The Lubricating Effect of Eye Drops Containing Hyaluronic Acid and Mallow Extract in Patients with Dry Eye Disease—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Products Used, Dosage and Administration
2.3. Efficacy and Safety Endpoints
- If you compare the first treatment period with the second one, have you perceived a difference?
- 2.
- Which product would you prefer?
2.4. Statistical Analysis
3. Results
3.1. Primary Variables
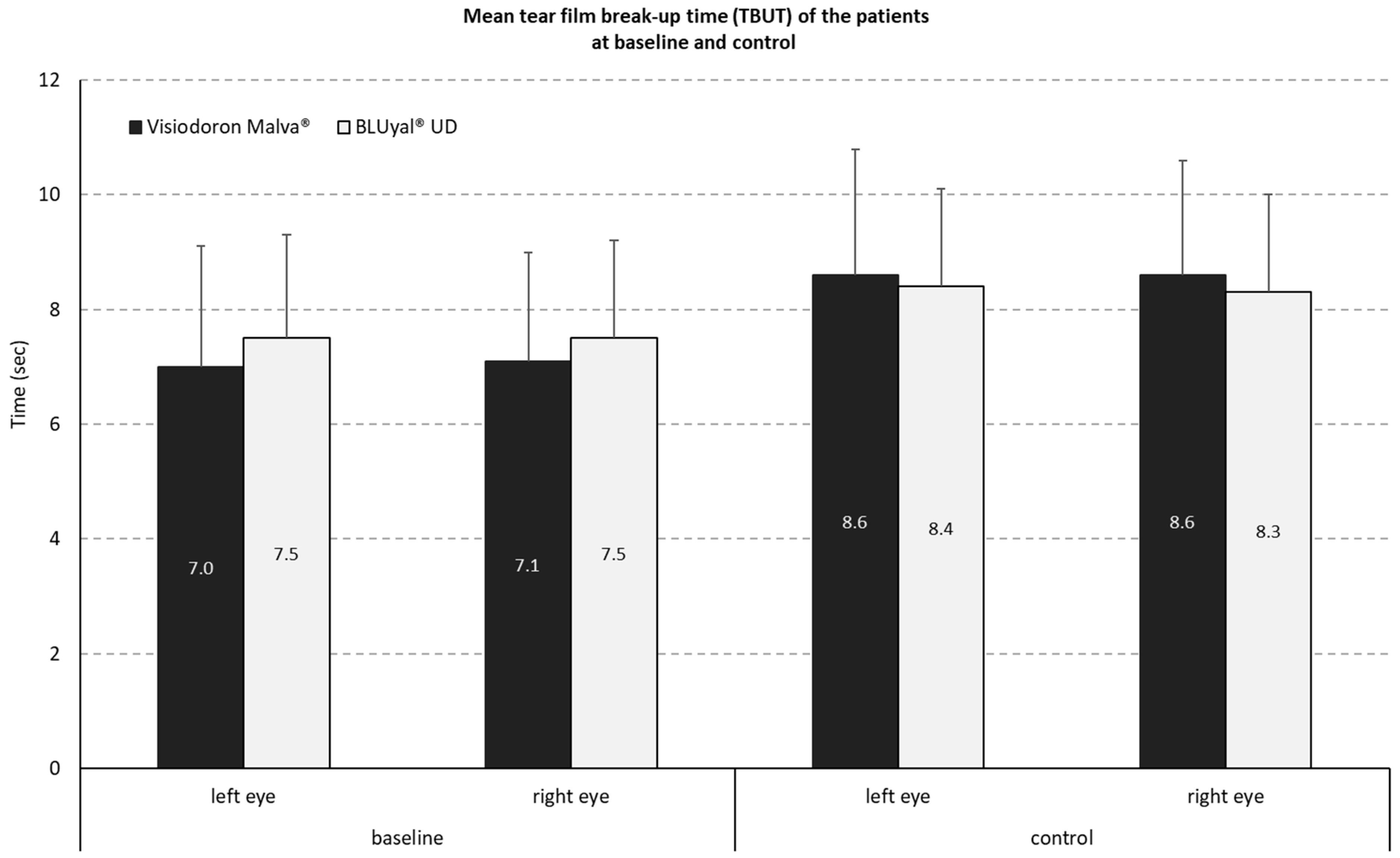
3.1.1. Measurement of Tear Film Breakup Time (TBUT)
3.1.2. Reduction of Lissamine Green Staining of the Ocular Surface (OS)
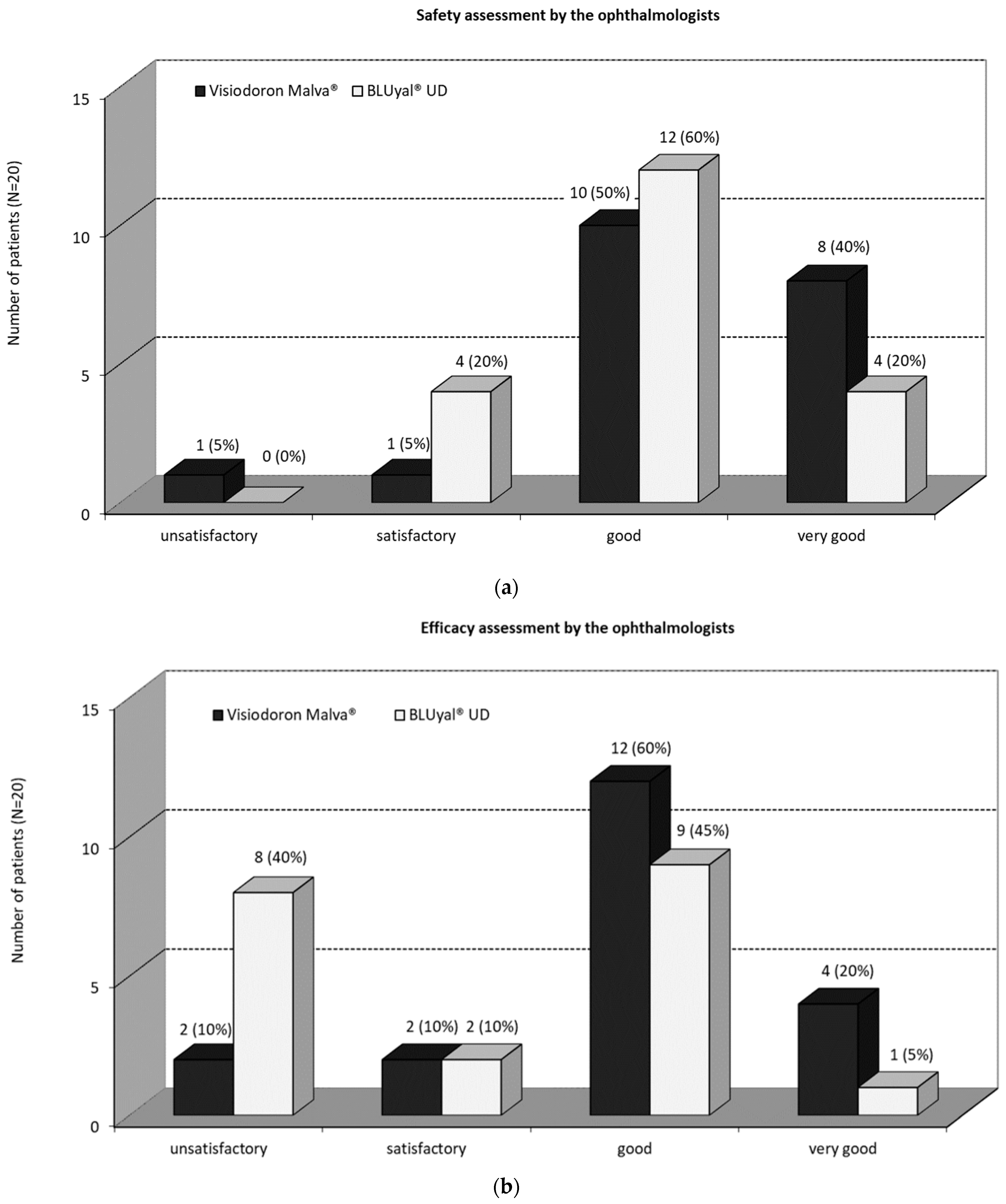
3.1.3. Safety and Efficacy Assessments by the Ophthalmologists
3.2. Secondary Variables
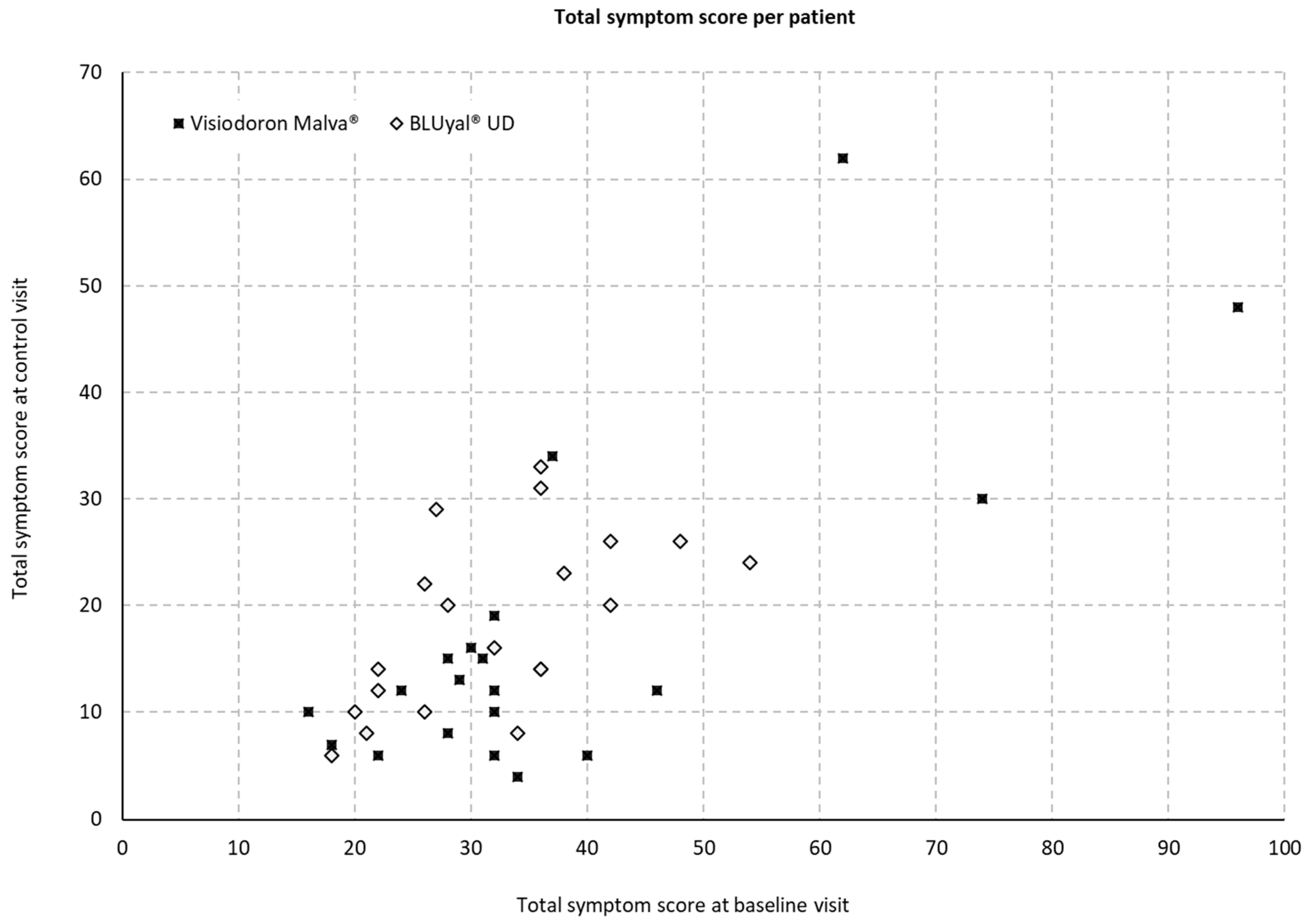
3.2.1. Total Symptom Score per Patient
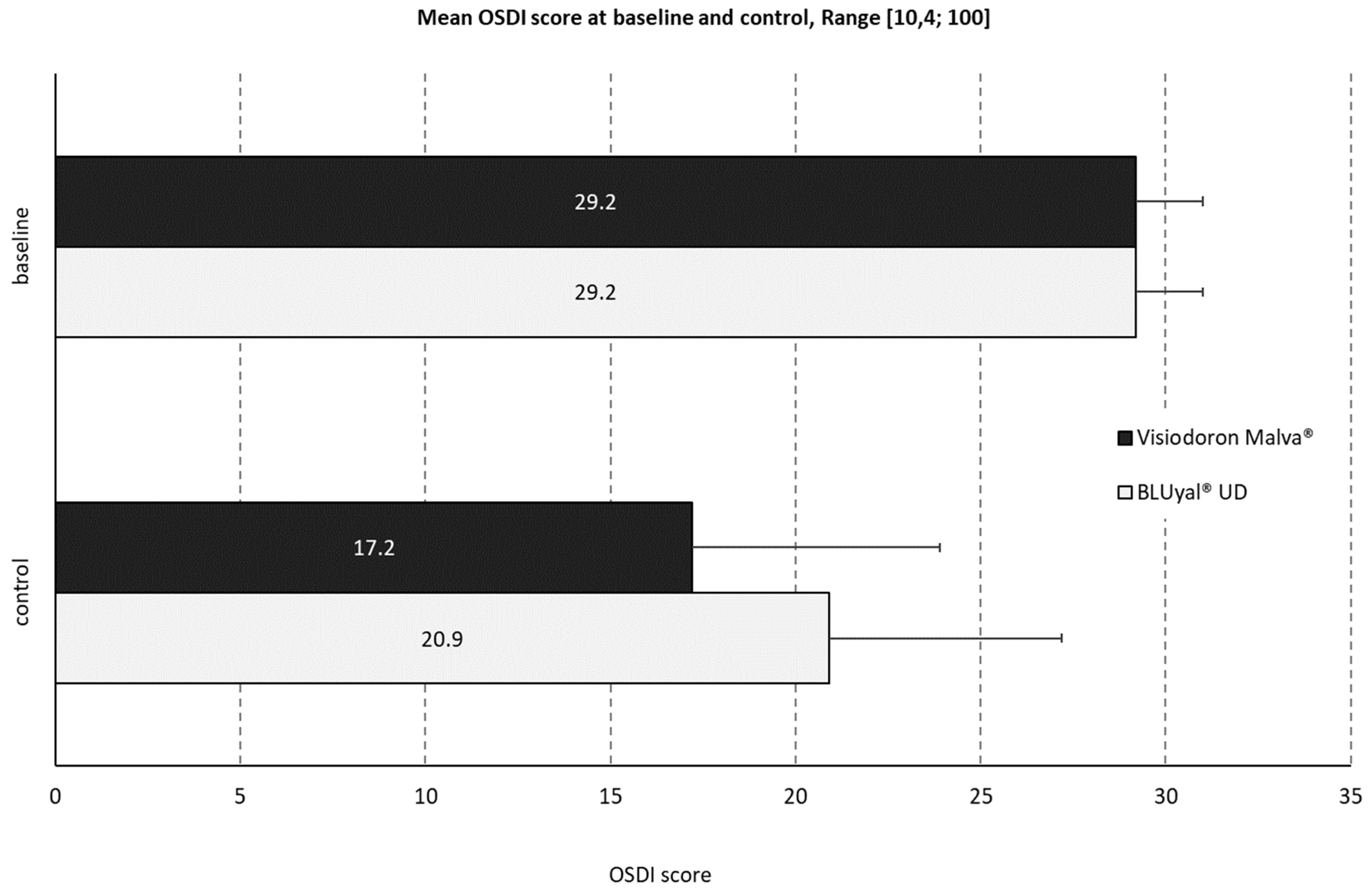
3.2.2. Ocular Surface Disease Index (OSDI)
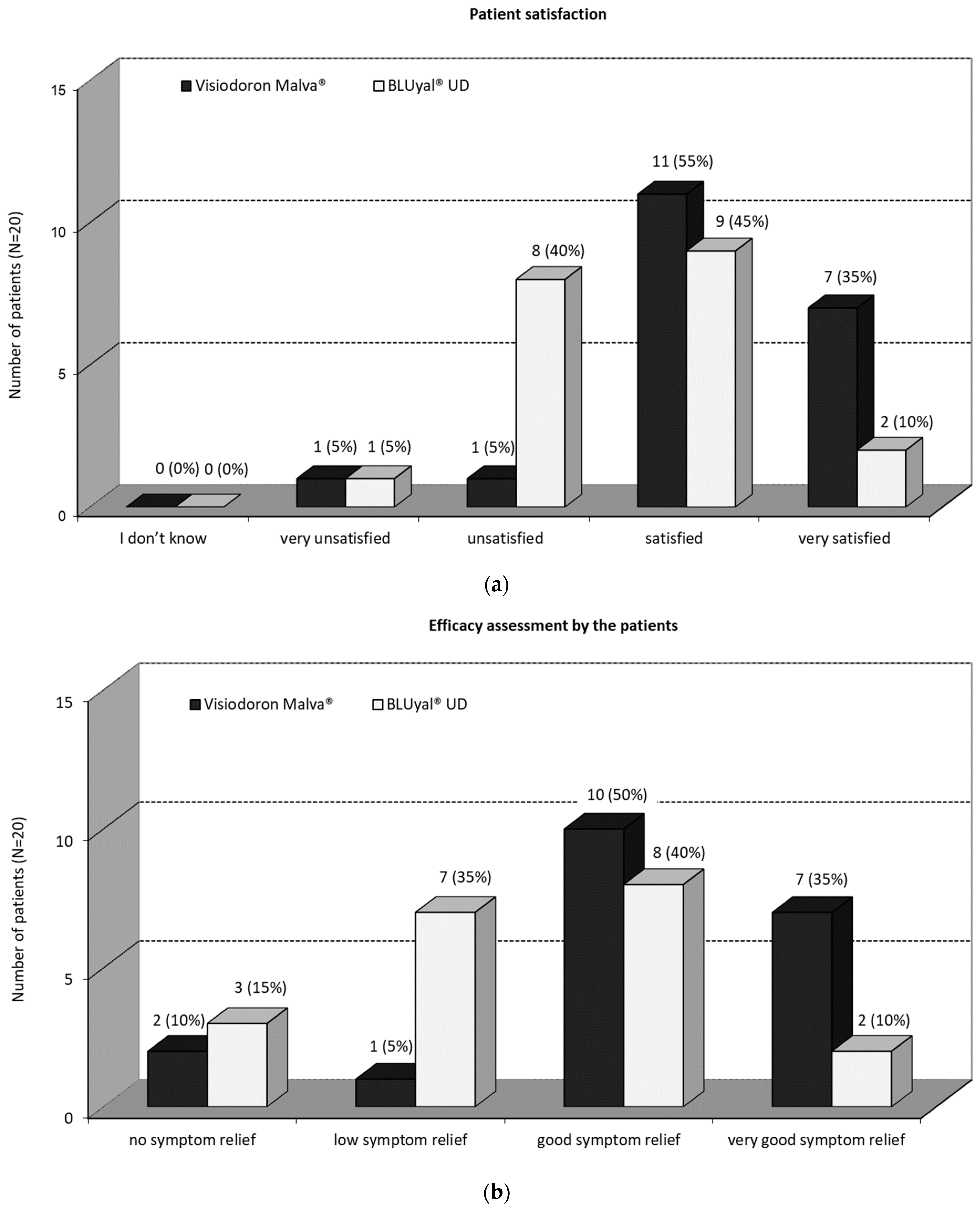
3.2.3. Assessment by the Patients
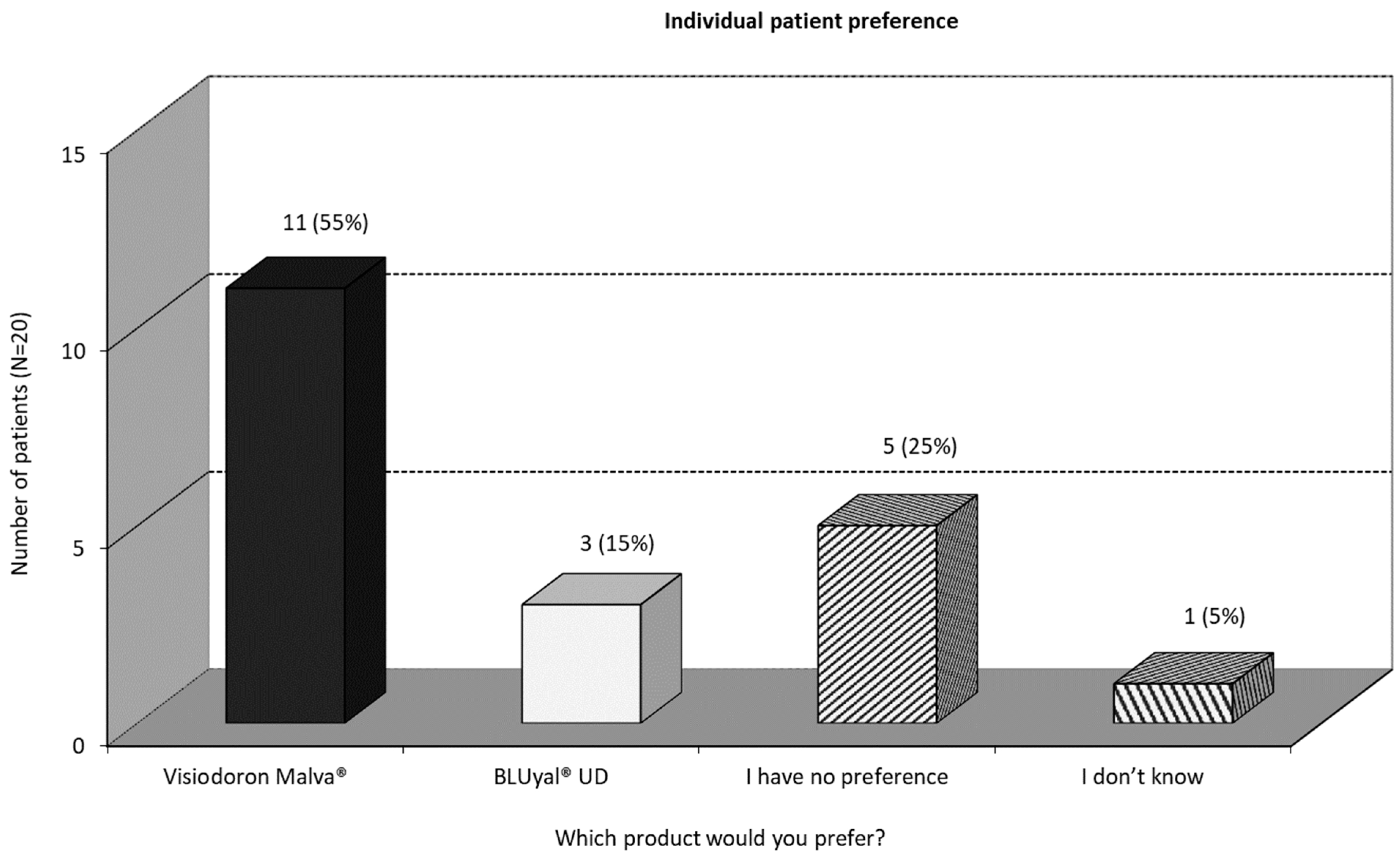
3.2.4. Individual Patient Preference
3.2.5. Safety Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weng, J.; Fink, M.K.; Sharma, A. A Critical Appraisal of the Physicochemical Properties and Biological Effects of Artificial Tear Ingredients and Formulations. Int. J. Mol. Sci. 2023, 24, 2758. [Google Scholar] [CrossRef]
- Murube, J. Andrew de Roetth (1893–1981): Dacryologist Who Introduced the Term Dry Eye. Ocul. Surf. 2004, 2, 225–227. [Google Scholar] [CrossRef]
- O’brien, P.D.; Collum, L.M.T. Dry eye: Diagnosis and current treatment strategies. Curr. Allergy Asthma Rep. 2004, 4, 314–319. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Vehof, J.; Snieder, H.; Jansonius, N.; Hammond, C.J. Prevalence and risk factors of dry eye in 79,866 participants of the population-based Lifelines cohort study in the Netherlands. Ocul. Surf. 2021, 19, 83–93. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Wolkoff, P.; Nøjgaard, J.K.; Troiano, P.; Piccoli, B. Eye complaints in the office environment: Precorneal tear film integrity influenced by eye blinking efficiency. Occup. Environ. Med. 2005, 62, 4–12. [Google Scholar] [CrossRef]
- Wolkoff, P.; Nojgaard, J.K.; Franck, C.; Skov, P. The modern office environment desiccates the eyes? Indoor Air 2006, 16, 258–265. [Google Scholar] [CrossRef]
- Khurana, A.K.; Choudhary, R.; Ahluwalia, B.K.; Gupta, S. Hospital epidemiology of dry eye. Indian J. Ophthalmol. 1991, 39, 55–58. [Google Scholar]
- Bazeer, S.; Jansonius, N.; Snieder, H.; Hammond, C.; Vehof, J. The relationship between occupation and dry eye. Ocul. Surf. 2019, 17, 484–490. [Google Scholar] [CrossRef]
- Vehof, J.; Wang, B.; Kozareva, D.; Hysi, P.G.; Snieder, H.; Hammond, C.J. The Heritability of Dry Eye Disease in a Female Twin Cohort. Investig. Opthalmology Vis. Sci. 2014, 55, 7278–7283. [Google Scholar] [CrossRef]
- Fariselli, C.; Giannaccare, G.; Fresina, M.; Versura, P. Trehalose/hyaluronate eyedrop effects on ocular surface inflammatory markers and mucin expression in dry eye patients. Clin. Ophthalmol. 2018, 12, 1293–1300. [Google Scholar] [CrossRef]
- Chiambaretta, F.; Doan, S.; Labetoulle, M.; Rocher, N.; El Fekih, L.; Messaoud, R.; Khairallah, M.; Baudouin, C.; HA-trehalose Study Group. A Randomized, Controlled Study of the Efficacy and Safety of a New Eyedrop Formulation for Moderate to Severe Dry Eye Syndrome. Eur. J. Ophthalmol. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- Juhlin, L. Hyaluronan in skin. J. Intern. Med. 1997, 242, 61–66. [Google Scholar] [CrossRef]
- Hamerman, D.; Schuster, H. Hyaluronate In Normal Human Synovial Fluid1. J. Clin. Investig. 1958, 37, 57–64. [Google Scholar] [CrossRef]
- Meyer, K.; Palmer, J.W. The Polysaccharide of The Vitreous Humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Weissmann, B.; Meyer, K. The Structure of Hyalobiuronic Acid and of Hyaluronic Acid from Umbilical Cord1,2. J. Am. Chem. Soc. 1954, 76, 1753–1757. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: A systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef]
- Gasparetto, J.C.; Martins, C.A.F.; Hayashi, S.S.; Otuky, M.F.; Pontarolo, R. Ethnobotanical and scientific aspects of Malva sylvestris L.: A millennial herbal medicine. J. Pharm. Pharmacol. 2011, 64, 172–189. [Google Scholar] [CrossRef]
- Classen, B.; Blaschek, W. High Molecular Weight Acidic Polysaccharides from Malva sylvestris and Alcea rosea. Planta Med. 1998, 64, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Hitoe, S.; Tanaka, J.; Shimoda, H. MaquiBright standardized maqui berry extract significantly increases tear fluid production and ameliorates dry eye-related symptoms in a clinical pilot trial. Panminerva Med. 2014, 56, 1–6. [Google Scholar] [PubMed]
- Kim, C.-S.; Jo, K.; Lee, I.-S.; Kim, J. Topical Application of Apricot Kernel Extract Improves Dry Eye Symptoms in a Unilateral Exorbital Lacrimal Gland Excision Mouse. Nutrients 2016, 8, 20161123. [Google Scholar] [CrossRef] [PubMed]
- Künstle, G.; Hufnagel, R.; Semaca, C.; Sterrantino, G.; Basile, A.; Suhr, C.; Schnelle, M.; Ammendola, A. Mallow extract-containing hyaluronic (HA) acid eye drops for the treatment of dry eyes is perceived superior over an HA-only product. Planta Med. 2019, 85, P-418. [Google Scholar] [CrossRef]
- DEWS. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and Validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Tsubota, K. Pharmacotherapy of dry eye. Expert Opin. Pharmacother. 2011, 12, 325–334. [Google Scholar] [CrossRef]
- Dogru, M.; Nakamura, M.; Shimazaki, J.; Tsubota, K. Changing trends in the treatment of dry-eye disease. Expert Opin. Investig. Drugs 2013, 22, 1581–1601. [Google Scholar] [CrossRef]
- Murube, J.; Paterson, A.; Murube, E. Classification of artificial tears. I: Composition and properties. Adv. Exp. Med. Biol. 1998, 438, 693–704. [Google Scholar] [CrossRef]
- Tong, L.; Petznick, A.; Lee, S.-Y.; Tan, J. Choice of artificial tear formulation for patients with dry eye: Where do we start? Cornea 2012, 31 (Suppl. S1), S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Murube, J.; Murube, A.; Zhuo, C. Classification of artificial tears. II: Additives and commercial formulas. Single Mol. Single Cell Seq. 1998, 438, 705–715. [Google Scholar]
- Pucker, A.D.; Ng, S.M.; Nichols, J.J. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst. Rev. 2016, 2, CD009729. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: A comparative study of the nutraceutical potential and composition. Food Chem. Toxicol. 2010, 48, 1466–1472. [Google Scholar] [CrossRef]
- Martins, C.A.F.; Weffort-Santos, A.M.; Gasparetto, J.C.; Trindade, A.C.L.B.; Otuki, M.F.; Pontarolo, R. Malva sylvestris L. extract suppresses desferrioxamine-induced PGE(2) and PGD(2) release in differentiated U937 cells: The development and validation of an LC-MS/MS method for prostaglandin quantification. Biomed. Chromatogr. 2014, 28, 986–993. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, A.A.; Mandelli, G.; Cendali, M.; Hufnagel, R. The Lubricating Effect of Eye Drops Containing Hyaluronic Acid and Mallow Extract in Patients with Dry Eye Disease—A Pilot Study. Medicina 2023, 59, 958. https://doi.org/10.3390/medicina59050958
Basile AA, Mandelli G, Cendali M, Hufnagel R. The Lubricating Effect of Eye Drops Containing Hyaluronic Acid and Mallow Extract in Patients with Dry Eye Disease—A Pilot Study. Medicina. 2023; 59(5):958. https://doi.org/10.3390/medicina59050958
Chicago/Turabian StyleBasile, Andrea Attilio, Giulia Mandelli, Magda Cendali, and Rebecca Hufnagel. 2023. "The Lubricating Effect of Eye Drops Containing Hyaluronic Acid and Mallow Extract in Patients with Dry Eye Disease—A Pilot Study" Medicina 59, no. 5: 958. https://doi.org/10.3390/medicina59050958
APA StyleBasile, A. A., Mandelli, G., Cendali, M., & Hufnagel, R. (2023). The Lubricating Effect of Eye Drops Containing Hyaluronic Acid and Mallow Extract in Patients with Dry Eye Disease—A Pilot Study. Medicina, 59(5), 958. https://doi.org/10.3390/medicina59050958






