Correlation between Cumulative Methotrexate Dose, Metabolic Syndrome and Hepatic Fibrosis Detected by FibroScan in Rheumatoid Arthritis Patients
Abstract
1. Introduction
2. Methods
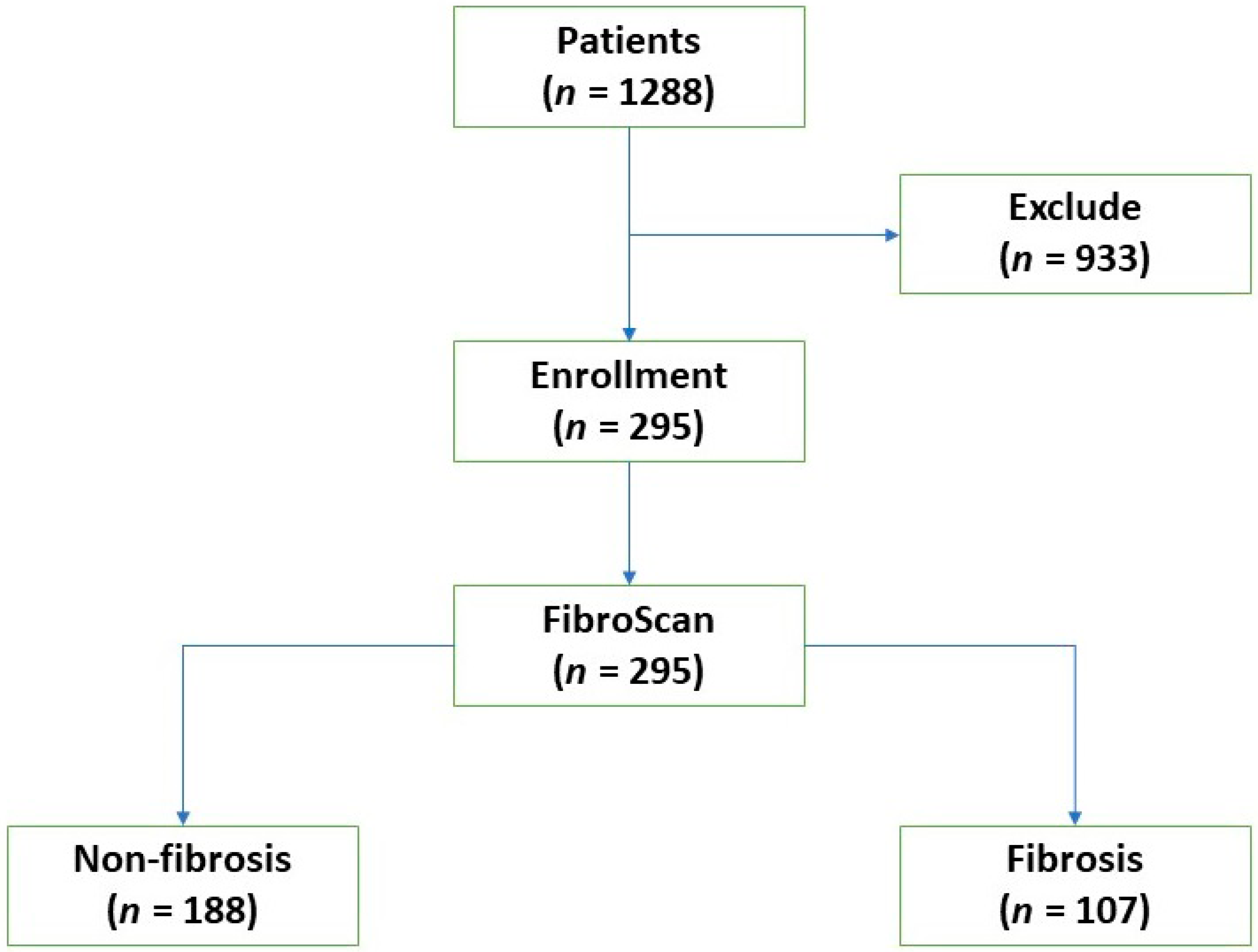
2.1. Study Population
2.2. Demographics and Clinical Parameters
2.3. Transient Elastography (TE)
2.4. Metabolic Syndrome
2.5. Statistical Analysis
2.6. Ethics Approval
3. Results
3.1. Baseline Characteristics
3.2. Rheumatoid Arthritis
3.3. Metabolic Syndrome
3.4. Factors Associated with Hepatic Fibrosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Landewe, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef] [PubMed]
- England, B.R.; Tiong, B.K.; Bergman, M.J.; Curtis, J.R.; Kazi, S.; Mikuls, T.R.; O’Dell, J.R.; Ranganath, V.K.; Limanni, A.; Suter, L.G.; et al. 2019 Update of the American College of Rheumatology Recommended Rheumatoid Arthritis Disease Activity Measures. Arthritis Care Res. 2019, 71, 1540–1555. [Google Scholar] [CrossRef]
- Ramiro, S.; Gaujoux-Viala, C.; Nam, J.L.; Smolen, J.S.; Buch, M.; Gossec, L.; Van Der Heijde, D.; Winthrop, K.; Landewé, R. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Salliot, C.; van der Heijde, D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: Systematic literature research. Ann. Rheum. Dis. 2009, 68, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Berends, M.A.; Snoek, J.; de Jong, E.M.; Van Krieken, J.H.; de Knegt, R.J.; van Oijen, M.G.; van de Kerkhof, P.C.; Drenth, J.P. Biochemical and biophysical assessment of MTX-induced liver fibrosis in psoriasis patients: Fibrotest predicts the presence and Fibroscan predicts the absence of significant liver fibrosis. Liver Int. 2007, 27, 639–645. [Google Scholar] [CrossRef]
- Rabinowich, L.; Shibolet, O. Drug Induced Steatohepatitis: An Uncommon Culprit of a Common Disease. Biomed. Res. Int. 2015, 2015, 168905. [Google Scholar] [CrossRef]
- Patel, V.; Sanyal, A.J. Drug-induced steatohepatitis. Clin. Liver Dis. 2013, 17, 533–vii. [Google Scholar] [CrossRef]
- Lertnawapan, R.; Chonprasertsuk, S.; Siramolpiwat, S. Association between cumulative methotrexate dose, non-invasive scoring system and hepatic fibrosis detected by Fibroscan in rheumatoid arthritis patients receiving methotrexate. Int. J. Rheum. Dis. 2019, 22, 214–221. [Google Scholar] [CrossRef]
- Park, S.H.; Choe, J.Y.; Kim, S.K. Assessment of liver fibrosis by transient elastography in rheumatoid arthritis patients treated with methotrexate. Joint Bone Spine 2010, 77, 588–592. [Google Scholar] [CrossRef]
- Whiting-O’Keefe, Q.E.; Fye, K.H.; Sack, K.D. Methotrexate and histologic hepatic abnormalities: A meta-analysis. Am. J. Med. 1991, 90, 711–716. [Google Scholar] [CrossRef]
- Schmajuk, G.; Miao, Y.; Yazdany, J.; Boscardin, W.J.; Daikh, D.I.; Steinman, M.A. Identification of risk factors for elevated transaminases in methotrexate users through an electronic health record. Arthritis Care Res. 2014, 66, 1159–1166. [Google Scholar] [CrossRef]
- Laharie, D.; Zerbib, F.; Adhoute, X.; Boue-Lahorgue, X.; Foucher, J.; Castera, L.; Rullier, A.; Bertet, J.; Couzigou, P.; Amouretti, M.; et al. Diagnosis of liver fibrosis by transient elastography (FibroScan) and non-invasive methods in Crohn’s disease patients treated with methotrexate. Aliment. Pharmacol. Ther. 2006, 23, 1621–1628. [Google Scholar] [CrossRef]
- van der Voort, E.A.; Koehler, E.M.; Nijsten, T.; Stricker, B.H.; Hofman, A.; Janssen, H.L.; Schouten, J.N.; Wakkee, M. Increased Prevalence of Advanced Liver Fibrosis in Patients with Psoriasis: A Cross-sectional Analysis from the Rotterdam Study. Acta Derm. Venereol. 2016, 96, 213–217. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Rahelic, D.; Turk-Wensween, T.; Ruzic, A.; Domislovic, V.; Hauser, G.; Matic, T.; Radic-Kristo, D.; Krznaric, Z.; Radic, M.; et al. Significant liver fibrosis, as assessed by fibroscan, is independently associated with chronic vascular complications of type 2 diabetes: A multicenter study. Diabetes Res. Clin. Pract. 2021, 177, 108884. [Google Scholar] [CrossRef]
- Hong, A.R.; Lim, S. Clinical characteristics of metabolic syndrome in Korea, and its comparison with other Asian countries. J. Diabetes Investig. 2015, 6, 508–515. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- American Academy of Dermatology Work Group; Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.; Lebwohl, M.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: Case-based presentations and evidence-based conclusions. J. Am. Acad. Dermatol. 2011, 65, 137–174. [Google Scholar] [CrossRef]
- Laharie, D.; Terrebonne, E.; Vergniol, J.; Chanteloup, E.; Chabrun, E.; Couzigou, P.; de Lédinghen, V. The liver and methotrexate. Gastroenterol. Clin. Biol. 2008, 32, 134–142. [Google Scholar] [CrossRef]
- Rosenberg, P.; Urwitz, H.; Johannesson, A.; Ros, A.M.; Lindholm, J.; Kinnman, N.; Hultcrantz, R. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J. Hepatol. 2007, 46, 1111–1118. [Google Scholar] [CrossRef]
- Chakravarty, K.; McDonald, H.; Pullar, T.; Taggart, A.; Chalmers, R.; Oliver, S.; Mooney, J.; Somerville, M.; Bosworth, A.; Kennedy, T.; et al. BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology 2008, 47, 924–925. [Google Scholar] [CrossRef]
- Nast, A.; Kopp, I.; Augustin, M.; Banditt, K.B.; Boehncke, W.H.; Follmann, M.; Friedrich, M.; Huber, M.; Kahl, C.; Klaus, J.; et al. German evidence-based guidelines for the treatment of Psoriasis Vulgaris (short version). Arch. Dermatol. Res. 2007, 299, 111–138. [Google Scholar] [CrossRef] [PubMed]
- West, S.G. Methotrexate hepatotoxicity. Rheum. Dis. Clin. N. Am. 1997, 23, 883–915. [Google Scholar] [CrossRef] [PubMed]
- Laharie, D.; Seneschal, J.; Schaeverbeke, T.; Doutre, M.S.; Longy-Boursier, M.; Pellegrin, J.L.; Chabrun, E.; Villars, S.; Zerbib, F.; de Lédinghen, V. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: A case-control study. J. Hepatol. 2010, 53, 1035–1040. [Google Scholar] [CrossRef]
- Alarcon, G.S.; Kremer, J.; Weinblatt, M. Monitoring guidelines for methotrexate-treated rheumatoid arthritis patients: Comment on the article by Yazici et al. Arthritis Rheum. 2004, 50, 2710. [Google Scholar] [CrossRef] [PubMed]
- Visser, K.; van der Heijde, D.M. Risk and management of liver toxicity during methotrexate treatment in rheumatoid and psoriatic arthritis: A systematic review of the literature. Clin. Exp. Rheumatol. 2009, 27, 1017–1025. [Google Scholar] [PubMed]
- Rajitha, P.; Biswas, R.; Sabitha, M.; Jayakumar, R. Methotrexate in the Treatment of Psoriasis and Rheumatoid Arthritis: Mechanistic Insights, Current Issues, and Novel Delivery Approaches. Curr. Pharm. Des. 2017, 23, 3550–3566. [Google Scholar] [CrossRef]
- Roenigk, H.H., Jr.; Auerbach, R.; Maibach, H.I. Methotrexate guidelines 2009? J. Am. Acad. Dermatol. 2010, 63, 344–345. [Google Scholar] [CrossRef]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Lindsay, K.; Fraser, A.D.; Layton, A.; Goodfield, M.; Gruss, H.; Gough, A. Liver fibrosis in patients with psoriasis and psoriatic arthritis on long-term, high cumulative dose methotrexate therapy. Rheumatology 2009, 48, 569–572. [Google Scholar] [CrossRef]
- Feuchtenberger, M.; Kraus, L.; Nigg, A.; Schulze-Koops, H.; Schafer, A. Methotrexate does not increase the risk of liver fibrosis in patients with rheumatoid arthritis: Assessment by ultrasound elastography (ARFI-MetRA study). Rheumatol. Int. 2021, 41, 1079–1087. [Google Scholar] [CrossRef]
- Mahajan, R.; Dogra, S.; Handa, S.; Razmi, T.M.; Narang, T.; Rathi, S.; Dhiman, R.K.; Saikia, B.; Karim, A. Metabolic syndrome and female gender, but not methotrexate, are the important associations of significant liver fibrosis in patients with moderate-to-severe psoriasis as detected by transient elastography. Indian. J. Dermatol. Venereol. Leprol. 2020, 86, 649–655. [Google Scholar]
| Parameters | Non-Fibrosis (N = 188) | Fibrosis (N = 107) | p-Value | |
|---|---|---|---|---|
| Sex (female) (%) | 154 (81.91) | 87 (81.31) | 1 | |
| Age (years) | 55.36 ± 15.12 | 59.49 ± 11.46 | 0.02 | |
| BWt (kg) | 52.37 ± 7.71 | 66.58 ± 1.00 | <0.001 | |
| Height (cm) | 157.77 ± 7.05 | 156.85 ± 8.94 | 0.33 | |
| Waist (cm) | 72.15 ± 6.06 | 86.24 ± 8.23 | <0.001 | |
| Hip (cm) | 91.14 ± 5.76 | 95.89 ± 7.51 | <0.001 | |
| Waist–hip ratio (WHR) | 1.21 ± 5.78 | 0.89 ± 0.05 | 0.58 | |
| BMI (kg/m2) | 20.99 ± 2.38 | 26.98 ± 2.59 | <0.001 | |
| Comorbidities | Alcohol consumption (%) | 2 (1.06) | 3 (2.80) | 0.36 |
| DM (%) | 13 (6.9) | 30 (28.04) | <0.001 | |
| IFG (%) | 18 (9.57) | 64 (59.81) | <0.001 | |
| Insulin resistance (%) | 31 (16.49) | 94 (87.85) | <0.001 | |
| HT (%) | 52 (27.66) | 74 (69.16) | <0.001 | |
| Fatty liver (%) | 10 (5.32) | 53 (49.53) | <0.001 | |
| HLP (%) | 53 (28.19) | 102 (95.33) | <0.001 | |
| Metabolic syndrome (%) | 3 (1.60) | 107 (100) | <0.001 | |
| HBV/HCV (%) | 0 (0) | 0 (0) | ||
| RA and MTX factors | RA duration (years) | 8.47 ± 4.09 | 10.07 ± 5.77 | 0.006 |
| AntiCCP (%) | 161 (85.64) | 98 (91.59) | 0.14 | |
| Rheumatoid factor (%) | 143 (76.06) | 88 (82.24) | 0.24 | |
| MTX duration (weeks) | 189.42 ± 136.65 | 291.63 ± 168.23 | 0.001 | |
| MTX average (mg/w) | 10.51 ± 3.01 | 11.07 ± 3.38 | 0.39 | |
| MTX cumulative(mg) | 2212.07 ± 1021.32 | 4661.4 ± 3192.51 | <0.001 | |
| MTX per weight(mg/kg) | 43.55 ± 22.35 | 70.46 ± 44.98 | <0.001 | |
| MTX average/wt (mg/w/kg) | 0.20 ± 0.07 | 0.18 ± 0.06 | 0.11 | |
| Concurrent medications | Other DMARDs (%) | 161 (85.64) | 98 (91.59) | 0.14 |
| Hydroxychloroquine (%) | 51 (27.13) | 34 (31.78) | 0.42 | |
| Sulfasalazine (%) | 75 (39.89) | 34 (31.78) | 0.17 | |
| Leflunomide (%) | 104 (55.32) | 78 (72.90) | 0.003 | |
| Azathioprine (%) | 6 (3.19) | 5 (4.67) | 0.54 | |
| Biologic agent (%) | 17 (9.04) | 11 (10.28) | 0.84 | |
| Steroid (%) | 56 (29.79) | 45 (42.06) | 0.04 | |
| Prednisolone dosage (mg/d) | 0.68 ± 1.51 | 0.98 ± 1.87 | 0.14 | |
| NSAIDs (%) | 39 (20.75) | 20 (18.69) | 0.84 | |
| Folic (%) | 188 (100) | 107 (100) | ||
| Statin (%) | 40 (21.28) | 62 (57.94) | <0.001 | |
| Laboratory parameters | ESR (mm/h) | 37.45 ± 21.21 | 42.90 ± 21.21 | 0.03 |
| CRP (mg/L) | 9.20 ± 20.03 | 7.75 ± 11.78 | 0.49 | |
| Hemoglobin (g/dL) | 11.75 ± 1.23 | 11.96 ± 1.40 | 0.35 | |
| WBC (×109/L) | 6478.56 ± 2279.88 | 6451.50 ± 1847.04 | 0.92 | |
| Platelet (×109/L) | 272,262.20 ± 77,879.23 | 269,411.20 ± 74,477.29 | 0.76 | |
| FBS (mg/dL) | 93.43 ± 14.10 | 110.06 ± 16.19 | <0.001 | |
| HbA1c (%) | 5.44 ± 0.87 | 6.05 ± 0.57 | <0.001 | |
| Albumin (g/dL) | 3.75 ± 0.47 | 3.89 ± 0.40 | 0.007 | |
| AST (IU/L) | 23.28 ± 5.92 | 26.94 ± 10.69 | 0.002 | |
| ALT (IU/L) | 20.56 ± 9.93 | 27.04 ± 17.51 | 0.001 | |
| Creatinine (mg/dL) | 0.74 ± 0.17 | 0.81 ± 0.20 | <0.001 | |
| INR | 1.00 ± 0.06 | 1.01 ± 0.07 | 0.02 | |
| Cholesterol (mg/dL) | 181.38 ± 30.27 | 229.79 ± 39.07 | <0.001 | |
| Triglyceride (mg/dL) | 88.36 ± 42.29 | 148.17 ± 58.94 | <0.001 | |
| HDL (mg/dL) | 62.25 ± 16.09 | 52.80 ± 15.63 | <0.001 | |
| LDL (mg/dL) | 101.95 ± 24.46 | 137.20 ± 28.87 | <0.001 | |
| Uric acid (mg/dL) | 4.55 ± 0.71 | 5.12 ± 0.92 | <0.001 | |
| Abnormal ultrasound (fatty liver by ultrasound) (%) | 10 (5.32) | 53 (49.53) | <0.001 | |
| CAP | 195.24 ± 30.47 | 249.43 ± 43.50 | <0.001 | |
| FibroScan (kPa) | 4.94 ± 0.89 | 9.09 ± 3.31 | <0.001 |
| Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| Age | 1.02 | 1.00–1.04 | 0.016 |
| BMI (kg/m2) | 8.29 | 4.46–15.42 | <0.001 |
| Waist (cm) | 1.32 | 1.24–1.40 | <0.001 |
| Hip (cm) | 1.12 | 1.08–1.17 | <0.001 |
| DM | 5.24 | 2.59–10.60 | <0.001 |
| IFG | 14.06 | 7.56–26.15 | <0.001 |
| Insulin resistance | 36.62 | 18.25–73.47 | <0.001 |
| HT | 5.86 | 3.49–9.87 | <0.001 |
| Fatty liver | 17.47 | 8.33–36.66 | <0.001 |
| HLP | 51.96 | 20.05–134.67 | <0.001 |
| RA duration (y) | 1.07 | 1.02–1.13 | 0.008 |
| MTX duration (w) | 1.00 | 1.00–1.01 | 0.003 |
| MTX cumulative dose (mg) | 2.01 | 1.02–2.13 | <0.001 |
| MTX/kg (mg/kg) | 1.03 | 1.02–1.05 | <0.001 |
| Leflunomide | 2.17 | 1.30–3.63 | 0.003 |
| Steroid | 1.71 | 1.04–2.81 | 0.03 |
| FBS | 1.08 | 1.06–1.11 | <0.001 |
| HbA1c | 13.33 | 6.82–26.04 | <0.001 |
| Albumin (g/dL) | 2.20 | 1.22–3.96 | 0.008 |
| AST (IU/L) | 1.07 | 1.03–1.11 | <0.001 |
| ALT (IU/L) | 1.04 | 1.02–1.07 | <0.001 |
| Creatinine (mg/dL) | 9.30 | 2.41–35.94 | 0.001 |
| INR | 79.67 | 2.06–3081.80 | 0.02 |
| Cholesterol (mg/dL) | 1.04 | 1.03–1.05 | <0.001 |
| Triglyceride (mg/dL) | 1.03 | 1.02–1.03 | <0.001 |
| HDL (mg/dL) | 0.95 | 0.94–0.97 | <0.001 |
| LDL (mg/dL) | 1.05 | 1.04–1.06 | <0.001 |
| Uric (mg/dL) | 2.58 | 1.81–3.67 | <0.001 |
| Fatty liver by ultrasound | 19.52 | 8.74–43.60 | <0.001 |
| ODDS Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|
| BMI (kg/m2) | 14.73 | 2.90–74.79 | 0.001 |
| Insulin resistance | 312.07 | 6.19–15,732.13 | 0.004 |
| HT | 5.35 | 0.29–99.52 | 0.26 |
| HLP | 5.50 | 0.34–89.45 | 0.23 |
| MTX cumulative dose (mg) | 1.03 | 1.01–1.10 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lertnawapan, R.; Chonprasertsuk, S.; Siramolpiwat, S.; Jatuworapruk, K. Correlation between Cumulative Methotrexate Dose, Metabolic Syndrome and Hepatic Fibrosis Detected by FibroScan in Rheumatoid Arthritis Patients. Medicina 2023, 59, 1029. https://doi.org/10.3390/medicina59061029
Lertnawapan R, Chonprasertsuk S, Siramolpiwat S, Jatuworapruk K. Correlation between Cumulative Methotrexate Dose, Metabolic Syndrome and Hepatic Fibrosis Detected by FibroScan in Rheumatoid Arthritis Patients. Medicina. 2023; 59(6):1029. https://doi.org/10.3390/medicina59061029
Chicago/Turabian StyleLertnawapan, Ratchaya, Soonthorn Chonprasertsuk, Sith Siramolpiwat, and Kanon Jatuworapruk. 2023. "Correlation between Cumulative Methotrexate Dose, Metabolic Syndrome and Hepatic Fibrosis Detected by FibroScan in Rheumatoid Arthritis Patients" Medicina 59, no. 6: 1029. https://doi.org/10.3390/medicina59061029
APA StyleLertnawapan, R., Chonprasertsuk, S., Siramolpiwat, S., & Jatuworapruk, K. (2023). Correlation between Cumulative Methotrexate Dose, Metabolic Syndrome and Hepatic Fibrosis Detected by FibroScan in Rheumatoid Arthritis Patients. Medicina, 59(6), 1029. https://doi.org/10.3390/medicina59061029





