Diagnostic and Prognostic Value of Serum Omentin-1 in Sepsis: A Prospective Study in Critically Ill Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients and Controls
3.2. Serum Omentin-1 in Patients and Controls
3.3. Serum Omentin-1 and Sepsis Severity
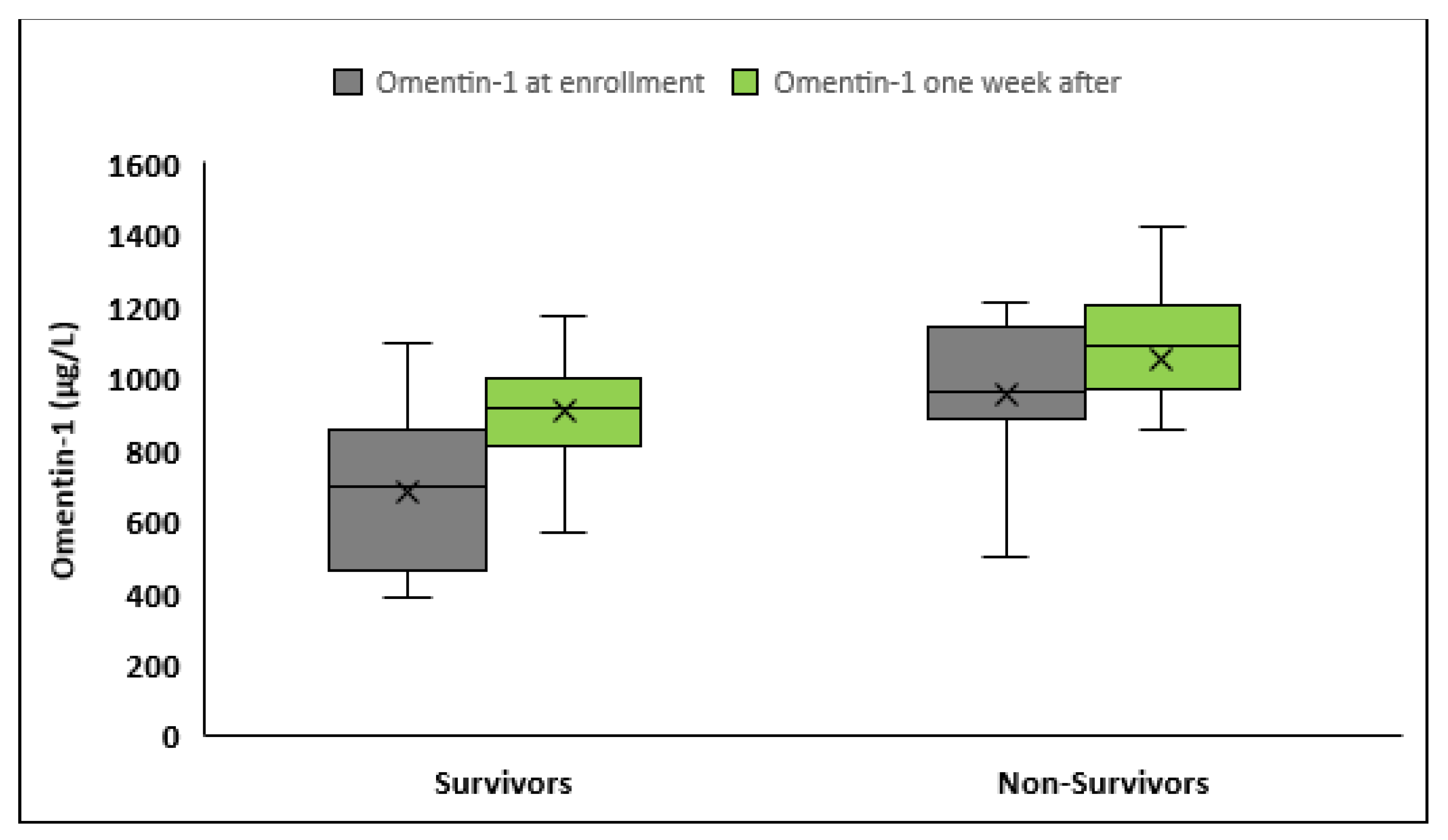
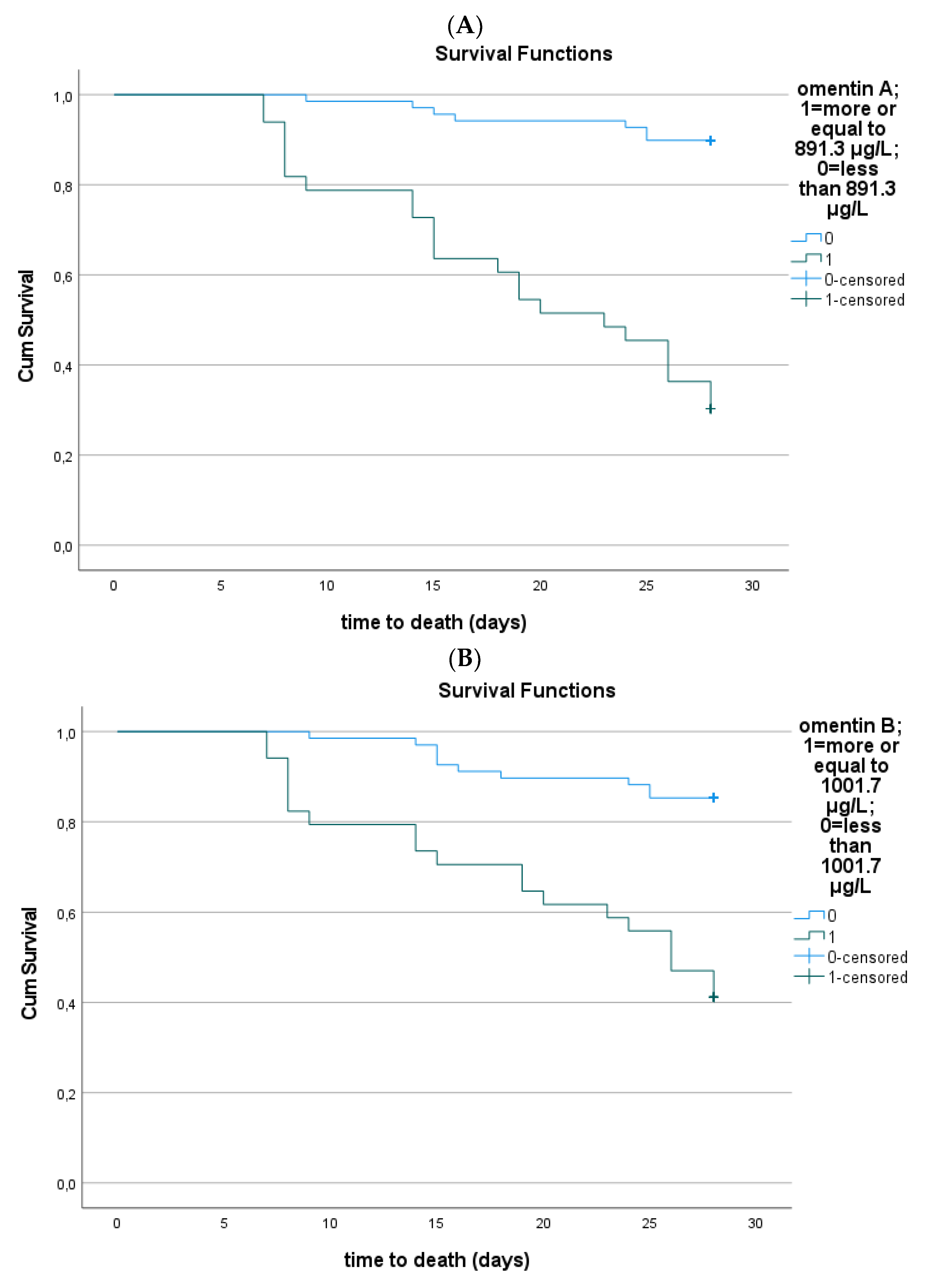
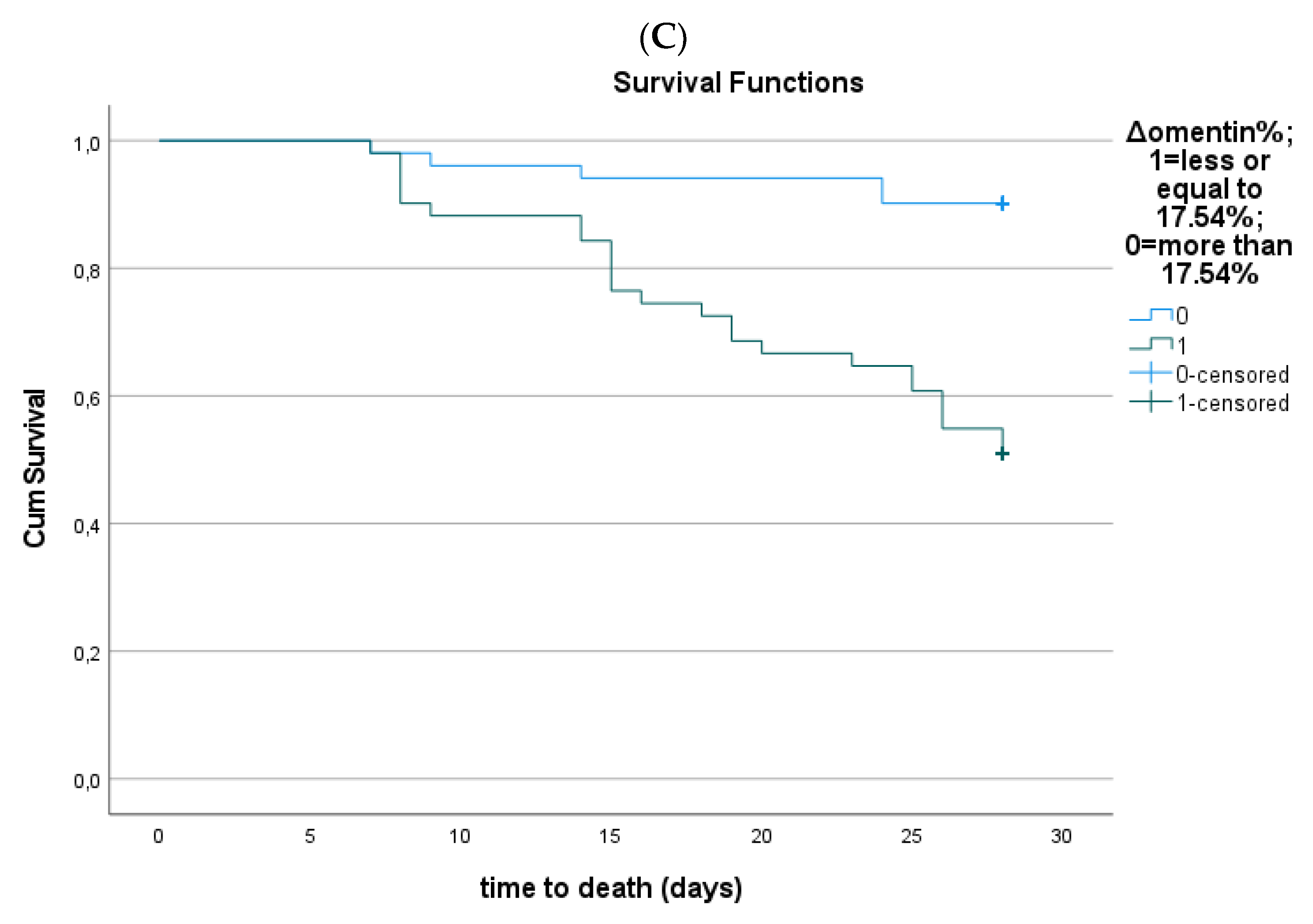
3.4. Serum Omentin-1 and Sepsis Outcome
3.5. Correlations of Serum Omentin-1 and Other Biomarkers
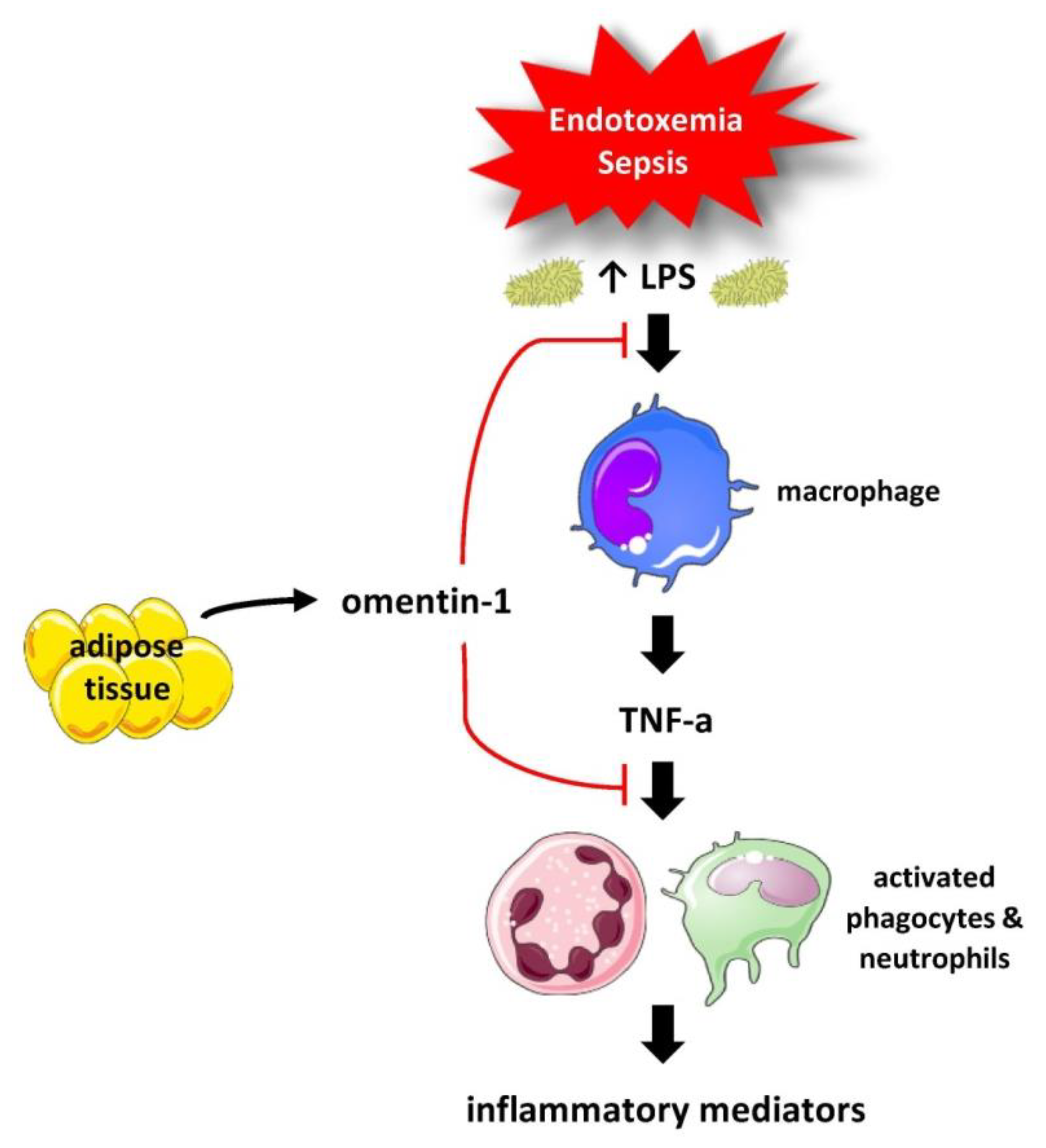
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schäffler, A.; Neumeier, M.; Herfarth, H.; Fürst, A.; Schölmerich, J.; Büchler, C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim. Biophys. Acta 2005, 1732, 96–102. [Google Scholar] [CrossRef]
- Nonnecke, E.B.; Castillo, P.A.; Dugan, A.E.; Almalki, F.; Underwood, M.A.; De La Motte, C.A.; Yuan, W.; Lu, W.; Shen, B.; Johansson, M.E.V.; et al. Human intelectin-1 (ITLN1) genetic variation and intestinal expression. Sci. Rep. 2021, 11, 12889. [Google Scholar] [CrossRef]
- Watanabe, T.; Watanabe-Kominato, K.; Takahashi, Y.; Kojima, M.; Watanabe, R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr. Physiol. 2017, 7, 765–781. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Randeva, H.S. Omentin: A novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc. Med. 2010, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- De Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol. Metab. 2014, 25, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.N.; Jung, C.H. The Role of Anti-Inflammatory Adipokines in Cardiometabolic Disorders: Moving beyond Adiponectin. Int. J. Mol. Sci. 2021, 22, 13529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Xiao, H.; Zhu, Y.; Liu, S.; Zhang, S.; Yang, Z.; Du, L.; Li, X.; Niu, X.; Wang, C.; et al. Omentin-1: A newly discovered warrior against metabolic related diseases. Expert. Opin. Ther. Targets 2022, 26, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Alam, R.; Ahsan, H.; Khan, S. Role of adipokines (omentin and visfatin) in coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X. Elucidating the pathophysiological significance of circulating omentin levels: Is higher better? Atherosclerosis 2016, 251, 522–524. [Google Scholar] [CrossRef]
- Miller, J.; Dreczkowski, G.; Ramage, M.I.; Wigmore, S.J.; Gallagher, I.J.; Skipworth, R.J.E. Adipose depot gene expression and intelectin-1 in the metabolic response to cancer and cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, N.; Avgerinos, K.I.; Mantzoros, C.S.; Dalamaga, M. Classic and Novel Adipocytokines at the Intersection of Obesity and Cancer: Diagnostic and Therapeutic Strategies. Curr. Obes. Rep. 2018, 7, 260–275. [Google Scholar] [CrossRef]
- Christodoulatos, G.S.; Antonakos, G.; Karampela, I.; Psallida, S.; Stratigou, T.; Vallianou, N.; Lekka, A.; Marinou, I.; Vogiatzakis, E.; Kokoris, S.; et al. Circulating Omentin-1 as a Biomarker at the Intersection of Postmenopausal Breast Cancer Occurrence and Cardiometabolic Risk: An Observational Cross-Sectional Study. Biomolecules 2021, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Christodoulatos, G.S.; Spyrou, N.; Kadillari, J.; Psallida, S.; Dalamaga, M. The Role of Adipokines in Breast Cancer: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 413–433. [Google Scholar] [CrossRef] [PubMed]
- Stratigou, T.; Muscogiuri, G.; Kotopouli, M.; Antonakos, G.; Christodoulatos, G.S.; Karampela, I.; Marinou, I.; Tsilingiris, D.; Vallianou, N.G.; Vogiatzakis, E.; et al. Lower circulating omentin-1 is independently linked to subclinical hypothyroidism reflecting cardiometabolic risk: An observational case-control and interventional, longitudinal study. Panminerva Med. 2022, 64, 452–464. [Google Scholar] [CrossRef]
- Katsi, V.; Vamvakou, G.; Lekakis, J.; Tousoulis, D.; Stefanadis, C.; Makris, T.; Kallikazaros, I. Omentin, fat and heart: Classical music with new instruments. Heart Lung Circ. 2014, 23, 802–806. [Google Scholar] [CrossRef]
- Radzik-Zając, J.; Wytrychowski, K.; Wiśniewski, A.; Barg, W. The role of the novel adipokines vaspin and omentin in chronic inflammatory diseases. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 48–52. [Google Scholar] [CrossRef]
- Yin, J.; Hou, P.; Wu, Z.; Nie, Y. Decreased levels of serum omentin-1 in patients with inflammatory bowel disease. Med. Sci. Monit. 2015, 21, 118–122. [Google Scholar] [CrossRef]
- Tan, Y.L.; Zheng, X.L.; Tang, C.K. The protective functions of omentin in cardiovascular diseases. Clin. Chim. Acta 2015, 448, 98–106. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Lin, F.; Han, K.; Wang, X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch. Biochem. Biophys. 2020, 679, 108187. [Google Scholar] [CrossRef]
- Tsuji, S.; Uehori, J.; Matsumoto, M.; Suzuki, Y.; Matsuhisa, A.; Toyoshima, K.; Seya, T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J. Biol. Chem. 2001, 276, 23456–23463. [Google Scholar] [CrossRef]
- Wesener, D.A.; Wangkanont, K.; McBride, R.; Song, X.; Kraft, M.B.; Hodges, H.L.; Zarling, L.C.; Splain, R.A.; Smith, D.F.; Cummings, R.D.; et al. Recognition of microbial glycans by human intelectin-1. Nat. Struct. Mol. Biol. 2015, 22, 603–610. [Google Scholar] [CrossRef]
- Karampela, I.; Christodoulatos, G.S.; Dalamaga, M. The Role of Adipose Tissue and Adipokines in Sepsis: Inflammatory and Metabolic Considerations, and the Obesity Paradox. Curr. Obes. Rep. 2019, 8, 434–457. [Google Scholar] [CrossRef]
- Sotiropoulos, G.P.; Dalamaga, M.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Kotopouli, M.; Karampela, I.; Christodoulatos, G.S.; Lekka, A.; Papavassiliou, A.G. Chemerin as a biomarker at the intersection of inflammation, chemotaxis, coagulation, fibrinolysis and metabolism in resectable non-small cell lung cancer. Lung Cancer 2018, 125, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Marouga, A.; Dalamaga, M.; Kastania, A.N.; Antonakos, G.; Thrasyvoulides, A.; Kontelia, G.; Dimas, C.; Vlahakos, D.V. Correlates of serum resistin in elderly, non-diabetic patients with chronic kidney disease. Clin. Lab. 2013, 59, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Hroussalas, G.; Kassi, E.; Dalamaga, M.; Delimaris, I.; Zachari, A.; Dionyssiou-Asteriou, A. Leptin, soluble leptin receptor, adiponectin and resistin in relation to OGTT in overweight/obese postmenopausal women. Maturitas 2008, 59, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Karmaniolas, K.; Chamberland, J.; Nikolaidou, A.; Lekka, A.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. Higher fetuin-A, lower adiponectin and free leptin levels mediate effects of excess body weight on insulin resistance and risk for myelodysplastic syndrome. Metabolism 2013, 62, 1830–1839. [Google Scholar] [CrossRef]
- Kassi, E.; Dalamaga, M.; Hroussalas, G.; Kazanis, K.; Merantzi, G.; Zachari, A.; Giamarellos-Bourboulis, E.J.; Dionyssiou-Asteriou, A. Adipocyte factors, high-sensitive C-reactive protein levels and lipoxidative stress products in overweight postmenopausal women with normal and impaired OGTT. Maturitas 2010, 67, 72–77. [Google Scholar] [CrossRef]
- Dalamaga, M.; Crotty, B.H.; Fargnoli, J.; Papadavid, E.; Lekka, A.; Triantafilli, M.; Karmaniolas, K.; Migdalis, I.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. B-cell chronic lymphocytic leukemia risk in association with serum leptin and adiponectin: A case-control study in Greece. Cancer Causes Control 2010, 21, 1451–1459. [Google Scholar] [CrossRef]
- Kassi, E.; Dalamaga, M.; Faviou, E.; Hroussalas, G.; Kazanis, K.; Nounopoulos, C.; Dionyssiou-Asteriou, A. Circulating oxidized LDL levels, current smoking and obesity in postmenopausal women. Atherosclerosis 2009, 205, 279–283. [Google Scholar] [CrossRef]
- Papadavid, E.; Gazi, S.; Dalamaga, M.; Stavrianeas, N.; Ntelis, V. Palmoplantar and scalp psoriasis occurring during anti-tumour necrosis factor-alpha therapy: A case series of four patients and guidelines for management. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Nikolaidou, A.; Karmaniolas, K.; Hsi, A.; Chamberland, J.; Dionyssiou-Asteriou, A.; Mantzoros, C.S. Circulating adiponectin and leptin in relation to myelodysplastic syndrome: A case-control study. Oncology 2007, 73, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Paroutoglou, K.; Papadavid, E.; Christodoulatos, G.S.; Dalamaga, M. Deciphering the Association Between Psoriasis and Obesity: Current Evidence and Treatment Considerations. Curr. Obes. Rep. 2020, 9, 165–178. [Google Scholar] [CrossRef]
- Dalamaga, M.; Christodoulatos, G.S.; Karampela, I.; Vallianou, N.; Apovian, C.M. Understanding the Co-Epidemic of Obesity and COVID-19: Current Evidence, Comparison with Previous Epidemics, Mechanisms, and Preventive and Therapeutic Perspectives. Curr. Obes. Rep. 2021, 10, 214–243. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Kandri, E.; Antonakos, G.; Vogiatzakis, E.; Christodoulatos, G.S.; Nikolaidou, A.; Dimopoulos, G.; Armaganidis, A.; Dalamaga, M. Kinetics of circulating fetuin-A may predict mortality independently from adiponectin, high molecular weight adiponectin and prognostic factors in critically ill patients with sepsis: A prospective study. J. Crit. Care 2017, 41, 78–85. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karampela, I. Fetuin-A to adiponectin ratio is a promising prognostic biomarker in septic critically ill patients. J. Crit. Care 2018, 44, 134–135. [Google Scholar] [CrossRef]
- Karampela, I.; Dalamaga, M. Comment to: Are fetuin-A levels beneficial for estimating timing of sepsis occurrence? Saudi Med. J. 2019, 40, 101–102. [Google Scholar] [CrossRef]
- Karampela, I.; Christodoulatos, G.S.; Kandri, E.; Antonakos, G.; Vogiatzakis, E.; Dimopoulos, G.; Armaganidis, A.; Dalamaga, M. Circulating eNampt and resistin as a proinflammatory duet predicting independently mortality in critically ill patients with sepsis: A prospective observational study. Cytokine 2019, 119, 62–70. [Google Scholar] [CrossRef]
- Karampela, I.; Dalamaga, M. Serum bilirubin to fetuin-A ratio as a prognostic biomarker in critically ill patients with sepsis. Metabol. Open 2021, 10, 100094. [Google Scholar] [CrossRef]
- Karampela, I.; Chrysanthopoulou, E.; Skyllas, G.; Christodoulatos, G.S.; Kandri, E.; Antonakos, G.; Stratigou, T.; Armaganidis, A.; Dalamaga, M. Circulating leptin, soluble leptin receptor and free leptin index in critically ill patients with sepsis: A prospective observational study. Minerva Anestesiol. 2021, 87, 880–890. [Google Scholar] [CrossRef]
- Karampela, I.; Christodoulatos, G.S.; Vallianou, N.; Tsilingiris, D.; Chrysanthopoulou, E.; Skyllas, G.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; et al. Circulating Chemerin and Its Kinetics May Be a Useful Diagnostic and Prognostic Biomarker in Critically Ill Patients with Sepsis: A Prospective Study. Biomolecules 2022, 12, 301. [Google Scholar] [CrossRef]
- Luedde, M.; Benz, F.; Niedeggen, J.; Vucur, M.; Hippe, H.J.; Spehlmann, M.E.; Schueller, F.; Loosen, S.; Frey, N.; Trautwein, C.; et al. Elevated Omentin Serum Levels Predict Long-Term Survival in Critically Ill Patients. Dis. Markers 2016, 2016, 3149243. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Loosen, S.H.; Koch, A.; Tacke, F.; Roderburg, C.; Luedde, T. The Role of Adipokines as Circulating Biomarkers in Critical Illness and Sepsis. Int. J. Mol. Sci. 2019, 20, 4820. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Carstensen, M.; Ouwens, D.M. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 39–50. [Google Scholar] [CrossRef]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur. J. Pharmacol. 2012, 686, 116–123. [Google Scholar] [CrossRef]
- Zhong, X.; Li, X.; Liu, F.; Tan, H.; Shang, D. Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NF-κB pathway. Biochem. Biophys. Res. Commun. 2012, 425, 401–406. [Google Scholar] [CrossRef]
- Zhou, Y.; Hao, C.; Li, C.; Huang, X.; Li, X.; Tang, Y.; Huang, Y.; Tang, S.; Liu, W.; Feng, D.; et al. Omentin-1 protects against bleomycin-induced acute lung injury. Mol. Immunol. 2018, 103, 96–105. [Google Scholar] [CrossRef]
- Qi, D.; Tang, X.; He, J.; Wang, D.; Zhao, Y.; Deng, W.; Deng, X.; Zhou, G.; Xia, J.; Zhong, X.; et al. Omentin protects against LPS-induced ARDS through suppressing pulmonary inflammation and promoting endothelial barrier via an Akt/eNOS-dependent mechanism. Cell. Death Dis. 2016, 7, e2360. [Google Scholar] [CrossRef]
- Vasamsetti, S.B.; Natarajan, N.; Sadaf, S.; Florentin, J.; Dutta, P. Regulation of cardiovascular health and disease by visceral adipose tissue-derived metabolic hormones. J. Physiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chondrogianni, M.; Lambadiari, V.; Katsanos, A.H.; Stefanou, M.I.; Palaiodimou, L.; Triantafyllou, A.S.; Karagiannis, G.; Konstantakos, V.; Ioakeimidis, M.; Triantafyllou, S.; et al. Omentin Is Independently Associated with Stroke Severity and Ipsilateral Carotid Artery Stenosis in Patients with Acute Cerebral Ischemia. J. Clin. Med. 2021, 10, 5797. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, K.B. Detector for organophosphorus compounds in liquid chromatography based on the cholinesterase inhibition reaction. J. Chromatogr. 1987, 389, 87–94. [Google Scholar] [CrossRef]
- Yan, X.; Wu, L.; Gao, M.; Yang, P.; Yang, J.; Deng, Y. Omentin inhibits the resistin-induced hypertrophy of H9c2 cardiomyoblasts by inhibiting the TLR4/MyD88/NF-κB signaling pathway. Exp. Ther. Med. 2022, 23, 292. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, X.; Zhang, C.; Zhou, Y.; Zhang, H. Omentin-1 attenuates inflammation and barrier damage in DSS-induced ulcerative colitis in mice by inhibiting endoplasmic reticulum stress. Gen. Physiol. Biophys. 2022, 41, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Cantarini, L.; Simonini, G.; Fioravanti, A.; Generoso, M.; Bacarelli, M.R.; Dini, E.; Galeazzi, M.; Cimaz, R. Circulating levels of the adipokines vaspin and omentin in patients with juvenile idiopathic arthritis, and relation to disease activity. Clin. Exp. Rheumatol. 2011, 29, 1044–1048. [Google Scholar]
- Senolt, L.; Polanská, M.; Filková, M.; Cerezo, L.A.; Pavelka, K.; Gay, S.; Haluzík, M.; Vencovsky, J. Vaspin and omentin: New adipokines differentially regulated at the site of inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1410–1411. [Google Scholar] [CrossRef]
- Yi, L.; Cheng, D.; Zhang, K.; Huo, X.; Mo, Y.; Shi, H.; Di, H.; Zou, Y.; Zhang, H.; Zhao, J.; et al. Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol. 2017, 10, 1491–1503. [Google Scholar] [CrossRef]
- Kerr, S.C.; Carrington, S.D.; Oscarson, S.; Gallagher, M.E.; Solon, M.; Yuan, S.; Ahn, J.N.; Dougherty, R.H.; Finkbeiner, W.E.; Peters, M.C.; et al. Intelectin-1 is a prominent protein constituent of pathologic mucus associated with eosinophilic airway inflammation in asthma. Am. J. Respir. Crit. Care Med. 2014, 189, 1005–1007. [Google Scholar] [CrossRef]
- Tsuji, S.; Yamashita, M.; Hoffman, D.R.; Nishiyama, A.; Shinohara, T.; Ohtsu, T.; Shibata, Y. Capture of heat-killed Mycobacterium bovis bacillus Calmette-Guérin by intelectin-1 deposited on cell surfaces. Glycobiology 2009, 19, 518–526. [Google Scholar] [CrossRef]
- Andresen, S.; Fantone, K.; Chapla, D.; Rada, B.; Moremen, K.W.; Pierce, M.; Szymanski, C.M. Human Intelectin-1 Promotes Cellular Attachment and Neutrophil Killing of Streptococcus pneumoniae in a Serotype-Dependent Manner. Infect. Immun. 2022, 90, e0068221. [Google Scholar] [CrossRef]
- Fang, R.; Uchiyama, R.; Sakai, S.; Hara, H.; Tsutsui, H.; Suda, T.; Mitsuyama, M.; Kawamura, I.; Tsuchiya, K. ASC and NLRP3 maintain innate immune homeostasis in the airway through an inflammasome-independent mechanism. Mucosal Immunol. 2019, 12, 1092–1103. [Google Scholar] [CrossRef]
- Kukla, M.; Menżyk, T.; Dembiński, M.; Winiarski, M.; Garlicki, A.; Bociąga-Jasik, M.; Skonieczna, M.; Hudy, D.; Maziarz, B.; Kusnierz-Cabala, B.; et al. Anti-inflammatory adipokines: Chemerin, vaspin, omentin concentrations and SARS-CoV-2 outcomes. Sci. Rep. 2021, 11, 21514. [Google Scholar] [CrossRef]
- Gültekin, Y.; Biri, İ.; Gojayev, A.; Yılmaz Işıkhan, S.; Akçin, O.P.; Kılıç, Y.A. Can omentin-1 be a prognostic marker in surgical intensive care patients? Turk. J. Med. Sci. 2021, 51, 2485–2493. [Google Scholar] [CrossRef]
- Sun, X.; Li, T.; Tian, Y.; Ren, S.; Li, L.; Li, P. Omentin as an Independent Predictor of Metabolic Syndrome and Obesity Among Adolescents in Northeast China. Diabetes Metab Syndr Obes. 2022, 15, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xue, J.; Hong, P. Relationships between serum omentin-1 concentration, body composition and physical activity levels in older women. Medicine (Baltim.) 2021, 10, e25020. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine 2013, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]


| Patients (n = 102) | Controls (n = 102) | p Value | |
|---|---|---|---|
| Age a, years | 64.7 ± 15.6 | 66.4 ± 10.3 | 0.35 |
| Gender, male, n (%) | 57 (55.9) | 57 (55.9) | 0.56 |
| BMI a, kg/m2 | 29.9 ± 8.5 | 28.1 ± 5.01 | 0.06 |
| Septic shock, n (%) | 42 (41.2) | - | |
| Death within 28 days, n (%) | 30 (29.4) | - | |
| Severity scores | |||
| APACHE II a | 23 ± 7.2 | - | |
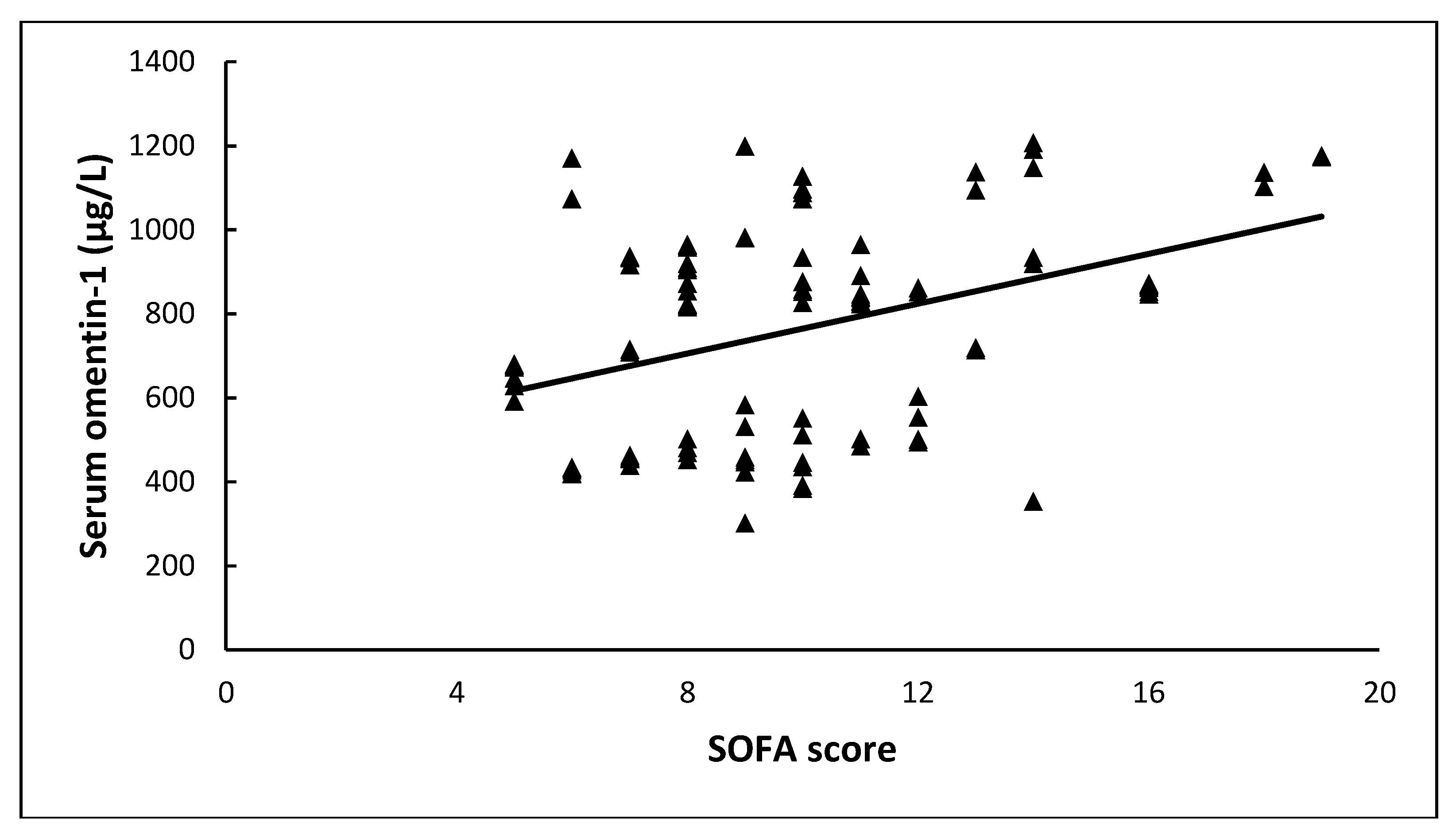
| SOFA a | 10 ± 3.3 | - | |
| Hematologic indices | |||
| Hemoglobin a, g/L | 93 ± 20 | 147.9 ± 16.3 | <0.001 |
| White blood cells a × 109/L | 14.1 ± 8.4 | 6.97 ± 1.8 | <0.001 |
| Platelets a × 109/L | 216.2 ± 118.8 | 243.8 ± 46.9 | 0.03 |
| Coagulation indices | |||
| Prothrombin time a, s | 14.3 ± 4.7 | 11.9 ± 0.8 | <0.001 |
| aPTT a, s | 38.9 ± 9.4 | 34.4 ± 7.3 | <0.001 |
| Fibrinogen a, μmol/L | 14.49 ± 5.26 | 9.06 ± 1.3 | <0.001 |
| Metabolic indices | |||
| Lactate b, mmol/L | 2.1 (1–9) | - | |
| Total Protein a, g/L | 50 ± 9 | 71 ± 4.2 | <0.001 |
| Albumin a, g/L | 24.6 ± 5.9 | 46.7 ± 5.6 | <0.001 |
| Creatinine a, μmol/L | 123.76 ± 70.72 | 74.26 ± 12.38 | 0.08 |
| Glucose a, mmol/L | 7.97 ± 2.9 | 5.32 ± 1.16 | <0.001 |
| Insulin b, pmol/L | 197.9 (88.2–402.8) | 73.13 (22.2–430.2) | <0.001 |
| HOMA-IR b | 8.9 (3.24–34.5) | 2.3 (0.65–23.5) | <0.001 |
| Inflammatory indices | |||
| CRP b, mg/L | 132 (7–431) | 3.4 (0.1–10.9) | <0.001 |
| Procalcitonin b, μg/L | 0.9 (0.1–100) | - | - |
| IL-1β b, ng/L | 5.9 (5.9–206) | - | - |
| IL-6 b ng/L | 27.4 (6–444) | - | - |
| IL-10 b, ng/L | 5 (5–300) | - | - |
| suPAR b, μg/L | 13 (2.1–16.8) | - | - |
| Omentin-1 a, μg/L | 763.3 ± 249.3 | 451.7 ± 122.3 | <0.001 |
| Upon Enrollment | One Week after Enrollment | |||||
|---|---|---|---|---|---|---|
| Sepsis (n = 60) | Septic Shock (n = 42) | p Value | Sepsis (n = 60) | Septic Shock (n = 42) | p Value | |
| CRP b, mg/L | 89 (7–218) | 174 (36–431) | <0.001 | 55 (8–282) | 101 (13–253) | 0.01 |
| Procalcitonin b, μg/L | 0.7 (0.09–47.7) | 4.8 (0.14–100) | 0.002 | 0.5 (0.06–15) | 1.4 (0.14–83) | 0.001 |
| IL-1β b, ng/L | 5.9 (5.9–207) | 8.8 (5.9–44.8) | 0.18 | 17 (5.9–499) | 8.8 (5.9–45) | 0.13 |
| IL-6 b, ng/L | 16.5 (6–385) | 74.4 (10–444) | 0.001 | 25 (4.6–419) | 20.5 (6–487) | 0.34 |
| IL-10 b, ng/L | 5 (5–300) | 6.9 (5–87) | 0.001 | 5 (5–300) | 5 (5–66) | 0.02 |
| suPAR b, μg/L | 10.5 (2.2–16.8) | 14.1 (4.4–16.8) | 0.04 | 11.3 (2.6–16.8) | 12.9 (5.2–16.8) | 0.68 |
| Omentin-1 a, μg/L | 683.1 ± 223.7 | 877.9 ± 241.2 | <0.001 | 901.7 ± 196.3 | 1020.4 ± 224.7 | 0.007 |
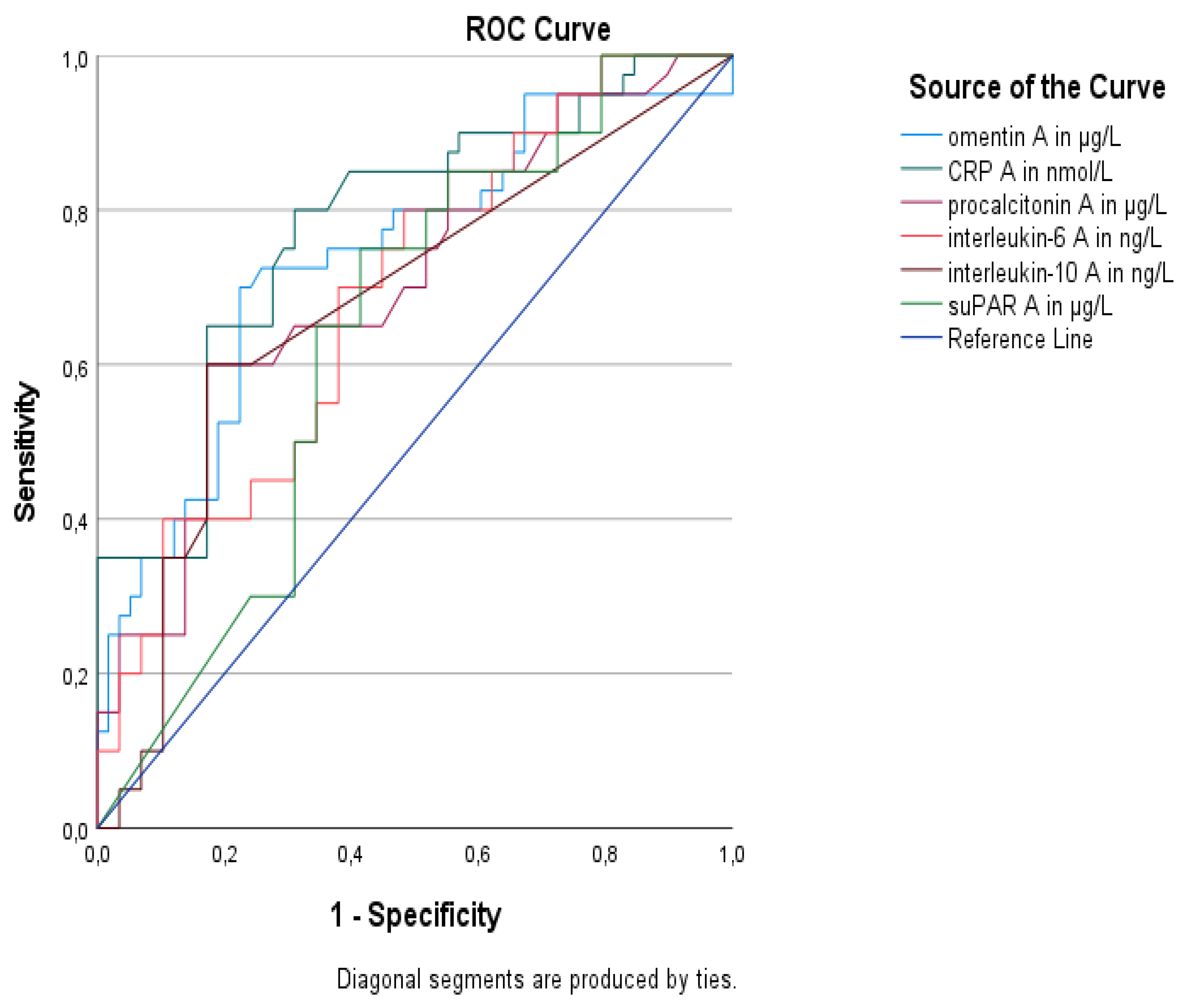
| Biomarkers | AUC (95% CI) | p Value | Sensitivity | Specificity | Youden’s Index | Cutoff Value | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|---|---|---|---|
| Omentin-1 | 0.74 (0.64–0.84) | <0.001 | 74% | 75% | 0.49 | 850.3 μg/L | 67.4% | 80.3% |
| CRP | 0.78 (0.68–0.87) | <0.001 | 80% | 69% | 0.49 | 132 mg/L | 64.4% | 83.1% |
| Procalcitonin | 0.71 (0.60–0.81) | 0.001 | 60% | 82.8% | 0.43 | 4.30 μg/L | 70.9% | 74.7% |
| IL-6 | 0.69 (0.58–0.79) | 0.001 | 70% | 62.1% | 0.32 | 24.50 ng/L | 56.4% | 74.7% |
| IL-10 | 0.68 (0.57–0.79) | 0.003 | 60% | 82.8% | 0.43 | 5.88 ng/L | 70.9% | 74.7% |
| suPAR | 0.64 (0.53–0.75) | 0.02 | 75% | 58.6% | 0.34 | 11.79 μg/L | 55.9% | 77% |
| b | SEb | Wald | df | p Value | HR | 95% for C.I. | |
|---|---|---|---|---|---|---|---|
| Independent predictors at enrollment | |||||||
| Omentin-1 | 0.81 | 0.32 | 6.59 | 1 | 0.01 * | 2.26 | 1.21–4.19 |
| CRP | 0.29 | 0.19 | 2.51 | 1 | 0.11 | 1.35 | 0.93–1.95 |
| IL-6 | −0.07 | 0.19 | 0.13 | 1 | 0.71 | 0.93 | 0.64–1.35 |
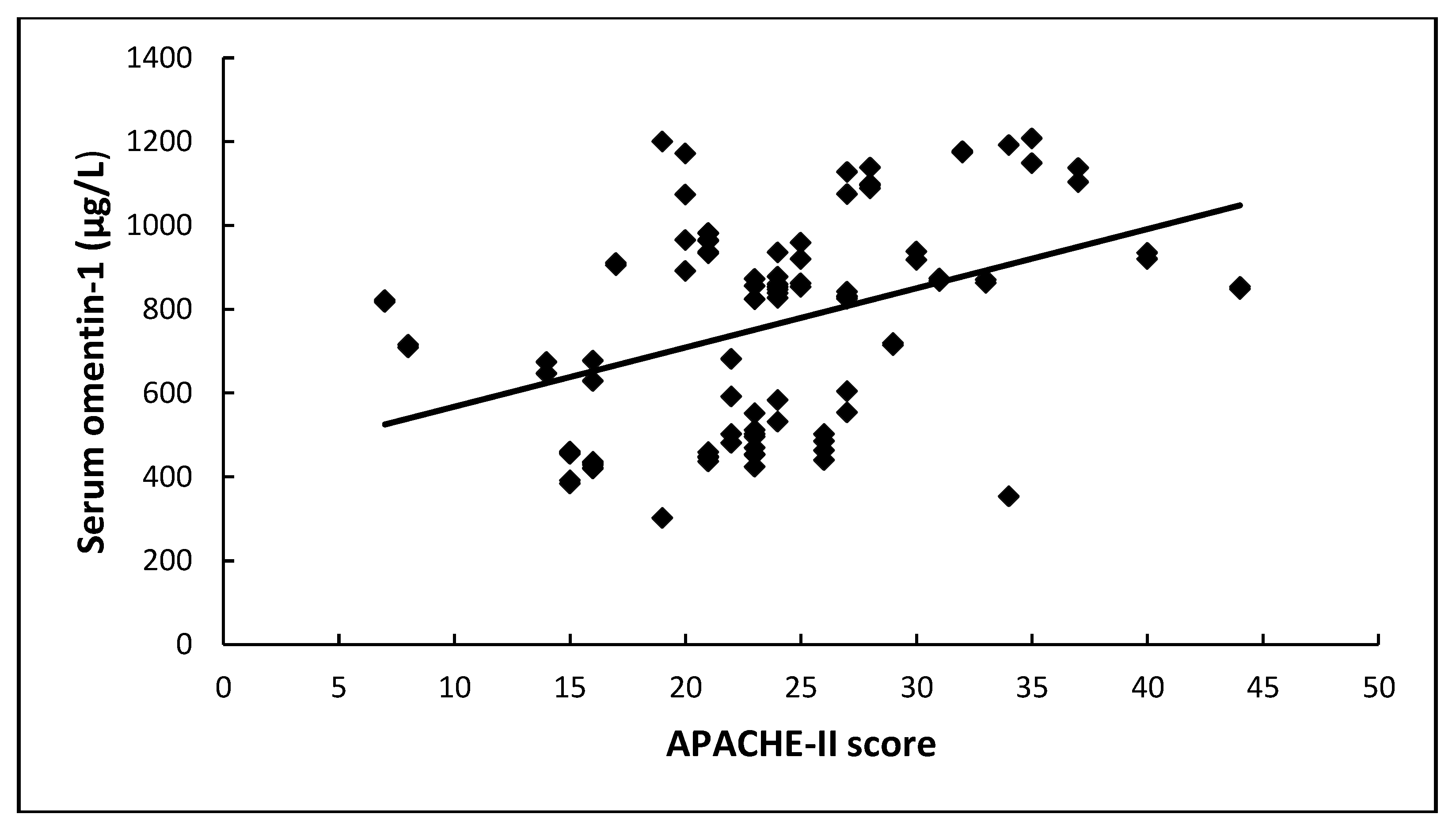
| APACHE II | 0.38 | 0.18 | 4.49 | 1 | 0.03 | 1.46 | 1.03–2.08 |
| Independent predictors 1 week after enrollment | |||||||
| Omentin-1 | 0.76 | 0.21 | 13.59 | 1 | <0.001 | 2.15 | 1.43–3.22 |
| CRP | −0.11 | 1.86 | 0.34 | 1 | 0.56 | 0.89 | 0.62–1.29 |
| IL-6 | 0.68 | 0.21 | 10.13 | 1 | 0.001 | 1.98 | 1.30–3.01 |
| APACHE II | 0.79 | 0.23 | 11.86 | 1 | <0.001 | 2.22 | 1.41–3.49 |
| At Enrollment | One Week after Enrollment | |||
|---|---|---|---|---|
| r | p | r | p | |
| Hematologic biomarkers | ||||
| Hemoglobin | −0.04 | 0.67 | −0.04 | 0.7 |
| White blood cells | 0.28 * | 0.004 | 0.23 | 0.02 |
| Neutrophils | 0.1 | 0.3 | 0.2 | 0.04 |
| Platelets | −0.03 | 0.76 | −0.1 | 0.29 |
| Coagulation biomarkers | ||||
| Prothrombin time | 0.4 | <0.001 | 0.31 | 0.002 |
| aPTT | 0.27 | 0.006 | 0.18 | 0.08 |
| Fibrinogen | 0.24 | 0.02 | 0.14 | 0.18 |
| Metabolic biomarkers | ||||
| Lactate | 0.19 | 0.05 | 0.16 | 0.11 |
| Total protein | −0.16 | 0.09 | −0.15 | 0.13 |
| Albumin | −0.13 | 0.2 | −0.14 | 0.16 |
| Creatinine | 0.24 | 0.01 | 0.16 | 0.11 |
| Glucose | 0.11 | 0.33 | - | - |
| Insulin | 0.28 | 0.15 | - | - |
| HOMA-IR | 0.3 | 0.002 | - | - |
| BMI | −0.07 | 0.48 | - | - |
| Inflammatory biomarkers | ||||
| CRP | 0.41 | <0.001 | 0.15 | 0.13 |
| Procalcitonin | 0.08 | 0.41 | −0.02 | 0.85 |
| IL-1β | 0.11 | 0.26 | −0.17 | 0.09 |
| IL-6 | 0.1 | 0.31 | −0.02 | 0.84 |
| IL-10 | 0.19 | 0.05 | −0.07 | 0.49 |
| suPAR | 0.09 | 0.34 | 0.05 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karampela, I.; Vallianou, N.G.; Tsilingiris, D.; Christodoulatos, G.S.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; Dalamaga, M. Diagnostic and Prognostic Value of Serum Omentin-1 in Sepsis: A Prospective Study in Critically Ill Patients. Medicina 2023, 59, 833. https://doi.org/10.3390/medicina59050833
Karampela I, Vallianou NG, Tsilingiris D, Christodoulatos GS, Antonakos G, Marinou I, Vogiatzakis E, Armaganidis A, Dalamaga M. Diagnostic and Prognostic Value of Serum Omentin-1 in Sepsis: A Prospective Study in Critically Ill Patients. Medicina. 2023; 59(5):833. https://doi.org/10.3390/medicina59050833
Chicago/Turabian StyleKarampela, Irene, Natalia G. Vallianou, Dimitrios Tsilingiris, Gerasimos Socrates Christodoulatos, Georgios Antonakos, Ioanna Marinou, Evaggelos Vogiatzakis, Apostolos Armaganidis, and Maria Dalamaga. 2023. "Diagnostic and Prognostic Value of Serum Omentin-1 in Sepsis: A Prospective Study in Critically Ill Patients" Medicina 59, no. 5: 833. https://doi.org/10.3390/medicina59050833
APA StyleKarampela, I., Vallianou, N. G., Tsilingiris, D., Christodoulatos, G. S., Antonakos, G., Marinou, I., Vogiatzakis, E., Armaganidis, A., & Dalamaga, M. (2023). Diagnostic and Prognostic Value of Serum Omentin-1 in Sepsis: A Prospective Study in Critically Ill Patients. Medicina, 59(5), 833. https://doi.org/10.3390/medicina59050833








