Multimodal Analgesia with Local Wound Infiltration and Intravenous Parecoxib for Thyroidectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Anesthesia Induction and Maintenance
2.3. Multimodal Analgesia and Study Protocol
2.4. Outcome Measurements
2.5. Statistic Analysis
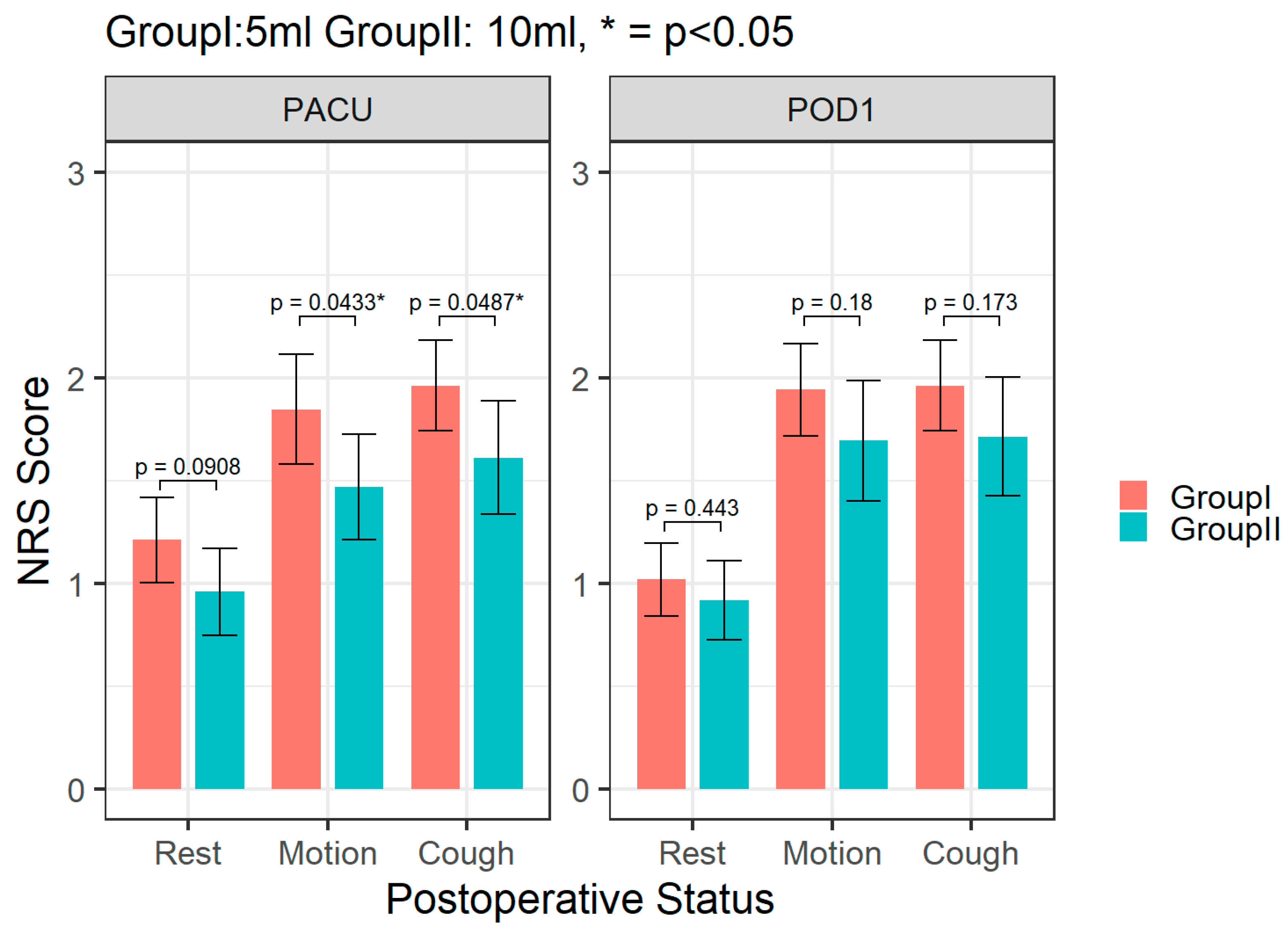
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coyle, M.J.; Main, B.; Hughes, C.; Craven, R.; Alexander, R.; Porter, G.; Thomas, S. Enhanced recovery after surgery (ERAS) for head and neck oncology patients. Clin. Otolaryngol. 2016, 41, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Chorath, K.; Luu, N.; Go, B.C.; Moreira, A.; Rajasekaran, K. ERAS Protocols for Thyroid and Parathyroid Surgery: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2022, 166, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Perttunen, K.; Tasmuth, T.; Kalso, E. Chronic pain after thoracic surgery: A follow-up study. Acta Anaesthesiol. Scand. 1999, 43, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Bayman, E.O.; Parekh, K.R.; Keech, J.; Selte, A.; Brennan, T.J. A Prospective Study of Chronic Pain after Thoracic Surgery. Anesthesiology 2017, 126, 938–951. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.K.; Stathakios, J.; Quan, D.; Pinto, J.; Lin, H.; Pashkova, A.A.; Svider, P.F. Perioperative Analgesia for Patients Undergoing Thyroidectomy and Parathyroidectomy: An Evidence-Based Review. Ann. Otol. Rhinol. Laryngol. 2020, 129, 949–963. [Google Scholar] [CrossRef]
- Dieudonne, N.; Gomola, A.; Bonnichon, P.; Ozier, Y.M. Prevention of postoperative pain after thyroid surgery: A double-blind randomized study of bilateral superficial cervical plexus blocks. Anesth. Analg. 2001, 92, 1538–1542. [Google Scholar] [CrossRef]
- Hoh, S.Y.; Doon, Y.K.; Chong, S.S.; Ng, K.L. Randomized controlled trial comparing bilateral superficial cervical plexus block and local wound infiltration for pain control in thyroid surgery. Asian J. Surg. 2019, 42, 1001–1008. [Google Scholar] [CrossRef]
- Simpson, J.C.; Bao, X.; Agarwala, A. Pain Management in Enhanced Recovery after Surgery (ERAS) Protocols. Clin. Colon Rectal Surg. 2019, 32, 121–128. [Google Scholar] [CrossRef]
- Ling, X.M.; Fang, F.; Zhang, X.G.; Ding, M.; Liu, Q.A.; Cang, J. Effect of parecoxib combined with thoracic epidural analgesia on pain after thoracotomy. J. Thorac. Dis. 2016, 8, 880–887. [Google Scholar] [CrossRef]
- Mulita, F.; Anjum, F. Thyroid Adenoma. In StatPearls [Internet]. Treasure Island (FL); StatPearls Publishing LLC: Tampa, FL, USA, 2022. [Google Scholar]
- Lu, I.C.; Hsu, C.D.; Chang, P.Y.; Wu, S.H.; Huang, T.Y.; Lin, Y.C.; Ko, H.Y.; Dionigi, G.; Chai, Y.J.; Chiang, F.Y.; et al. A Surgeon-Centered Neuromuscular Block Protocol Improving Intraoperative Neuromonitoring Outcome of Thyroid Surgery. Front. Endocrinol. 2022, 13, 817476. [Google Scholar] [CrossRef]
- Wu, C.W.; Dionigi, G.; Barczynski, M.; Chiang, F.Y.; Dralle, H.; Schneider, R.; Al-Quaryshi, Z.; Angelos, P.; Brauckhoff, K.; Brooks, J.A.; et al. International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope 2018, 128 (Suppl. S3), S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.W.; Dralle, H.; International Intraoperative Monitoring Study Group; Abdullah, H.; Barczynski, M.; Bellantone, R.; Brauckhoff, M.; Carnaille, B.; Cherenko, S.; Chiang, F.Y.; et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: International standards guideline statement. Laryngoscope 2011, 121 (Suppl. S1), S1–S16. [Google Scholar] [CrossRef] [PubMed]
- Torp, K.D.; Metheny, E.; Simon, L.V. Lidocaine Toxicity. In StatPearls [Internet]. Treasure Island (FL); StatPearls Publishing LLC: Tampa, FL, USA, 2022. [Google Scholar]
- Chiang, F.Y.; Wu, C.W.; Chang, P.Y.; Wu, S.H.; Chen, H.Y.; Lin, Y.C.; Huang, T.Y.; Zesendavaa, E.; Lu, I.C. Trans-thyroid cartilage recording for neural monitoring of the recurrent laryngeal nerve in thyroid surgery. Laryngoscope 2020, 130, E280–E283. [Google Scholar] [CrossRef] [PubMed]
- Lu, I.C.; Lin, I.H.; Wu, C.W.; Chen, H.Y.; Lin, Y.C.; Chiang, F.Y.; Chang, P.Y. Preoperative, intraoperative and postoperative anesthetic prospective for thyroid surgery: What’s new. Gland Surg. 2017, 6, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Yip, L.; Carty, S.E.; Holder-Murray, J.M.; Recker, A.; Nicholson, K.J.; Boisen, M.L.; Esper, S.A.; McCoy, K.L. A specific enhanced recovery protocol decreases opioid use after thyroid and parathyroid surgery. Surgery 2021, 169, 197–201. [Google Scholar] [CrossRef]
- Warschkow, R.; Tarantino, I.; Jensen, K.; Beutner, U.; Clerici, T.; Schmied, B.M.; Steffen, T. Bilateral superficial cervical plexus block in combination with general anesthesia has a low efficacy in thyroid surgery: A meta-analysis of randomized controlled trials. Thyroid 2012, 22, 44–52. [Google Scholar] [CrossRef]
- Miu, M.; Royer, C.; Gaillat, C.; Schaup, B.; Menegaux, F.; Langeron, O.; Riou, B.; Aubrun, F. Lack of Analgesic Effect Induced by Ropivacaine Wound Infiltration in Thyroid Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Anesth. Analg. 2016, 122, 559–564. [Google Scholar] [CrossRef]
- Emery, G.; Handley, G.; Davies, M.J.; Mooney, P.H. Incidence of phrenic nerve block and hypercapnia in patients undergoing carotid endarterectomy under cervical plexus block. Anaesth. Intensive Care 1998, 26, 377–381. [Google Scholar] [CrossRef]
- Opperer, M.; Kaufmann, R.; Meissnitzer, M.; Enzmann, F.K.; Dinges, C.; Hitzl, W.; Nawratil, J.; Kokofer, A. Depth of cervical plexus block and phrenic nerve blockade: A randomized trial. Reg. Anesth. Pain Med. 2022, 47, 205–211. [Google Scholar] [CrossRef]
- Cheer, S.M.; Goa, K.L. Parecoxib (parecoxib sodium). Drugs 2001, 61, 1133–1141, discussion 1142–1133. [Google Scholar] [CrossRef]
- Marret, E.; Kurdi, O.; Zufferey, P.; Bonnet, F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: Meta-analysis of randomized controlled trials. Anesthesiology 2005, 102, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.F.; Chang, P.Y.; Chen, Y.C.; Tseng, K.Y.; Hsu, H.T.; Cheng, K.I.; Lu, I.C. Single bolus parecoxib attenuates sore throat after laryngeal microsurgery: A randomized double-blind control study. Kaohsiung J. Med. Sci. 2014, 30, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.Y.; Wang, L.C.; Qian, W.W.; Lin, J.; Jin, J.; Peng, H.M.; Weng, X.S. Role of Parecoxib Sodium in the Multimodal Analgesia after Total Knee Arthroplasty: A Randomized Double-blinded Controlled Trial. Orthop. Surg. 2018, 10, 321–327. [Google Scholar] [CrossRef]
- Mulita, F.; Verras, G.I.; Iliopoulos, F.; Kaplanis, C.; Liolis, E.; Tchabashvili, L.; Tsilivigkos, C.; Perdikaris, I.; Sgourou, A.; Papachatzopoulou, A.; et al. Analgesic effect of paracetamol monotherapy vs. the combination of paracetamol/parecoxib vs. the combination of pethidine/paracetamol in patients undergoing thyroidectomy. Prz. Menopauzalny 2021, 20, 226–230. [Google Scholar] [CrossRef] [PubMed]


| Group I (n = 52) | Group II (n = 49) | p | |
|---|---|---|---|
| Female/Male (n) | 38/14 | 37/12 | 0.959 |
| Age (y/o) | 48.9 ± 12.8 | 53.0 ± 12.8 | 0.109 |
| ASA status 1 (n) | 0.195 | ||
| I | 3 (52.2%) | 1 (2.0%) | |
| II | 45 (43.5%) | 39 (79.6%) | |
| III | 4 (43.5%) | 9 (18.4%) | |
| Operation (n) | 0.169 | ||
| Total thyroidectomy | 27 (51.9%) | 33 (67.3%) | |
| Total lobectomy | 25 (48.1%) | 16 (32.7%) | |
| Operation time (minutes) | 72.9 ± 16.3 | 75.2 ± 17.2 | 0.508 |
| Nerve at risk (n) | 79 | 82 | |
| Temporary vocal palsy | 1 (1.9%) | 0 (0%) | 0.306 |
| Permanent vocal palsy | 0 (0%) | 0 (0%) | 1 |
| Group I (n = 52) | Group II (n = 49) | p | |
|---|---|---|---|
| Rescue analgesics at PACU 1 | 2 (3.8 %) | 0 (0 %) | 0.166 |
| Pain intensity at OPD 2 | 0.026 | ||
| None (NRS 3 = 0) | 27 (51.9%) | 38 (77.6%) | |
| Mild (NRS = 1~3) | 23 (44.2%) | 10 (20.4%) | |
| Moderate (NRS > 3) | 2 (3.9 %) | 1 (2.0 %) | |
| Nausea/vomiting | |||
| at PACU (n) | 13 (25%) | 12 (24.5%) | 0.952 |
| at POD 1 4 (n) | 1 (1.9%) | 1 (2.0%) | 0.966 |
| Dizziness | |||
| at PACU (n) | 14 (26.9%) | 14 (28.6%) | 0.853 |
| at POD 1 (n) | 3 (5.8%) | 2 (4.1%) | 0.94 |
| Pruritus (n) | 0 (0%) | 0 (0%) | 1 |
| Sore throat (n) | 9 (17.3%) | 12 (24.5%) | 0.374 |
| Airway events 5 (n) | 0 (0%) | 0 (0%) | 1 |
| Pulmonary complications (n) | 0 (0%) | 0 (0%) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gau, T.-P.; Wu, S.-H.; Huang, J.-M.; Lu, W.-L.; Huang, T.-Y.; Lu, I.-C.; Wu, C.-W. Multimodal Analgesia with Local Wound Infiltration and Intravenous Parecoxib for Thyroidectomy. Medicina 2023, 59, 855. https://doi.org/10.3390/medicina59050855
Gau T-P, Wu S-H, Huang J-M, Lu W-L, Huang T-Y, Lu I-C, Wu C-W. Multimodal Analgesia with Local Wound Infiltration and Intravenous Parecoxib for Thyroidectomy. Medicina. 2023; 59(5):855. https://doi.org/10.3390/medicina59050855
Chicago/Turabian StyleGau, Tz-Ping, Sheng-Hua Wu, Jui-Mei Huang, Wen-Ling Lu, Tzu-Yen Huang, I-Cheng Lu, and Che-Wei Wu. 2023. "Multimodal Analgesia with Local Wound Infiltration and Intravenous Parecoxib for Thyroidectomy" Medicina 59, no. 5: 855. https://doi.org/10.3390/medicina59050855
APA StyleGau, T.-P., Wu, S.-H., Huang, J.-M., Lu, W.-L., Huang, T.-Y., Lu, I.-C., & Wu, C.-W. (2023). Multimodal Analgesia with Local Wound Infiltration and Intravenous Parecoxib for Thyroidectomy. Medicina, 59(5), 855. https://doi.org/10.3390/medicina59050855






