] [CrossRef]
- Song, S.J.; Kim, K.I.; Bae, D.K.; Park, C.H. Mid-term lifetime survivals of octogenarians following primary and revision total knee arthroplasties were satisfactory: A retrospective single center study in contemporary period. Knee Surg. Relat. Res. 2020, 32, 50. [Google Scholar] [CrossRef]
- Misra, D.; Fielding, R.A.; Felson, D.T.; Niu, J.; Brown, C.; Nevitt, M.; Lewis, C.E.; Torner, J.; Neogi, T.; MOST study. Risk of knee osteoarthritis with obesity, sarcopenic obesity, and sarcopenia. Arthritis Rheumatol. 2019, 71, 232–237. [Google Scholar] [CrossRef]
- Papalia, R.; Zampogna, B.; Torre, G.; Lanotte, A.; Vasta, S.; Albo, E.; Tecame, A.; Denaro, V. Sarcopenia and its relationship with osteoarthritis: Risk factor or direct consequence? Musculoskelet. Surg. 2014, 98, 9–14. [Google Scholar] [CrossRef]
- Von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Babu, J.M.; Kalagara, S.; Durand, W.; Antoci, V.; Deren, M.E.; Cohen, E. Sarcopenia as a risk factor for prosthetic infection after total hip or knee arthroplasty. J. Arthroplast. 2019, 34, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, C.J.; Jung, M.-G.; Choi, Y.H.; Park, K.-S.; Koh, H.S. High prevalence of sarcopenia in asian female patients awaiting primary total knee arthroplasty: Application of updated diagnostic tools from the asian working group for sarcopenia. J. Orthop. Surg. 2022, 30, 10225536221113034. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Granic, A.; Davies, K.; Kirkwood, T.B.; Jagger, C.; Sayer, A.A. Prevalence and incidence of sarcopenia in the very old: Findings from the newcastle 85+ study. J. Cache Sarcop Muscle 2017, 8, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wong, M.; Leung, J.; Lee, J.; Auyeung, T.W.; Woo, J. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older c hinese adults. Geriatr. Gerontol. Int. 2014, 14, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-R.; Cho, S.-K.; Im, S.G.; Jung, S.-Y.; Kim, D.; Jang, E.J.; Sung, Y.-K. Treatment patterns of knee osteoarthritis patients in Korea. Korean J. Intern. Med. 2019, 34, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).