Hypotensive Effect of Electric Stimulation of Caudal Ventrolateral Medulla in Freely Moving Rats
Abstract
1. Introduction
2. Materials and Methods
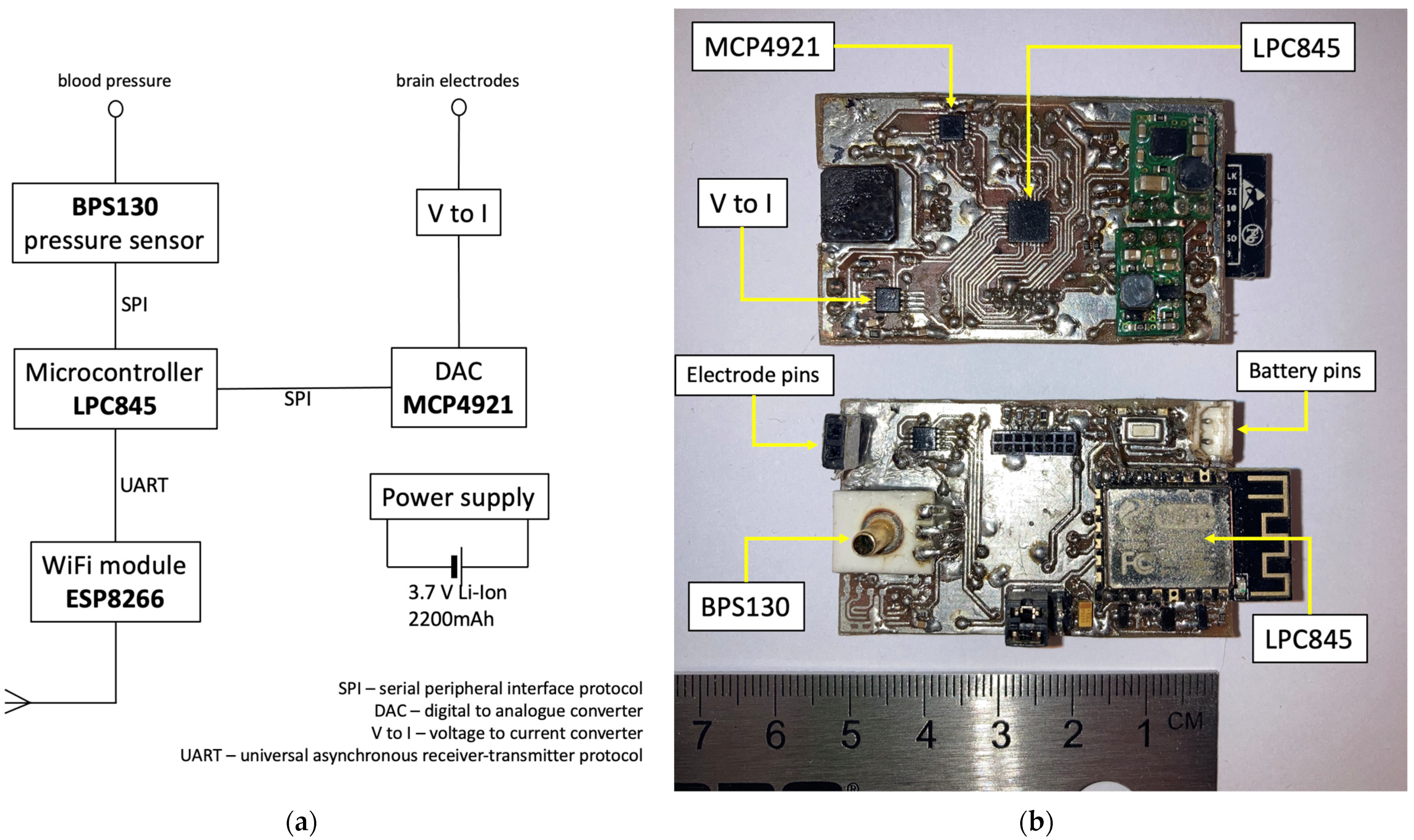
2.1. Design of the Device
2.2. Preoperative Period
2.3. Surgery
2.4. Postoperative Period and Data Analysis
3. Results
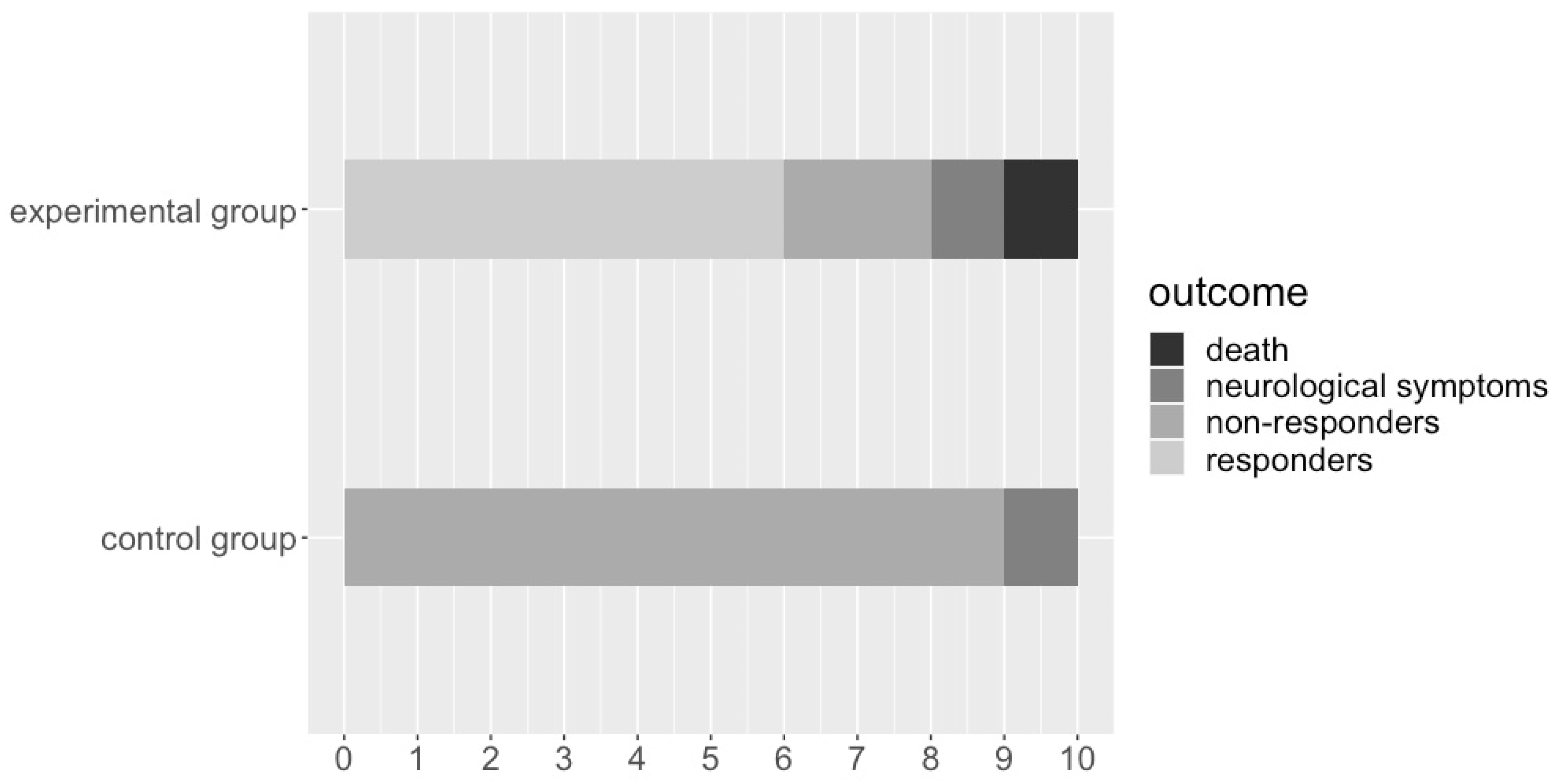
3.1. Survivability and Neurological Compromise
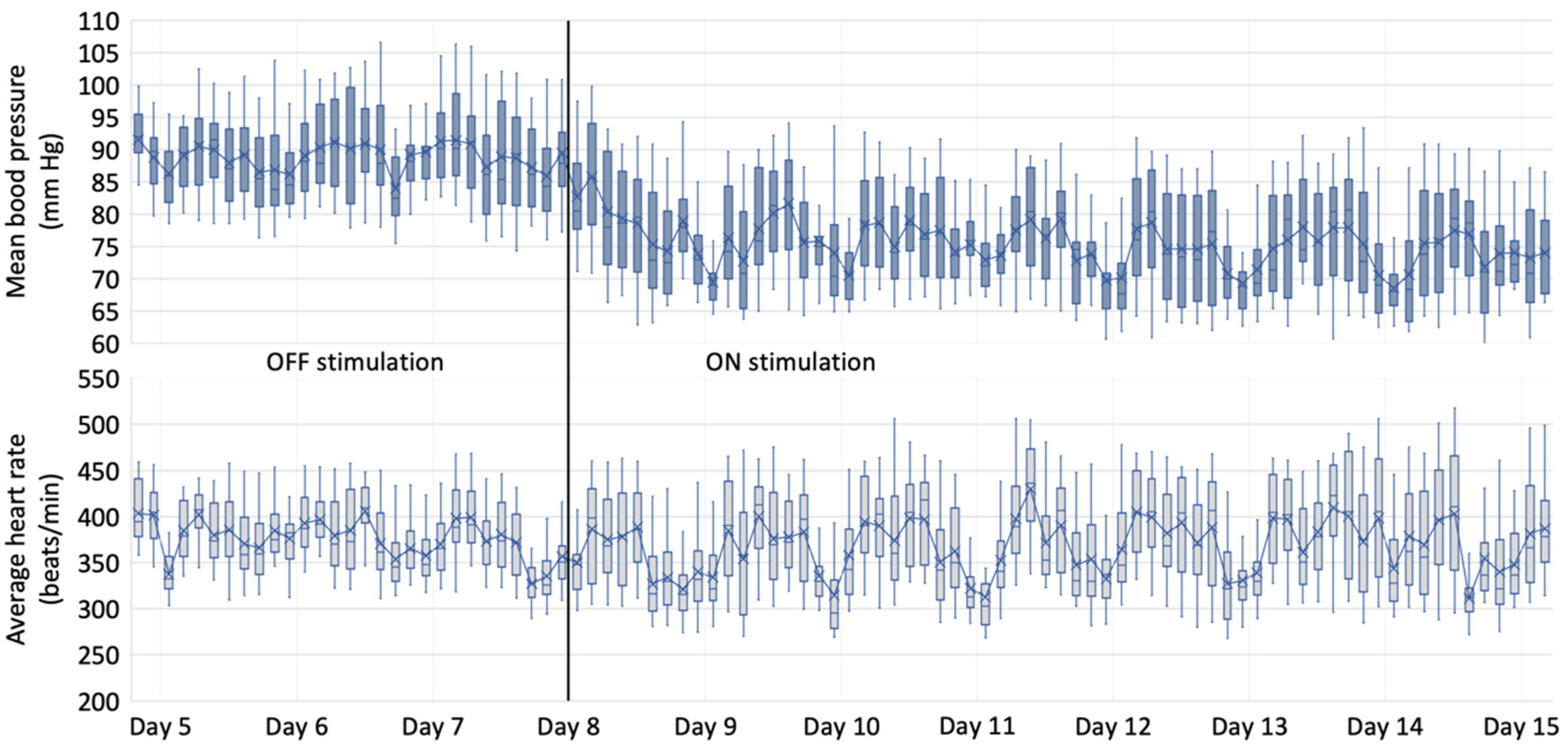
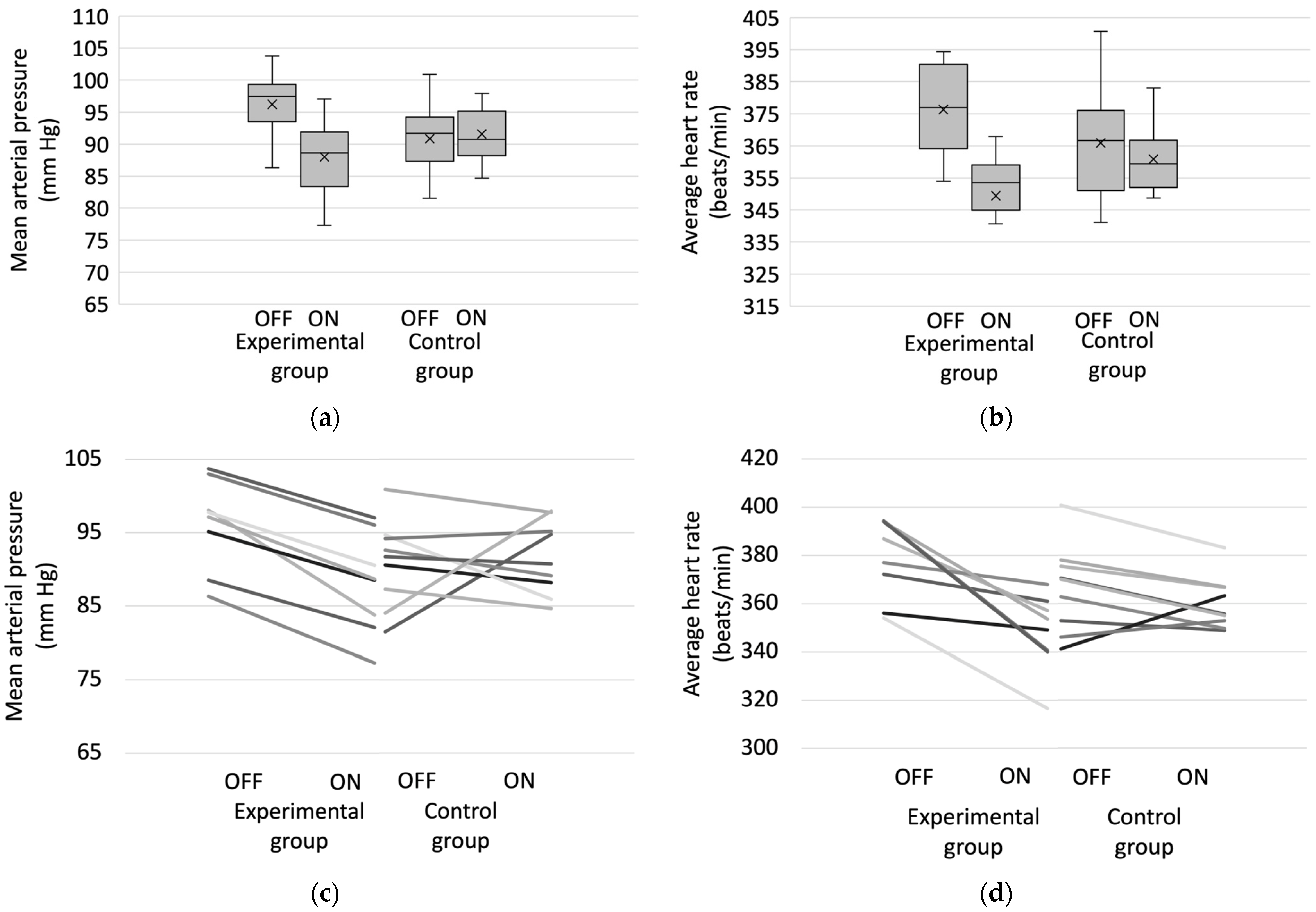
3.2. Hemodynamic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Limousin, P.; Pollak, P.; Benazzouz, A.; Hoffmann, D.; Le Bas, J.F.; Broussolle, E.; Perret, J.E.; Benabid, A.L. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet 1995, 345, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Mallet, L.; Polosan, M.; Jaafari, N.; Baup, N.; Welter, M.L.; Fontaine, D.; du Montcel, S.T.; Yelnik, J.; Chéreau, I.; Arbus, C.; et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N. Engl. J. Med. 2008, 359, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Halpern, C.H.; Wolf, J.A.; Bale, T.L.; Stunkard, A.J.; Danish, S.F.; Grossman, M.; Jaggi, J.L.; Grady, M.S.; Baltuch, G.H. Deep brain stimulation in the treatment of obesity. J. Neurosurg. 2008, 109, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Lessmann, L.; Unterbrink, D.; Popa, R.C.; Gasser, T.; Schulz, J.B. Lesion of the pedunculopontine nucleus reverses hyperactivity of the subthalamic nucleus and substantia nigra pars reticulata in a 6-hydroxydopamine rat model. Eur. J. Neurosci. 2006, 24, 2275–2282. [Google Scholar] [CrossRef]
- Sani, S.; Jobe, K.; Smith, A.; Kordower, J.H.; Bakay, R.A. Deep brain stimulation for treatment of obesity in rats. J. Neurosurg. 2007, 107, 809–813. [Google Scholar] [CrossRef]
- Harmsen, I.E.; Elias, G.J.B.; Beyn, M.E.; Boutet, A.; Pancholi, A.; Germann, J.; Mansouri, A.; Lozano, C.S.; Lozano, A.M. Clinical trials for deep brain stimulation: Current state of affairs. Brain Stimul. 2020, 13, 378–385. [Google Scholar] [CrossRef]
- Dougherty, D.D.; Rezai, A.R.; Carpenter, L.L.; Howland, R.H.; Bhati, M.T.; O’Reardon, J.P.; Eskandar, E.N.; Baltuch, G.H.; Machado, A.D.; Kondziolka, D.; et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Biol. Psychiatry 2015, 78, 240–248. [Google Scholar] [CrossRef]
- Hsu, T.I.; Nguyen, A.; Gupta, N.; Godbole, N.; Perisetla, N.; Hatter, M.J.; Beyer, R.S.; Bui, N.E.; Jagan, J.; Yang, C.; et al. Effectiveness of Deep Brain Stimulation in Treatment of Anorexia Nervosa and Obesity: A Systematic Review. World Neurosurg. 2022, 168, 179–189. [Google Scholar] [CrossRef]
- Chudy, D.; Deletis, V.; Almahariq, F.; Marčinković, P.; Škrlin, J.; Paradžik, V. Deep brain stimulation for the early treatment of the minimally conscious state and vegetative state: Experience in 14 patients. J. Neurosurg. 2018, 128, 1189–1198. [Google Scholar] [CrossRef]
- Pereira, E.A.; Wang, S.; Paterson, D.J.; Stein, J.F.; Aziz, T.Z.; Green, A.L. Sustained reduction of hypertension by deep brain stimulation. J. Clin. Neurosci. 2010, 17, 124–127. [Google Scholar] [CrossRef]
- O’Callaghan, E.L.; Hart, E.C.; Sims-Williams, H.; Javed, S.; Burchell, A.E.; Papouchado, M.; Tank, J.; Heusser, K.; Jordan, J.; Menne, J.; et al. Chronic Deep Brain Stimulation Decreases Blood Pressure and Sympathetic Nerve Activity in a Drug- and Device-Resistant Hypertensive Patient. Hypertension 2017, 69, 522–528. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks, 1st ed.; World Health Organization: Geneva, Switzerland, 2009; p. V. [Google Scholar]
- Zhou, B.; Bentham, J.; Di Cesare, M.; Bixby, H.; Danaei, G.; Cowan, M.J.; Paciorek, C.J.; Singh, G.; Hajifathalian, K.; Bennett, J.E.; et al. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef]
- DeLalio, L.J.; Sved, A.F.; Stocker, S.D. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can. J. Cardiol. 2020, 36, 712–720. [Google Scholar] [CrossRef]
- Mann, S.J. Neurogenic hypertension: Pathophysiology, diagnosis and management. Clin. Auton. Res. 2018, 28, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G. Sympathomodulatory Effects of Antihypertensive Drug Treatment. Am. J. Hypertens. 2016, 29, 665–675. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Novotny, M.; Swain, G.M.; Fink, G.D. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Osborn, J.W.; Fink, G.D. Region-specific changes in sympathetic nerve activity in angiotensin II-salt hypertension in the rat. Exp. Physiol. 2010, 95, 61–68. [Google Scholar] [CrossRef]
- Armitage, J.A.; Burke, S.L.; Prior, L.J.; Barzel, B.; Eikelis, N.; Lim, K.; Head, G.A. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension 2012, 60, 163–171. [Google Scholar] [CrossRef]
- Bisognano, J.D.; Bakris, G.; Nadim, M.K.; Sanchez, L.; Kroon, A.A.; Schafer, J.; de Leeuw, P.W.; Sica, D.A. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: Results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J. Am. Coll. Cardiol. 2011, 58, 765–773. [Google Scholar] [CrossRef]
- Mahfoud, F.; Schlaich, M.P.; Lobo, M.D. Device Therapy of Hypertension. Circ. Res. 2021, 128, 1080–1099. [Google Scholar] [CrossRef]
- Ems, R.; Garg, A.; Ostergard, T.A.; Miller, J.P. Potential Deep Brain Stimulation Targets for the Management of Refractory Hypertension. Front. Neurosci. 2019, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Keay, K.A.; Bandler, R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci. Biobehav. Rev. 2001, 25, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Hyam, J.A.; Williams, C.; Wang, S.; Shlugman, D.; Stein, J.F.; Paterson, D.J.; Aziz, T.Z. Intra-operative deep brain stimulation of the periaqueductal grey matter modulates blood pressure and heart rate variability in humans. Neuromodulation 2010, 13, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Blessing, W.W.; Reis, D.J. Inhibitory cardiovascular function of neurons in the caudal ventrolateral medulla of the rabbit: Relationship to the area containing A1 noradrenergic cells. Brain Res. 1982, 253, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Stornetta, R.L.; Souza, G.M.P.R.; Abbott, S.B.G.; Brooks, V.L. Neuronal Networks in Hypertension: Recent Advances. Hypertension 2020, 76, 300–311. [Google Scholar] [CrossRef]
- Mayorov, D.N.; Head, G.A. Influence of rostral ventrolateral medulla on renal sympathetic baroreflex in conscious rabbits. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, 577–587. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kuo, T.B.; Hsieh, I.T.; Yang, C.C. Electrical stimulation of the rostral ventrolateral medulla promotes wakefulness in rats. Sleep Med. 2013, 14, 1076–1084. [Google Scholar] [CrossRef]
- Chomanskis, Ž.; Jonkus, V.; Danielius, T.; Paulauskas, T.; Orvydaitė, M.; Melaika, K.; Rukšėnas, O.; Hendrixson, V.; Ročka, S. Remotely Programmable Deep Brain Stimulator Combined with an Invasive Blood Pressure Monitoring System for a Non-Tethered Rat Model in Hypertension Research. Brain Sci. 2023, 13, 504. [Google Scholar] [CrossRef]
- Bourns.com. Available online: https://www.bourns.com/docs/product-datasheets/bps130.pdf (accessed on 13 February 2023).
- Goodchild, A.K.; Moon, E.A. Maps of cardiovascular and respiratory regions of rat ventral medulla: Focus on the caudal medulla. J. Chem. Neuroanat. 2009, 38, 209–221. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Cutsforth-Gregory, J.K.; Benarroch, E.E. Nucleus of the solitary tract, medullary reflexes, and clinical implications. Neurology 2017, 88, 1187–1196. [Google Scholar] [CrossRef]
- Dampney, R.A. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef] [PubMed]
- Sata, Y.; Head, G.A.; Denton, K.; May, C.N.; Schlaich, M.P. Role of the Sympathetic Nervous System and Its Modulation in Renal Hypertension. Front. Med. 2018, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Sved, A.F.; Blessing, W.W.; Reis, D.J. Caudal ventrolateral medulla can alter vasopressin and arterial pressure. Brain Res. Bull. 1985, 14, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Willette, R.N.; Barcas, P.P.; Krieger, A.J.; Sapru, H.N. Vasopressor and depressor areas in the rat medulla. Identification by microinjection of L-glutamate. Neuropharmacology 1983, 22, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, E.L.; McBryde, F.D.; Patel, N.K.; Paton, J.F.R. Examination of the periaqueductal gray as a site for controlling arterial pressure in the conscious spontaneously hypertensive rat. Auton. Neurosci. 2022, 240, 102984. [Google Scholar] [CrossRef]
- Nelson, A.J.; Juraska, J.M.; Musch, T.I.; Iwamoto, G.A. Neuroplastic adaptations to exercise: Neuronal remodeling in cardiorespiratory and locomotor areas. J. Appl. Physiol. 2005, 99, 2312–2322. [Google Scholar] [CrossRef]
- Jannetta, P.J.; Segal, R.; Wolfson, S.K., Jr. Neurogenic hypertension: Etiology and surgical treatment. I. Observations in 53 patients. Ann. Surg. 1985, 201, 391–398. [Google Scholar] [CrossRef]
- Patel, S.; Krishna, V.; Nicholas, J.; Welzig, C.M.; Vera, C. Preliminary observations on the vasomotor responses to electrical stimulation of the ventrolateral surface of the human medulla. J. Neurosurg. 2012, 117, 150–155. [Google Scholar] [CrossRef]
- Hamasaki, T.; Yamakawa, T.; Fujiwara, K.; Harashima, H.; Nakamura, K.; Ikuta, Y.; Yamamoto, T.; Hasegawa, Y.; Takezaki, T.; Mukasa, A. Sympathetic hyperactivity, hypertension, and tachycardia induced by stimulation of the ponto-medullary junction in humans. Clin. Neurophysiol. 2021, 132, 1264–1273. [Google Scholar] [CrossRef]
- Feinstein, B.; Gleason, C.A.; Libet, B. Stimulation of locus coeruleus in man. Preliminary trials for spasticity and epilepsy. Stereotact. Funct. Neurosurg. 1989, 52, 26–41. [Google Scholar] [CrossRef]
- Young, R.F.; Tronnier, V.; Rinaldi, P.C. Chronic stimulation of the Kölliker-Fuse nucleus region for relief of intractable pain in humans. J. Neurosurg. 1992, 76, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn-Smith, I.J.; Verberne, A.J.M. Central Regulation of Autonomic Functions, 2nd ed.; Oxford University Press: Oxford, UK, 2011; pp. 3–22. [Google Scholar]
- Green, A.L.; Wang, S.; Owen, S.L.; Xie, K.; Liu, X.; Paterson, D.J.; Stein, J.F.; Bain, P.G.; Aziz, T.Z. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport 2005, 16, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L.; Wang, S.; Bittar, R.G.; Owen, S.L.; Paterson, D.J.; Stein, J.F.; Bain, P.G.; Shlugman, D.; Aziz, T.Z. Deep brain stimulation: A new treatment for hypertension? J. Clin. Neurosci. 2007, 14, 592–595. [Google Scholar] [CrossRef]
- Mayyas, F.; Sturey, T.; Van Wagoner, D.R. Baroreflex stimulation vs. renal denervation for treatment of hypertension: What constitutes a logical comparison of these interventions on atrial electrophysiology? J. Cardiovasc. Electrophysiol. 2013, 24, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chomanskis, Ž.; Jonkus, V.; Danielius, T.; Paulauskas, T.; Orvydaitė, M.; Melaika, K.; Rukšėnas, O.; Hendrixson, V.; Ročka, S. Hypotensive Effect of Electric Stimulation of Caudal Ventrolateral Medulla in Freely Moving Rats. Medicina 2023, 59, 1046. https://doi.org/10.3390/medicina59061046
Chomanskis Ž, Jonkus V, Danielius T, Paulauskas T, Orvydaitė M, Melaika K, Rukšėnas O, Hendrixson V, Ročka S. Hypotensive Effect of Electric Stimulation of Caudal Ventrolateral Medulla in Freely Moving Rats. Medicina. 2023; 59(6):1046. https://doi.org/10.3390/medicina59061046
Chicago/Turabian StyleChomanskis, Žilvinas, Vytautas Jonkus, Tadas Danielius, Tomas Paulauskas, Monika Orvydaitė, Kazimieras Melaika, Osvaldas Rukšėnas, Vaiva Hendrixson, and Saulius Ročka. 2023. "Hypotensive Effect of Electric Stimulation of Caudal Ventrolateral Medulla in Freely Moving Rats" Medicina 59, no. 6: 1046. https://doi.org/10.3390/medicina59061046
APA StyleChomanskis, Ž., Jonkus, V., Danielius, T., Paulauskas, T., Orvydaitė, M., Melaika, K., Rukšėnas, O., Hendrixson, V., & Ročka, S. (2023). Hypotensive Effect of Electric Stimulation of Caudal Ventrolateral Medulla in Freely Moving Rats. Medicina, 59(6), 1046. https://doi.org/10.3390/medicina59061046





