Endocrine Disorders in a Newborn with Heterozygous Galactosemia, Down Syndrome and Complex Cardiac Malformation: Case Report
Abstract
1. Introduction
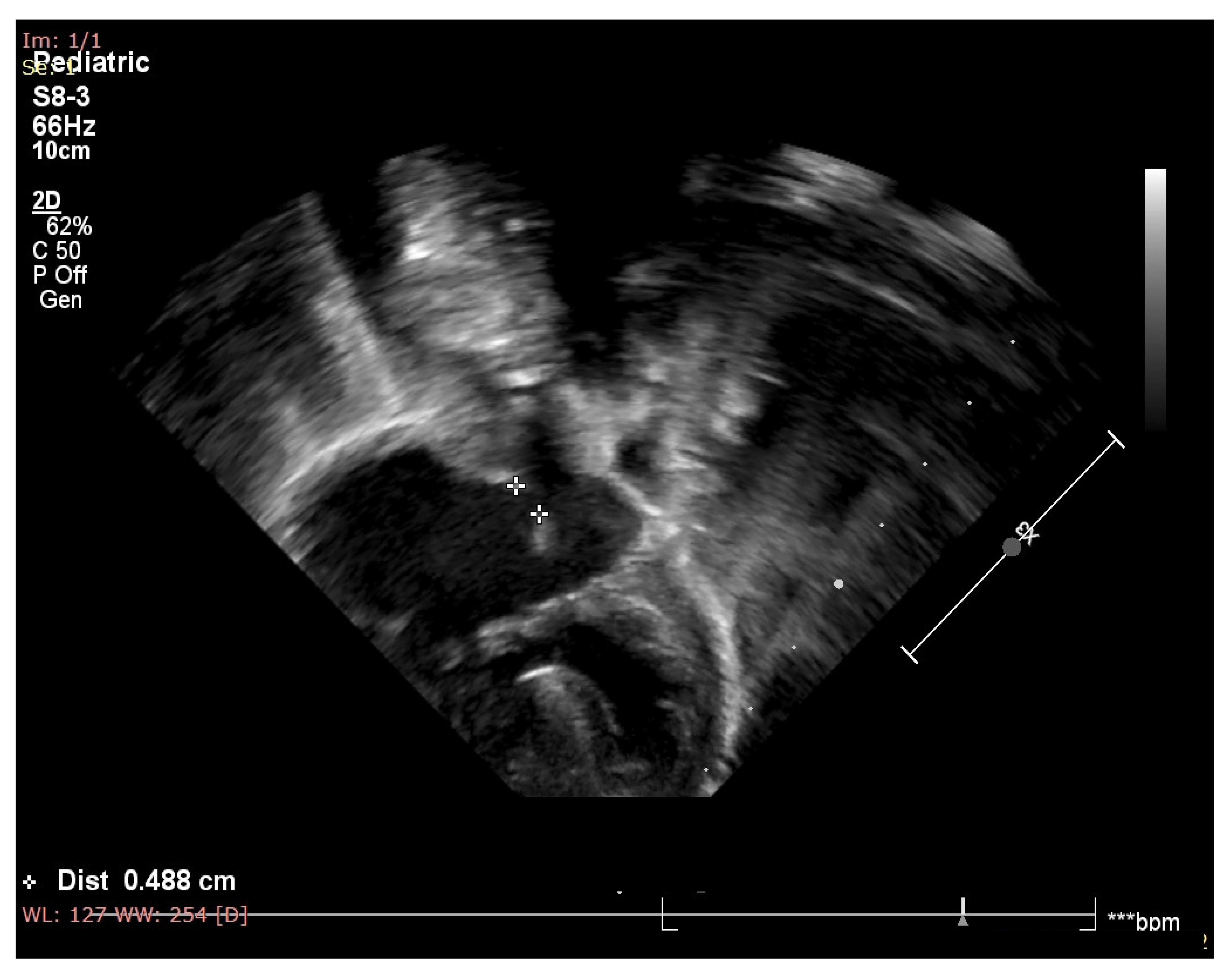
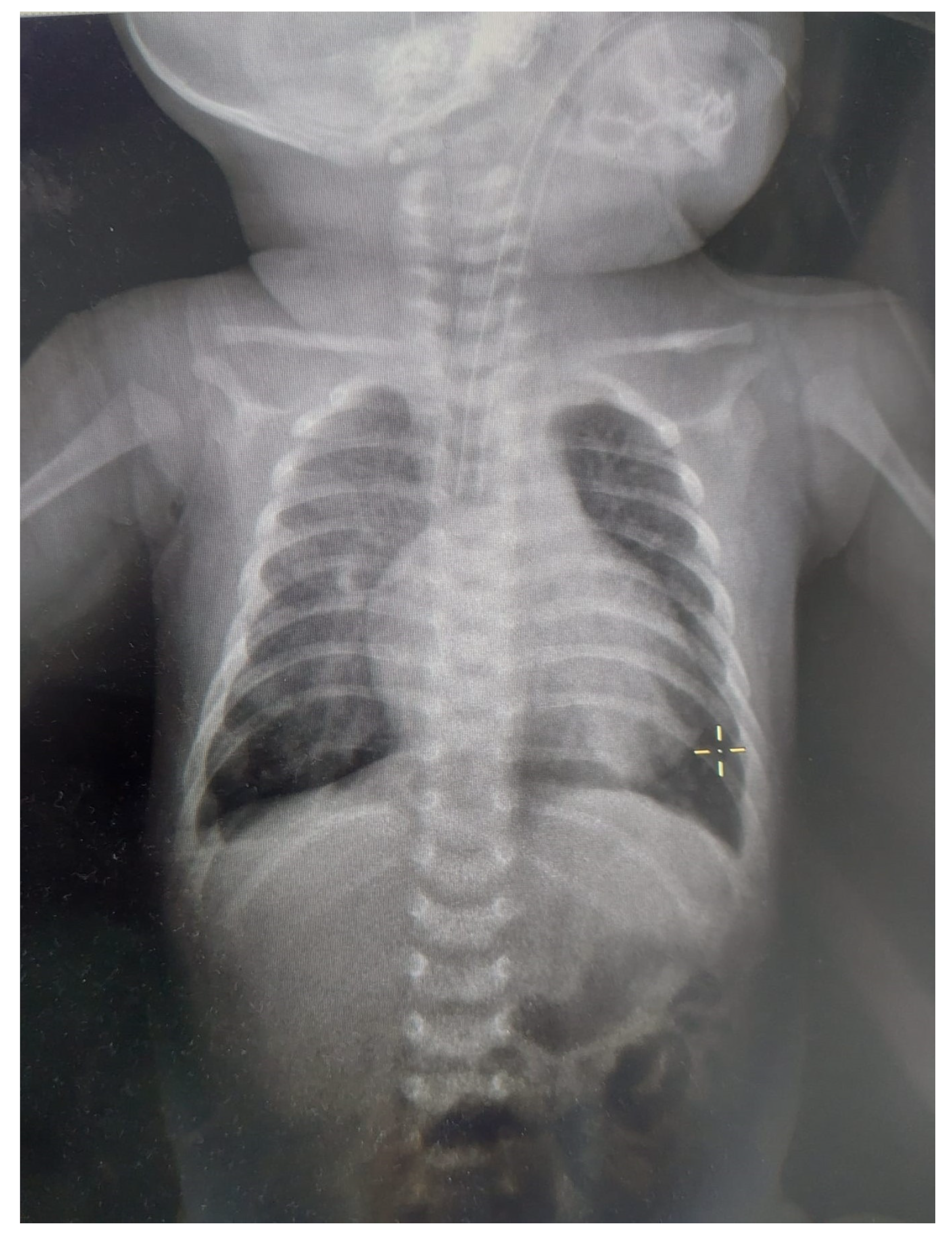
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranweiler, R. Assessment and Care of the Newborn with Down Syndrome. Adv. Neonatal Care Off. J. Natl. Assoc. Neonatal Nurses 2009, 9, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down Syndrome. Nat. Rev. Dis. Prim. 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J. Down Syndrome. N. Engl. J. Med. 2020, 382, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Agarwal Gupta, N.; Kabra, M. Diagnosis and Management of Down Syndrome. Indian J. Pediatr. 2014, 81, 560–567. [Google Scholar] [CrossRef]
- de Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the Number of People with Down Syndrome in Europe. Eur. J. Hum. Genet. 2021, 29, 402–410. [Google Scholar] [CrossRef]
- Whooten, R.; Schmitt, J.; Schwartz, A. Endocrine Manifestations of Down Syndrome. Curr. Opin. Endocrinol. Diabetes. Obes. 2018, 25, 61–66. [Google Scholar] [CrossRef]
- Asim, A.; Kumar, A.; Muthuswamy, S.; Jain, S.; Agarwal, S. Down Syndrome: An Insight of the Disease. J. Biomed. Sci. 2015, 22, 41. [Google Scholar] [CrossRef]
- Venegas-Zamora, L.; Bravo-Acuña, F.; Sigcho, F.; Gomez, W.; Bustamante-Salazar, J.; Pedrozo, Z.; Parra, V. New Molecular and Organelle Alterations Linked to Down Syndrome Heart Disease. Front. Genet. 2022, 12, 2734. [Google Scholar] [CrossRef]
- De Rubens Figueroa, J.; del Pozzo Magaña, B.; Pablos Hach, J.L.; Calderón Jiménez, C.; Castrejón Urbina, R. Heart malformations in children with Down syndrome. Rev. Esp. Cardiol. 2003, 56, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Mogra, R.; Zidere, V.; Allan, L.D. Prenatally Detectable Congenital Heart Defects in Fetuses with Down Syndrome. Ultrasound Obstet. Gynecol. 2011, 38, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Dott, B.; Alembik, Y.; Roth, M.-P. Associated Congenital Anomalies among Cases with Down Syndrome. Eur. J. Med. Genet. 2015, 58, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Metwalley, K.A.; Farghaly, H.S. Endocrinal Dysfunction in Children with Down Syndrome. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 15–21. [Google Scholar] [CrossRef]
- Lagan, N.; Huggard, D.; Mc Grane, F.; Leahy, T.R.; Franklin, O.; Roche, E.; Webb, D.; O’ Marcaigh, A.; Cox, D.; El-Khuffash, A.; et al. Multiorgan Involvement and Management in Children with Down Syndrome. Acta Paediatr. 2020, 109, 1096–1111. [Google Scholar] [CrossRef]
- Guaraldi, F.; Rossetto Giaccherino, R.; Lanfranco, F.; Motta, G.; Gori, D.; Arvat, E.; Ghigo, E.; Giordano, R. Endocrine Autoimmunity in Down’s Syndrome. Front. Horm. Res. 2017, 48, 133–146. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, G.; Buckley, F.; Skotko, B.G. Estimation of the Number of People with Down Syndrome in the United States. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017, 19, 439–447. [Google Scholar] [CrossRef]
- Calabrò, R.; Limongelli, G. Complete Atrioventricular Canal. Orphanet J. Rare Dis. 2006, 1, 8. [Google Scholar] [CrossRef]
- Nicolescu, A.; Cinteză, E. Esențialul În Cardiologia Pediatrica; Amaltea: Bucharest, Romania, 2022; ISBN 9789731622255. [Google Scholar]
- Maria, S.; Andreea-Luciana, A. Urgențe Neonatale; Tehnopress: Iasi, Romania, 2018; ISBN 978-606-687-363-5. [Google Scholar]
- Florea, I. Tratat de Pediatrie; ALL: Bucharest, Romania, 2019; ISBN 978-606-587-550-0. [Google Scholar]
- Eichenwald, E.C.; Hansen, R.A.; Martin, C.R.; Stark, A.R. Cloherty and Stark’s Manual of Neonatal Care; Wolters Kluwer: Mumbai, India, 2021; Volume 8, ISBN 9788194864554. [Google Scholar]
- Colvin, K.L.; Yeager, M.E. What People with Down Syndrome Can Teach Us about Cardiopulmonary Disease. Eur. Respir. Rev. 2017, 26, 160098. [Google Scholar] [CrossRef]
- Santoro, J.D.; Pagarkar, D.; Chu, D.T.; Rosso, M.; Paulsen, K.C.; Levitt, P.; Rafii, M.S. Neurologic Complications of Down Syndrome: A Systematic Review. J. Neurol. 2021, 268, 4495–4509. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, D.; Coelho, A.I.; Rubio-Gozalbo, M.E.; Berry, G.T. Hereditary Galactosemia. Metabolism 2018, 83, 188–196. [Google Scholar] [CrossRef]
- Rubio-Gozalbo, M.E.; Haskovic, M.; Bosch, A.M.; Burnyte, B.; Coelho, A.I.; Cassiman, D.; Couce, M.L.; Dawson, C.; Demirbas, D.; Derks, T.; et al. The Natural History of Classic Galactosemia: Lessons from the GalNet Registry. Orphanet J. Rare Dis. 2019, 14, 86. [Google Scholar] [CrossRef]
- Succoio, M.; Sacchettini, R.; Rossi, A.; Parenti, G.; Ruoppolo, M. Galactosemia: Biochemistry, Molecular Genetics, Newborn Screening, and Treatment. Biomolecules 2022, 12, 968. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.I.; Rubio-Gozalbo, M.E.; Vicente, J.B.; Rivera, I. Sweet and Sour: An Update on Classic Galactosemia. J. Inherit. Metab. Dis. 2017, 40, 325–342. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Tran, C.; Demirbas, D.; Gubbels, C.S.; Hsiao, M.; Daesety, V.; Berry, G.T. Transient Developmental Delays in Infants with Duarte-2 Variant Galactosemia. Mol. Genet. Metab. 2021, 134, 132–138. [Google Scholar] [CrossRef]
- Carlock, G.; Fischer, S.T.; Lynch, M.E.; Potter, N.L.; Coles, C.D.; Epstein, M.P.; Mulle, J.G.; Kable, J.A.; Barrett, C.E.; Edwards, S.M.; et al. Developmental Outcomes in Duarte Galactosemia. Pediatrics 2019, 143, e20182516. [Google Scholar] [CrossRef]
- Vlachopapadopoulou, E.-A.; Bonataki, M. Diagnosis of Hypoaldosteronism in Infancy. In Renin-Angiotensin Aldosterone System; McFarlane, S.I., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Rivelli, A.; Fitzpatrick, V.; Wales, D.; Chicoine, L.; Jia, G.; Rzhetsky, A.; Chicoine, B. Prevalence of Endocrine Disorders Among 6078 Individuals With Down Syndrome in the United States. J. Patient-Cent. Res. Rev. 2022, 9, 70–74. [Google Scholar] [CrossRef]
- Graber, E.; Chacko, E.; Regelmann, M.O.; Costin, G.; Rapaport, R. Down Syndrome and Thyroid Function. Endocrinol. Metab. Clin. N. Am. 2012, 41, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Fort, P.; Lifshitz, F.; Bellisario, R.; Davis, J.; Lanes, R.; Pugliese, M.; Richman, R.; Post, E.M.; David, R. Abnormalities of Thyroid Function in Infants with Down Syndrome. J. Pediatr. 1984, 104, 545–549. [Google Scholar] [CrossRef]
- Bull, M.J. Health Supervision for Children with Down Syndrome. Pediatrics 2011, 128, 393–406. [Google Scholar] [CrossRef]
- Karlsson, B.; Gustafsson, J.; Hedov, G.; Ivarsson, S.A.; Annerén, G. Thyroid Dysfunction in Down’s Syndrome: Relation to Age and Thyroid Autoimmunity. Arch. Dis. Child. 1998, 79, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Van Cleve, S.N.; Cohen, W.I. Part I: Clinical Practice Guidelines for Children with Down Syndrome from Birth to 12 Years. J. Pediatr. Health Care Off. Publ. Natl. Assoc. Pediatr. Nurse Assoc. Pract. 2006, 20, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gomella, T.L.; Cunningham, M.D.; Eyal, F.G.; Tuttle, D. Neonatology: Management, Procedures, on-Call Problems, Diseases, and Drugs, 7th ed.; Fried, A.K., Lebowitz, H., Eds.; McGraw-Hill: New York, NY, USA, 2013; ISBN 978-1-259-64481-8. [Google Scholar]
- Maria, S. Aspecte Practice in Nutritia Neonatala, 1st ed.; Editura Universitară: Carol Davila, Romania, 2013. [Google Scholar]
- Santoro, S.L.; Steffensen, E.H. Congenital Heart Disease in Down Syndrome—A Review of Temporal Changes. J. Congenit. Cardiol. 2021, 5, 1. [Google Scholar] [CrossRef]
- Ujuanbi Amenawon, S.; Onyeka Adaeze, C. Prevalence and Pattern of Congenital Heart Disease among Children with Down Syndrome Seen in a Federal Medical Centre in the Niger Delta Region, Nigeria. J. Cardiol. Cardiovasc. Med. 2022, 7, 030–035. [Google Scholar] [CrossRef]
- Ko, J.M. Genetic Syndromes Associated with Congenital Heart Disease. Korean Circ. J. 2015, 45, 357–361. [Google Scholar] [CrossRef]
- Benhaourech, S.; Drighil, A.; Hammiri, A. El Congenital Heart Disease and Down Syndrome: Various Aspects of a Confirmed Association. Cardiovasc. J. Afr. 2016, 27, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Amr, N.H. Thyroid Disorders in Subjects with Down Syndrome: An Update. Acta Biomed. 2018, 89, 132–139. [Google Scholar] [CrossRef]
- Caracausi, M.; Ghini, V.; Locatelli, C.; Mericio, M.; Piovesan, A.; Antonaros, F.; Pelleri, M.C.; Vitale, L.; Vacca, R.A.; Bedetti, F.; et al. Plasma and Urinary Metabolomic Profiles of Down Syndrome Correlate with Alteration of Mitochondrial Metabolism. Sci. Rep. 2018, 8, 2977. [Google Scholar] [CrossRef] [PubMed]
- Cooke, E.; Coles, L.; Staton, S.; Thorpe, K.; Chawla, J. Communicating the Complex Lives of Families That Include a Child with Down Syndrome. Health Sociol. Rev. J. Health Sect. Aust. Sociol. Assoc. 2023, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosca, I.; Turenschi, A.; Nicolescu, A.; Constantin, A.T.; Canciu, A.M.; Dica, A.D.; Bratila, E.; Coroleuca, C.A.; Nastase, L. Endocrine Disorders in a Newborn with Heterozygous Galactosemia, Down Syndrome and Complex Cardiac Malformation: Case Report. Medicina 2023, 59, 856. https://doi.org/10.3390/medicina59050856
Rosca I, Turenschi A, Nicolescu A, Constantin AT, Canciu AM, Dica AD, Bratila E, Coroleuca CA, Nastase L. Endocrine Disorders in a Newborn with Heterozygous Galactosemia, Down Syndrome and Complex Cardiac Malformation: Case Report. Medicina. 2023; 59(5):856. https://doi.org/10.3390/medicina59050856
Chicago/Turabian StyleRosca, Ioana, Alina Turenschi, Alin Nicolescu, Andreea Teodora Constantin, Adina Maria Canciu, Alice Denisa Dica, Elvira Bratila, Ciprian Andrei Coroleuca, and Leonard Nastase. 2023. "Endocrine Disorders in a Newborn with Heterozygous Galactosemia, Down Syndrome and Complex Cardiac Malformation: Case Report" Medicina 59, no. 5: 856. https://doi.org/10.3390/medicina59050856
APA StyleRosca, I., Turenschi, A., Nicolescu, A., Constantin, A. T., Canciu, A. M., Dica, A. D., Bratila, E., Coroleuca, C. A., & Nastase, L. (2023). Endocrine Disorders in a Newborn with Heterozygous Galactosemia, Down Syndrome and Complex Cardiac Malformation: Case Report. Medicina, 59(5), 856. https://doi.org/10.3390/medicina59050856





