The Effect of Maternal Exposure to Air Pollutants and Heavy Metals during Pregnancy on the Risk of Neurological Disorders Using the National Health Insurance Claims Data of South Korea
Abstract
1. Introduction
2. Materials and Methods
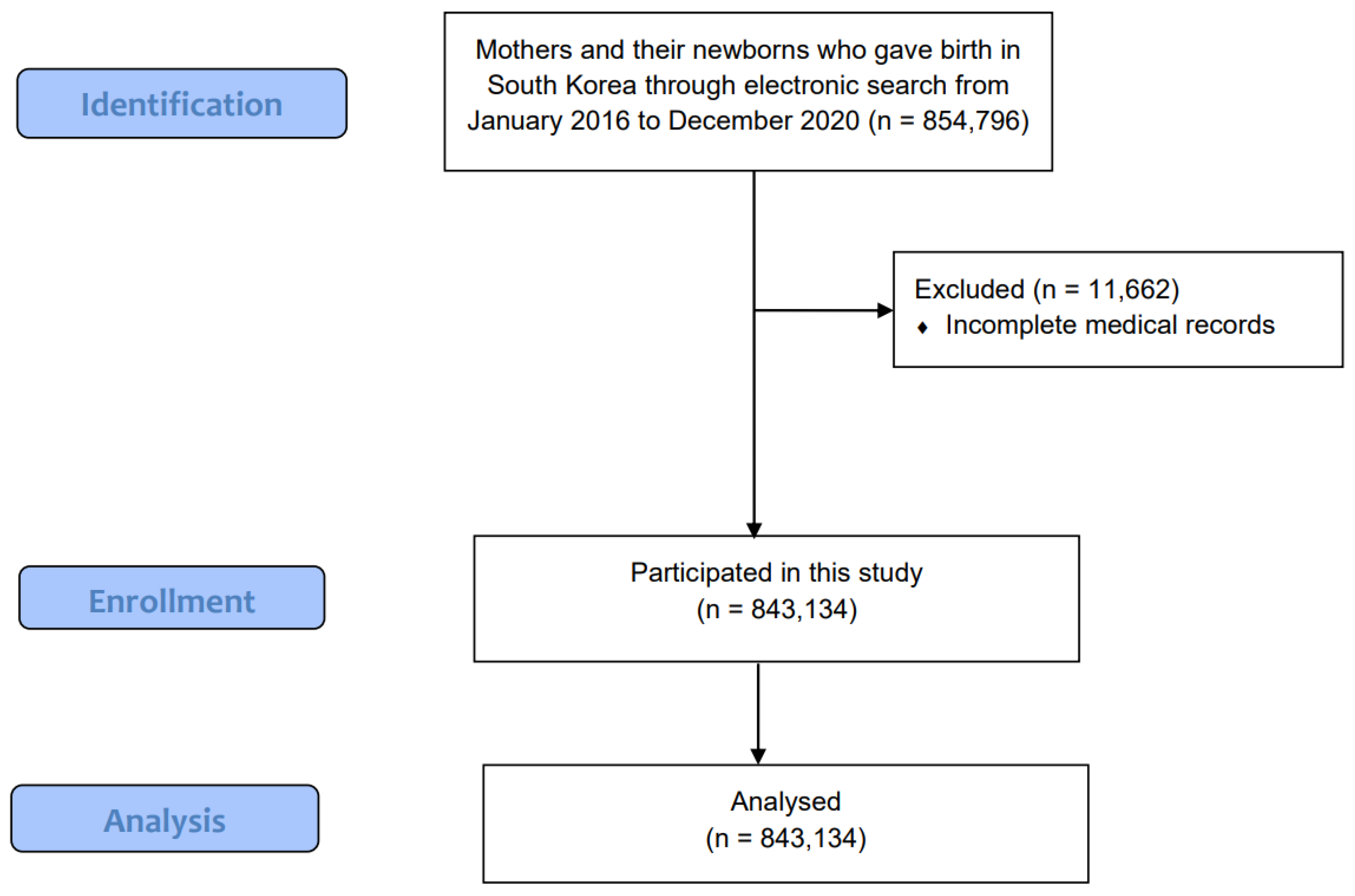
2.1. Study Population
2.2. Statistical Analysis
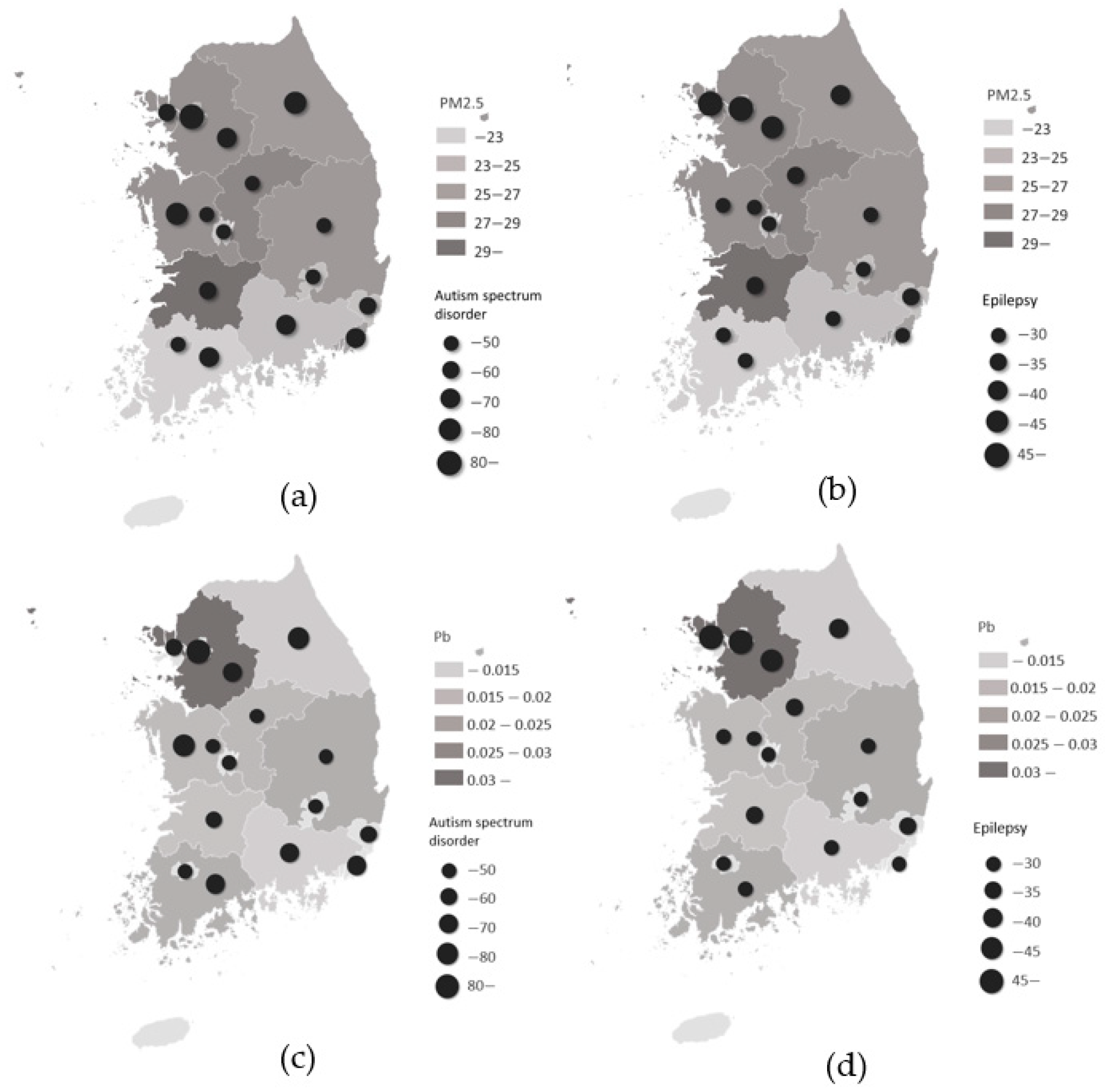
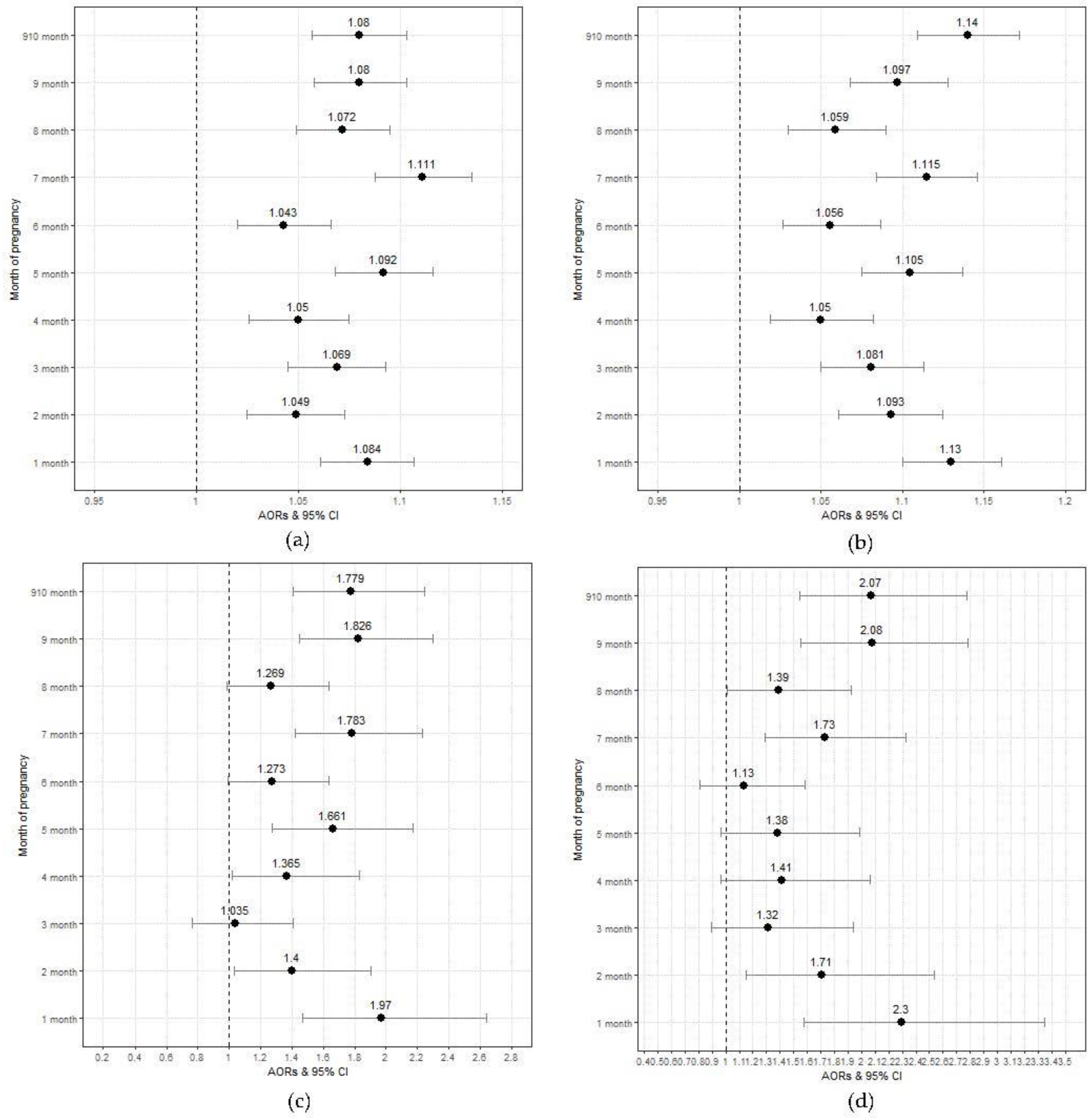
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, J.; Qin, Y.; Chen, Z.; Li, J.; Guo, P.; Shan, L.; Li, Y.; Hao, Y.; Jiao, M.; et al. Global attributed burden of death for air pollution: Demographic decomposition and birth cohort effect. Sci. Total Environ. 2023, 860, 160444. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, E.; Martin, R.; Yin, H.; Brauer, M. Global Burden of Disease from Major Air Pollution Sources (GBD MAPS): A Global Approach. Res. Rep. Health Eff. Inst. 2021, 2021, 1–45. [Google Scholar]
- Ju, M.J.; Oh, J.; Choi, Y.H. Changes in air pollution levels after COVID-19 outbreak in Korea. Sci. Total Environ. 2021, 750, 141521. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Z.; Xue, B.; Ma, J.; Chen, X.; Lu, C. Economic growth and pollution emission in China: Structural path analysis. Sustainability 2018, 10, 2569. [Google Scholar] [CrossRef]
- Ngarambe, J.; Joen, S.J.; Han, C.H.; Yun, G.Y. Exploring the relationship between particulate matter, CO, SO(2), NO(2), O(3) and urban heat island in Seoul, Korea. J. Hazard. Mater. 2021, 403, 123615. [Google Scholar] [CrossRef] [PubMed]
- Guaman, M.; Roberts-Semple, D.; Aime, C.; Shin, J.; Akinremi, A. Traffic Density and Air Pollution: Spatial and Seasonal Variations of Nitrogen Dioxide and Ozone in Jamaica, New York. Atmosphere 2022, 13, 2042. [Google Scholar] [CrossRef]
- Choi, E.; Yi, S.M.; Lee, Y.S.; Jo, H.; Baek, S.O.; Heo, J.B. Sources of airborne particulate matter-bound metals and spatial-seasonal variability of health risk potentials in four large cities, South Korea. Environ. Sci. Pollut. Res. Int. 2022, 29, 28359–28374. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlahogianni, T.; Bakeas, E.B.; Triantafillaki, S.; Paraskevopoulou, V.; Dassenakis, M. Characterization of atmospheric particulates, particle-bound transition metals and polycyclic aromatic hydrocarbons of urban air in the centre of Athens (Greece). Chemosphere 2006, 65, 760–768. [Google Scholar] [CrossRef]
- Querol, X.; Viana, M.; Alastuey, A.; Amato, F.; Moreno, T.; Castillo, S.; Pey, J.; Rosa, J.d.l.; Campa, A.S.d.l.; Artíñano, B.; et al. Source origin of trace elements in PM from regional background, urban and industrial sites of Spain. Atmos. Environ. 2007, 41, 7219–7231. [Google Scholar] [CrossRef]
- Meng, X.; Ma, Y.; Chen, R.; Zhou, Z.; Chen, B.; Kan, H. Size-fractionated particle number concentrations and daily mortality in a Chinese city. Environ. Health Perspect. 2013, 121, 1174–1178. [Google Scholar] [CrossRef]
- Samoli, E.; Atkinson, R.W.; Analitis, A.; Fuller, G.W.; Beddows, D.; Green, D.C.; Mudway, I.S.; Harrison, R.M.; Anderson, H.R.; Kelly, F.J. Differential health effects of short-term exposure to source-specific particles in London, U.K. Environ. Int. 2016, 97, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, H.; Fakhri, S.; Derakhshanfar, H.; Hosseini-Zijoud, S.M.; Safari, S.; Hatamabadi, H.R. The Effects of Air Pollution on Ischemic Stroke Admission Rate. Chonnam Med. J. 2016, 52, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Simoncic, V.; Enaux, C.; Deguen, S.; Kihal-Talantikite, W. Adverse Birth Outcomes Related to NO(2) and PM Exposure: European Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8116. [Google Scholar] [CrossRef]
- Lertxundi, A.; Andiarena, A.; Martinez, M.D.; Ayerdi, M.; Murcia, M.; Estarlich, M.; Guxens, M.; Sunyer, J.; Julvez, J.; Ibarluzea, J. Prenatal exposure to PM(2.5) and NO(2) and sex-dependent infant cognitive and motor development. Environ. Res. 2019, 174, 114–121. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoylaerts, M.F.; Hoet, P.H.; Nemery, B. Possible mechanisms of the cardiovascular effects of inhaled particles: Systemic translocation and prothrombotic effects. Toxicol. Lett. 2004, 149, 243–253. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Cao, F.; Liu, S.; Zou, X.; Li, G.; Yang, H.; Jiao, Y. Number 2 Feibi Recipe Reduces PM2.5-Induced Lung Injury in Rats. Evid. Based Complement Alternat. Med. 2018, 2018, 3674145. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.H.; Fan, Y.; Nguyen, T.V.; Shin, H.S.; Kim, H.T.; Song, C.H.; Chai, O.H. PM(2.5) Exacerbates Oxidative Stress and Inflammatory Res.ponse through the Nrf2/NF-kappaB Signaling Pathway in OVA-Induced Allergic Rhinitis Mouse Model. Int. J. Mol. Sci. 2021, 22, 8173. [Google Scholar] [CrossRef]
- Thornburg, K.L.; Kolahi, K.; Pierce, M.; Valent, A.; Drake, R.; Louey, S. Biological features of placental programming. Placenta 2016, 48 (Suppl. 1), S47–S53. [Google Scholar] [CrossRef]
- Siddika, N.; Balogun, H.A.; Amegah, A.K.; Jaakkola, J.J. Prenatal ambient air pollution exposure and the risk of stillbirth: Systematic review and meta-analysis of the empirical evidence. Occup. Environ. Med. 2016, 73, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Enkhmaa, D.; Warburton, N.; Javzandulam, B.; Uyanga, J.; Khishigsuren, Y.; Lodoysamba, S.; Enkhtur, S.; Warburton, D. Seasonal ambient air pollution correlates strongly with spontaneous abortion in Mongolia. BMC Pregnancy Childbirth 2014, 14, 146. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 19, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Eyre, H.; Baune, B.T. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology 2012, 37, 1397–1416. [Google Scholar] [CrossRef]
- Goel, R.; Bhat, S.A.; Hanif, K.; Nath, C.; Shukla, R. Angiotensin II Receptor Blockers Attenuate Lipopolysaccharide-Induced Memory Impairment by Modulation of NF-kappaB-Mediated BDNF/CREB Expression and Apoptosis in Spontaneously Hypertensive Rats. Mol. Neurobiol. 2018, 55, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Saenen, N.D.; Plusquin, M.; Bijnens, E.; Janssen, B.G.; Gyselaers, W.; Cox, B.; Fierens, F.; Molenberghs, G.; Penders, J.; Vrijens, K.; et al. In Utero Fine Particle Air Pollution and Placental Expression of Genes in the Brain-Derived Neurotrophic Factor Signaling Pathway: An ENVIRONAGE Birth Cohort Study. Environ. Health Perspect. 2015, 123, 834–840. [Google Scholar] [CrossRef]
- Wei, H.; Liang, F.; Meng, G.; Nie, Z.; Zhou, R.; Cheng, W.; Wu, X.; Feng, Y.; Wang, Y. Redox/methylation mediated abnormal DNA methylation as regulators of ambient fine particulate matter-induced neurodevelopment related impairment in human neuronal cells. Sci. Rep. 2016, 6, 33402. [Google Scholar] [CrossRef]
- Chen, M.; Li, B.; Sang, N. Particulate matter (PM(2.5)) exposure season-dependently induces neuronal apoptosis and synaptic injuries. J. Environ. Sci. (China) 2017, 54, 336–345. [Google Scholar] [CrossRef]
- Folstein, S.; Rutter, M. Infantile autism: A genetic study of 21 twin pairs. J. Child Psychol. Psychiatry 1977, 18, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Steffenburg, S.; Gillberg, C.; Hellgren, L.; Andersson, L.; Gillberg, I.C.; Jakobsson, G.; Bohman, M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J. Child Psychol. Psychiatry 1989, 30, 405–416. [Google Scholar] [CrossRef]
- Jeste, S.S.; Geschwind, D.H. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat. Rev. Neurol. 2014, 10, 74–81. [Google Scholar] [CrossRef]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Larsson, H.; Hultman, C.M.; Reichenberg, A. The familial risk of autism. JAMA 2014, 311, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, K.; van Luijtelaar, G. The impact of early-life environment on absence epilepsy and neuropsychiatric comorbidities. IBRO Neurosci. Rep. 2022, 13, 436–468. [Google Scholar] [CrossRef]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ. Health Perspect. 2006, 114, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Seltenrich, N. PM2.5 Exposure and Intrauterine Inflammation: A Possible Mechanism for Preterm and Underweight Birth. Environ. Health Perspect. 2016, 124, A190. [Google Scholar] [CrossRef] [PubMed]
- van den Hooven, E.H.; Pierik, F.H.; de Kluizenaar, Y.; Hofman, A.; van Ratingen, S.W.; Zandveld, P.Y.; Russcher, H.; Lindemans, J.; Miedema, H.M.; Steegers, E.A.; et al. Air pollution exposure and markers of placental growth and function: The generation R study. Environ. Health Perspect. 2012, 120, 1753–1759. [Google Scholar] [CrossRef]
- Abraham, E.; Rousseaux, S.; Agier, L.; Giorgis-Allemand, L.; Tost, J.; Galineau, J.; Hulin, A.; Siroux, V.; Vaiman, D.; Charles, M.A.; et al. Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environ. Int. 2018, 118, 334–347. [Google Scholar] [CrossRef]
- Hung, T.H.; Hsu, T.Y.; Tung, T.H.; Tsai, C.C.; Ou, C.Y.; Chung, F.F.; Wan, G.H. The association between maternal exposure to outdoor air pollutants, inflammatory response, and birth weight in healthy women. Environ. Res. 2021, 196, 110921. [Google Scholar] [CrossRef]
- Cao, Z.; Meng, L.; Zhao, Y.; Liu, C.; Yang, Y.; Su, X.; Fu, Q.; Wang, D.; Hua, J. Maternal exposure to ambient fine particulate matter and fetal growth in Shanghai, China. Environ. Health 2019, 18, 49. [Google Scholar] [CrossRef]
- Rich, D.Q.; Liu, K.; Zhang, J.; Thurston, S.W.; Stevens, T.P.; Pan, Y.; Kane, C.; Weinberger, B.; Ohman-Strickland, P.; Woodruff, T.J.; et al. Differences in Birth Weight Associated with the 2008 Beijing Olympics Air Pollution Reduction: Res.ults from a Natural Experiment. Environ. Health Perspect. 2015, 123, 880–887. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zhou, Y.; Xia, B.; Zhu, Q.; Ge, W.; Li, J.; Shi, H.; Xiao, X.; Zhang, Y. Prenatal fine particulate matter exposure, placental DNA methylation changes, and fetal growth. Environ. Int. 2021, 147, 106313. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Cheng, Y.Y.; Guo, H.R.; Tseng, Y.C. Air Pollution during Pregnancy and Childhood Autism Spectrum Disorder in Taiwan. Int. J. Environ. Res. Public Health 2021, 18, 9784. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, S.K.; Lin, K.C.; Ho, W.C.; Hsieh, W.S.; Shu, B.C.; Chen, P.C. Multilevel analysis of air pollution and early childhood neurobehavioral development. Int. J. Environ. Res. Public Health 2014, 11, 6827–6841. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhou, L.; Xu, J.; Kan, H.; Chen, R.; Chen, S.; Hua, H.; Liu, Z.; Yan, C. Effects of prenatal exposures to air sulfur dioxide/nitrogen dioxide on toddler neurodevelopment and effect modification by ambient temperature. Ecotoxicol. Environ. Saf. 2021, 230, 113118. [Google Scholar] [CrossRef]
- Yargicoglu, P.; Agar, A.; Gumuslu, S.; Bilmen, S.; Oguz, Y. Age-related alterations in antioxidant enzymes, lipid peroxide levels, and somatosensory-evoked potentials: Effect of sulfur dioxide. Arch. Environ. Contam. Toxicol. 1999, 37, 554–560. [Google Scholar] [CrossRef]
- Kucukatay, V.; Agar, A.; Yargicoglu, P.; Gumuslu, S.; Aktekin, B. Changes in somatosensory evoked potentials, lipid peroxidation, and antioxidant enzymes in experimental diabetes: Effect of sulfur dioxide. Arch. Environ. Health 2003, 58, 14–22. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, B. Oxidative damage of sulfur dioxide inhalation on brains and livers of mice. Environ. Toxicol. Pharmacol. 2003, 13, 1–8. [Google Scholar] [CrossRef]
- Yun, Y.; Li, H.; Li, G.; Sang, N. SO2 inhalation modulates the expression of apoptosis-related genes in rat hippocampus via its derivatives in vivo. Inhal. Toxicol. 2010, 22, 919–929. [Google Scholar] [CrossRef]
- Choi, Y.J.; Cho, J.; Hong, Y.C.; Lee, D.W.; Moon, S.; Park, S.J.; Lee, K.S.; Shin, C.H.; Lee, Y.A.; Kim, B.N.; et al. DNA methylation is associated with prenatal exposure to sulfur dioxide and childhood attention-deficit hyperactivity disorder symptoms. Sci. Rep. 2023, 13, 3501. [Google Scholar] [CrossRef]
- Gunnison, A.F.; Benton, A.W. Sulfur dioxide: Sulfite. Interaction with mammalian serum and plasma. Arch. Environ. Health 1971, 22, 381–388. [Google Scholar] [CrossRef]
- Gunnison, A.F. Sulphite toxicity: A critical review of in vitro and in vivo data. Food Cosmet. Toxicol. 1981, 19, 667–682. [Google Scholar] [CrossRef]
- Muller, F.; Massey, V. Flavin-sulfite complexes and their structures. J. Biol. Chem. 1969, 244, 4007–4016. [Google Scholar] [CrossRef] [PubMed]
- Kamogawa, A.; Fukui, T. Inhibition of -glucan phosphorylase by bisulfite competition at the phosphate binding site. Biochim. Biophys. Acta 1973, 302, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Xie, J.; Jiao, X.; Hu, H.; Zhang, Y.; Tao, R.; Tao, F.; Zhu, P. Foetal 25-hydroxyvitamin D moderates the association of prenatal air pollution exposure with foetal glucolipid metabolism disorder and systemic inflammatory responses. Environ. Int. 2021, 151, 106460. [Google Scholar] [CrossRef] [PubMed]
- Volk, H.E.; Lurmann, F.; Penfold, B.; Hertz-Picciotto, I.; McConnell, R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 2013, 70, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Dalman, C.; Wicks, S.; Dal, H.; Magnusson, C.; Lundholm, C.; Almqvist, C.; Pershagen, G. Perinatal Exposure to Traffic-Related Air Pollution and Autism Spectrum Disorders. Environ. Health Perspect. 2017, 125, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Almqvist, C.; Bolte, S.; Lichtenstein, P.; Anckarsater, H.; Lind, T.; Lundholm, C.; Pershagen, G. Exposure to air pollution from traffic and neurodevelopmental disorders in Swedish twins. Twin Res. Hum. Genet. 2014, 17, 553–562. [Google Scholar] [CrossRef]
- Cheng, J.; Su, H.; Song, J.; Wang, X. Short-term effect of air pollution on childhood epilepsy in eastern China: A space-time-stratified case-crossover and pooled analysis. Environ. Int. 2022, 170, 107591. [Google Scholar] [CrossRef]
- Cakmak, S.; Dales, R.E.; Vidal, C.B. Air pollution and hospitalization for epilepsy in Chile. Environ. Int. 2010, 36, 501–505. [Google Scholar] [CrossRef]
- Clemente, D.B.P.; Casas, M.; Janssen, B.G.; Lertxundi, A.; Santa-Marina, L.; Iniguez, C.; Llop, S.; Sunyer, J.; Guxens, M.; Nawrot, T.S.; et al. Prenatal ambient air pollution exposure, infant growth and placental mitochondrial DNA content in the INMA birth cohort. Environ. Res. 2017, 157, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Decrue, F.; Gorlanova, O.; Salem, Y.; Vienneau, D.; de Hoogh, K.; Gisler, A.; Usemann, J.; Korten, I.; Nahum, U.; Sinues, P.; et al. Increased Impact of Air Pollution on Lung Function in Preterm versus Term Infants: The BILD Study. Am. J. Respir. Crit. Care Med. 2022, 205, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gong, X.; Han, B.; Chu, M.; Gong, C.; Yang, J.; Chen, L.; Wang, J.; Bai, Z.; Zhang, Y. Ambient PM(2.5) exposures and systemic inflammation in women with early pregnancy. Sci. Total Environ. 2022, 829, 154564. [Google Scholar] [CrossRef] [PubMed]
- Gyllenhammer, L.E.; Rasmussen, J.M.; Bertele, N.; Halbing, A.; Entringer, S.; Wadhwa, P.D.; Buss, C. Maternal Inflammation During Pregnancy and Offspring Brain Development: The Role of Mitochondria. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 498–509. [Google Scholar] [CrossRef]
- Barron, A.; McCarthy, C.M.; O’Keeffe, G.W. Preeclampsia and Neurodevelopmental Outcomes: Potential Pathogenic Roles for Inflammation and Oxidative Stress? Mol. Neurobiol. 2021, 58, 2734–2756. [Google Scholar] [CrossRef]
- Valencia, A.; Moran, J. Role of oxidative stress in the apoptotic cell death of cultured cerebellar granule neurons. J. Neurosci. Res. 2001, 64, 284–297. [Google Scholar] [CrossRef]
- Xu, W.; Chi, L.; Row, B.W.; Xu, R.; Ke, Y.; Xu, B.; Luo, C.; Kheirandish, L.; Gozal, D.; Liu, R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 2004, 126, 313–323. [Google Scholar] [CrossRef]
- Singh, J. Nitrogen dioxide exposure alters neonatal development. Neurotoxicology 1988, 9, 545–549. [Google Scholar]
- Michikawa, T.; Morokuma, S.; Yamazaki, S.; Fukushima, K.; Kato, K.; Nitta, H. Exposure to air pollutants during the early weeks of pregnancy, and placenta praevia and placenta accreta in the western part of Japan. Environ. Int. 2016, 92–93, 464–470. [Google Scholar] [CrossRef]
- Park, J.; Kim, W.J.; Kim, J.; Jeong, C.Y.; Park, H.; Hong, Y.C.; Ha, M.; Kim, Y.; Won, S.; Ha, E. Prenatal Exposure to Traffic-Related Air Pollution and the DNA Methylation in Cord Blood Cells: MOCEH Study. Int. J. Environ. Res. Public Health 2022, 19, 3292. [Google Scholar] [CrossRef]
- Zhai, Q.; Cen, S.; Jiang, J.; Zhao, J.; Zhang, H.; Chen, W. Disturbance of trace element and gut microbiota profiles as indicators of autism spectrum disorder: A pilot study of Chinese children. Environ. Res. 2019, 171, 501–509. [Google Scholar] [CrossRef]
- Lakshmi Priya, M.D.; Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 2011, 142, 148–158. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Suren, P.; Oie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ. Int. 2021, 152, 106468. [Google Scholar] [CrossRef] [PubMed]
- Sasmaz, S.; Uz, E.; Pinar, T.; Vural, H.s.; Eiri, M.c.; Ilihan, A.; Akyol, m. Hair lead and cadmium concentrations in patients with epilepsy and migraine. Neurosci. Res. Commun. 2003, 32, 107–114. [Google Scholar] [CrossRef]
- Eubig, P.A.; Aguiar, A.; Schantz, S.L. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ. Health Perspect. 2010, 118, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, H.; Wang, C.; Gao, D.; Zhou, Y.; Zuo, Z. Maternal exposure to the water soluble fraction of crude oil, lead and their mixture induces autism-like behavioral deficits in zebrafish (Danio rerio) larvae. Ecotoxicol. Environ. Saf. 2016, 134, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Xu, H.; Zheng, H.; Bai, C.; Pan, W.; Zhou, H.; Liao, M.; Huang, C.; Dong, Q. Maternal exposure to low dose BDE209 and Pb mixture induced neurobehavioral anomalies in C57BL/6 male offspring. Toxicology 2019, 418, 70–80. [Google Scholar] [CrossRef]
- Su, P.; Wang, D.; Cao, Z.; Chen, J.; Zhang, J. The role of NLRP3 in lead-induced neuroinflammation and possible underlying mechanism. Environ. Pollut. 2021, 287, 117520. [Google Scholar] [CrossRef]
- Liu, J.T.; Chen, B.Y.; Zhang, J.Q.; Kuang, F.; Chen, L.W. Lead exposure induced microgliosis and astrogliosis in hippocampus of young mice potentially by triggering TLR4-MyD88-NFkappaB signaling cascades. Toxicol. Lett. 2015, 239, 97–107. [Google Scholar] [CrossRef]
- Smith, M.R.; Yevoo, P.; Sadahiro, M.; Austin, C.; Amarasiriwardena, C.; Awawda, M.; Arora, M.; Dudley, J.T.; Morishita, H. Integrative bioinformatics identifies postnatal lead (Pb) exposure disrupts developmental cortical plasticity. Sci. Rep. 2018, 8, 16388. [Google Scholar] [CrossRef]
- Gundacker, C.; Hengstschlager, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.F.; Farooqui, Z.; Faulk, C.D.; Barks, A.K.; Jones, T.; Dolinoy, D.C.; Bakulski, K.M. Perinatal Lead (Pb) Exposure and Cortical Neuron-Specific DNA Methylation in Male Mice. Genes 2019, 10, 274. [Google Scholar] [CrossRef]
- Montrose, L.; Faulk, C.; Francis, J.; Dolinoy, D.C. Perinatal lead (Pb) exposure results in sex and tissue-dependent adult DNA methylation alterations in murine IAP transposons. Environ. Mol. Mutagen 2017, 58, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- Huel, G.; Everson, R.B.; Menger, I. Increased hair cadmium in newborns of women occupationally exposed to heavy metals. Environ. Res. 1984, 35, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Doyle, P.; Wang, D.; Hwang, Y.H.; Chen, P.C. Does prenatal cadmium exposure affect fetal and child growth? Occup. Environ. Med. 2011, 68, 641–646. [Google Scholar] [CrossRef]
- Grover, C.A.; Nation, J.R.; Reynolds, K.M.; Benzick, A.E.; Bratton, G.R.; Rowe, L.D. The effects of cadmium on ethanol self-administration using a sucrose-fading procedure. Neurotoxicology 1991, 12, 235–243. [Google Scholar]
- Pelletier, M.R.; Satinder, K.P. Low-level cadmium exposure increases one-way avoidance in juvenile rats. Neurotoxicol. Teratol. 1991, 13, 657–662. [Google Scholar] [CrossRef]
- Kim, Y.; Ha, E.H.; Park, H.; Ha, M.; Kim, Y.; Hong, Y.C.; Kim, E.J.; Kim, B.N. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: The Mothers and Children’s Environ.mental Health (MOCEH) study. Neurotoxicology 2013, 35, 15–22. [Google Scholar] [CrossRef]
- Forns, J.; Fort, M.; Casas, M.; Caceres, A.; Guxens, M.; Gascon, M.; Garcia-Esteban, R.; Julvez, J.; Grimalt, J.O.; Sunyer, J. Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. Neurotoxicology 2014, 40, 16–22. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Chisolm, J.J., Jr. The susceptibility of the fetus and child to chemical pollutants. Heavy metal exposures: Toxicity from metal-metal interactions, and behavioral effects. Pediatrics 1974, 53, 841–843. [Google Scholar] [PubMed]
- Gorini, F.; Muratori, F.; Morales, M.A. The Role of Heavy Metal Pollution in Neurobehavioral Disorders: A Focus on Autism. Rev. J. Autism Dev. Disord. 2014, 1, 354–372. [Google Scholar] [CrossRef]
| Total | With Autism Spectrum Disorder | With Epilepsy | |
|---|---|---|---|
| Maternal age (years) *† | |||
| total | 843,134 | 5493 | 3190 |
| <20 | 68 (0.0) | 0 (0.0) | 1 (0.0) |
| 20–30 | 70,501 (8.4) | 460 (8.4) | 246 (7.7) |
| 30–40 | 616,720 (73.1) | 3740 (68.1) | 2182 (68.4) |
| 40< | 155,845 (18.5) | 1293 (23.5) | 761 (23.9) |
| Occupational status * | |||
| Worked outside home | 323,250 (38.3) | 1945 (35.4) | 1187 (37.2) |
| Household income * | |||
| Low (40%) | 292,469 (34.7) | 1819 (33.1) | 1095 (34.3) |
| Middle (40–95%) | 531,455 (63.0) | 3526 (64.2) | 2006 (62.9) |
| High (95% <) | 19,210 (2.3) | 148 (2.7) | 89 (2.8) |
| Infant sex *† | |||
| Male | 428,873 (51.2) | 3934 (71.6) | 1765 (55.3) |
| Season *† | |||
| Winter | 224,344 (26.6) | 1850 (33.7) | 1170 (36.7) |
| Spring | 218,963 (26.0) | 1332 (24.2) | 716 (22.4) |
| Summer | 204,840 (24.3) | 1196 (21.8) | 679 (21.3) |
| Fall | 194,987 (23.1) | 1115 (20.3) | 625 (19.6) |
| Premature (27–36 weeks) *† | |||
| <36 weeks | 44,060 (5.3) | 829 (15.1) | 430 (13.5) |
| Multiple birth * | |||
| twin (Z38.3–Z38.5) | 18,734 (2.2) | 193 (3.5) | 77 (2.4) |
| Air Pollutants and Heavy Metals | Mean | Median |
|---|---|---|
| PM10 (μg/m3) | 45.35 ± 11.07 | 45.07 (36.67–52.68) |
| PM2.5 (μg/m3) | 25.83 ± 6.17 | 25.33 (21.36–29.71) |
| SO2 (ppm)) | 0.005 ± 0.001 | 0.005 (0.004–0.005) |
| NO2 (ppm) | 0.023 ± 0.007 | 0.023 (0.018–0.028) |
| O3 (ppm) | 0.028 ± 0.010 | 0.028 (0.020–0.035) |
| CO (ppm) | 0.493 ± 0.110 | 0.468 (0.412–0.575) |
| Pb (μg/m3) | 0.024 ± 0.012 | 0.022 (0.015–0.033) |
| Cd (μg/m3) | 0.001 ± 0.001 | 0.001 (0.001–0.001) |
| Cr (μg/m3) | 0.005 ± 0.004 | 0.004 (0.002–0.006) |
| Cu (μg/m3) | 0.025 ± 0.018 | 0.021 (0.013–0.034) |
| Mn (μg/m3) | 0.032 ± 0.017 | 0.029 (0.020–0.040) |
| Fe (μg/m3) | 0.672 ± 0.345 | 0.650 (0.429–0.838) |
| Ni (μg/m3) | 0.005 ± 0.003 | 0.004 (0.003–0.006) |
| As (μg/m3) | 0.004 ± 0.003 | 0.003 (0.002–0.005) |
| With Autism Spectrum Disorder | With Epilepsy | ||
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| PM10 | 1st stage | 0.992 (0.988–0.996) | 0.992 (0.987–0.998) |
| 2nd stage | 1.023 (1.019–1.027) | 1.021 (1.016–1.027) | |
| 3rd stage | 1.022 (1.018–1.026) | 1.020 (1.015–1.026) | |
| total | 1.023 (1.017–1.029) | 1.023 (1.015–1.030) | |
| PM2.5 | 1st stage | 0.995 (0.989–1.003) | 0.996 (0.987–1.005) |
| 2nd stage | 1.036 (1.029–1.043) | 1.033 (1.024–1.043) | |
| 3rd stage | 1.039 (1.031–1.046) | 1.033 (1.024–1.043) | |
| total | 1.048 (1.037–1.059) | 1.047 (1.033–1.061) | |
| SO2 | 1st stage | 2.843 (2.240–3.609) | 2.900 (2.129–3.950) |
| 2nd stage | 4.149 (3.183–5.408) | 3.443 (2.434–4.871) | |
| 3rd stage | 5.060 (3.819–6.703) | 5.670 (3.920–8.202) | |
| total | 6.060 (4.449–8.256) | 6.039 (4.033–9.041) | |
| NO2 | 1st stage | 1.281 (1.222–1.343) | 1.485 (1.396–1.580) |
| 2nd stage | 1.463 (1.396–1.533) | 1.587 (1.492–1.688) | |
| 3rd stage | 1.394 (1.329–1.462) | 1.577 (1.481–1.680) | |
| total | 1.439 (1.367–1.515) | 1.669 (1.559–1.786) | |
| O3 | 1st stage | 0.719 (0.679–0.761) | 0.679 (0.629–0.732) |
| 2nd stage | 0.764 (0.723–0.807) | 0.716 (0.666–0.770) | |
| 3rd stage | 0.862 (0.813–0.914) | 0.653 (0.603–0.707) | |
| total | 0.572 (0.522–0.626) | 0.404 (0.358–0.457) | |
| CO | 1st stage | 1.008 (1.004–1.012) | 1.014 (1.009–1.019) |
| 2nd stage | 1.022 (1.018–1.026) | 1.025 (1.020–1.030) | |
| 3rd stage | 1.021 (1.017–1.025) | 1.032 (1.026–1.037) | |
| total | 1.027 (1.022–1.033) | 1.040 (1.033–1.047) | |
| Pb | 1st stage | 1.097 (1.069–1.126) | 1.147 (1.108–1.186) |
| 2nd stage | 1.088 (1.060–1.117) | 1.101 (1.063–1.139) | |
| 3rd stage | 1.139 (1.110–1.169) | 1.164 (1.125–1.205) | |
| total | 1.143 (1.109–1.178) | 1.181 (1.136–1.228) | |
| Cd | 1st stage | 2.038 (1.350–3.076) | 3.045 (1.789–5.181) |
| 2nd stage | 1.874 (1.316–2.667) | 1.750 (1.101–2.782) | |
| 3rd stage | 2.458 (1.815–3.328) | 3.288 (2.263–4.777) | |
| total | 3.287 (2.113–5.113) | 5.389 (3.061–9.487) | |
| Cr | 1st stage | 1.075 (0.980–1.180) | 1.059 (0.936–1.197) |
| 2nd stage | 1.121 (1.020–1.233) | 1.054 (0.929–1.195) | |
| 3rd stage | 1.102 (0.995–1.221) | 1.122 (0.982–1.282) | |
| total | 1.153 (1.026–1.297) | 1.112 (0.954–1.297) | |
| Cu | 1st stage | 1.028 (1.010–1.046) | 1.071 (1.047–1.094) |
| 2nd stage | 1.045 (1.026–1.064) | 1.088 (1.063–1.114) | |
| 3rd stage | 1.059 (1.039–1.078) | 1.103 (1.077–1.129) | |
| total | 1.052 (1.031–1.073) | 1.109 (1.080–1.137) | |
| Mn | 1st stage | 1.016 (0.997–1.036) | 1.003 (0.978–1.029) |
| 2nd stage | 1.042 (1.023–1.061) | 1.023 (0.999–1.048) | |
| 3rd stage | 1.031 (1.012–1.051) | 1.031 (1.005–1.056) | |
| total | 1.036 (1.014–1.058) | 1.024 (0.996–1.054) | |
| Fe | 1st stage | 1.001 (1.000–1.002) | 1.003 (1.001–1.004) |
| 2nd stage | 1.004 (1.003–1.005) | 1.005 (1.004–1.007) | |
| 3rd stage | 1.004 (1.003–1.005) | 1.004 (1.003–1.006) | |
| total | 1.004 (1.003–1.005) | 1.006 (1.004–1.007) | |
| Ni | 1st stage | 1.054 (0.945–1.176) | 1.020 (0.883–1.177) |
| 2nd stage | 1.202 (1.078–1.340) | 1.191 (1.032–1.374) | |
| 3rd stage | 1.394 (1.248–1.558) | 1.296 (1.120–1.501) | |
| total | 1.302 (1.144–1.481) | 1.248 (1.054–1.479) | |
| As | 1st stage | 1.565 (1.390–1.763) | 1.311 (1.118–1.537) |
| 2nd stage | 1.912 (1.700–2.152) | 1.481 (1.260–1.741) | |
| 3rd stage | 1.660 (1.488–1.852) | 1.687 (1.461–1.947) | |
| total | 2.622 (2.222–3.095) | 2.039 (1.642–2.532) |
| With Autism Spectrum Disorder | ||
|---|---|---|
| SO2 + NO2 + Pb + Cd | OR (95%CI) | |
| SO2 | Total | 3.288 (2.306–4.687) |
| 1st stage | 1.770 (1.338–2.342) | |
| 2nd stage | 2.128 (1.557–2.909) | |
| 3rd stage | 2.723 (1.971–3.761) | |
| NO2 | Total | 1.322 (1.244–1.403) |
| 1st stage | 1.233 (1.165–1.304) | |
| 2nd stage | 1.421 (1.346–1.501) | |
| 3rd stage | 1.267 (1.197–1.341) | |
| Pb | Total | 1.079 (1.017–1.145) |
| 1st stage | 1.041 (0.993–1.092) | |
| 2nd stage | 0.980 (0.936–1.026) | |
| 3rd stage | 1.063 (1.019–1.110) | |
| Cd | Total | 0.441 (0.184–1.057) |
| 1st stage | 1.233 (1.165–1.304) | |
| 2nd stage | 1.421 (1.346–1.501) | |
| 3rd stage | 1.267 (1.197–1.341) | |
| With Epilepsy | ||
|---|---|---|
| SO2 + NO2 + Pb + Cd + As | OR (95%CI) | |
| SO2 | Total | 3.702 (2.25–6.089) |
| 1st stage | 2.106 (1.403–3.163) | |
| 2nd stage | 1.648 (1.061–2.560) | |
| 3rd stage | 2.897 (1.855–4.522) | |
| NO2 | Total | 1.869 (1.696–2.059) |
| 1st stage | 1.542 (1.422–1.672) | |
| 2nd stage | 1.680 (1.553–1.817) | |
| 3rd stage | 1.548 (1.427–1.681) | |
| Pb | Total | 1.064 (0.983–1.152) |
| 1st stage | 1.109 (1.043–1.179) | |
| 2nd stage | 1.039 (0.976–1.107) | |
| 3rd stage | 1.031 (0.974–1.092) | |
| Cd | Total | 2.591 (0.738–9.102) |
| 1st stage | 0.857 (0.328–2.243) | |
| 2nd stage | 0.757 (0.308–1.859) | |
| 3rd stage | 2.193 (1.074–4.477) | |
| As | Total | 0.273 (0.187–0.399) |
| 1st stage | 0.461 (0.361–0.589) | |
| 2nd stage | 0.568 (0.436–0.741) | |
| 3rd stage | 0.613 (0.480–0.783) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.S.; Min, W.K.; Choi, Y.J.; Jin, S.; Park, K.H.; Kim, S. The Effect of Maternal Exposure to Air Pollutants and Heavy Metals during Pregnancy on the Risk of Neurological Disorders Using the National Health Insurance Claims Data of South Korea. Medicina 2023, 59, 951. https://doi.org/10.3390/medicina59050951
Lee KS, Min WK, Choi YJ, Jin S, Park KH, Kim S. The Effect of Maternal Exposure to Air Pollutants and Heavy Metals during Pregnancy on the Risk of Neurological Disorders Using the National Health Insurance Claims Data of South Korea. Medicina. 2023; 59(5):951. https://doi.org/10.3390/medicina59050951
Chicago/Turabian StyleLee, Kuen Su, Won Kee Min, Yoon Ji Choi, Sejong Jin, Kyu Hee Park, and Suhyun Kim. 2023. "The Effect of Maternal Exposure to Air Pollutants and Heavy Metals during Pregnancy on the Risk of Neurological Disorders Using the National Health Insurance Claims Data of South Korea" Medicina 59, no. 5: 951. https://doi.org/10.3390/medicina59050951
APA StyleLee, K. S., Min, W. K., Choi, Y. J., Jin, S., Park, K. H., & Kim, S. (2023). The Effect of Maternal Exposure to Air Pollutants and Heavy Metals during Pregnancy on the Risk of Neurological Disorders Using the National Health Insurance Claims Data of South Korea. Medicina, 59(5), 951. https://doi.org/10.3390/medicina59050951







