Prognostic Value for Mortality of Plasma Bioactive Adrenomedullin in Patients with Pulmonary Arterial Hypertension: A Sub Analysis of the Biomarker Study in the COHARD-PH Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects’ Enrollment
2.2. Biomarker Procedures
2.3. Mortality Outcomes
2.4. Statistical Analysis
3. Results
3.1. Bio-ADM
3.2. Bio-ADM and Mortality
4. Discussion
The Study’s Limitations
- (1)
- A rather small number of subjects with I/H PAH were enrolled, leading to the need to confirm these data in a new multicentric prospective study with more patients from these groups.
- (2)
- The biomarkers’ results were obtained analyzing blood samples that were stored and frozen for a long period, and we cannot exclude the possibility that this could have affected the results. Consequently, it will be necessary to confirm the results of biomarker data obtained from this study and analyze fresh plasma samples in the future.
- (3)
- The use of medication with PAH-specific drugs was quite limited in our COHARD-PH registry, since not all PAH-specific drugs are available in Indonesia.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef]
- Di Somma, S.; Magrini, L.; Travaglino, F.; Lalle, I.; Fiotti, N.; Cervellin, G.; Avanzi, G.C.; Lupia, E.; Maisel, A.; Hein, F.; et al. Opinion paper on innovative approach of biomarkers for infectious diseases and sepsis management in the emergency department. Clin. Chem. Lab. Med. 2013, 51, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Struck, J.; Maisel, A.S.; Magrini, L.; Bergmann, A.; Di Somma, S. Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Crit. Care. 2014, 18, R34. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Ter Maaten, J.M.; Voors, A.A. Bio-adrenomedullin as a potential quick, reliable, and objective marker of congestion in heart failure. Eur. J. Heart Fail. 2018, 20, 1363–1365. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Kremer, D.; Demissei, B.G.; Struck, J.; Bergmann, A.; Anker, S.D.; Ng, L.L.; Dickstein, K.; Metra, M.; Samani, N.J.; et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur. J. Heart Fail. 2019, 21, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Molvin, J.; Jujic, A.; Navarin, S.; Melander, O.; Zoccoli, G.; Hartmann, O.; Bergmann, A.; Struck, J.; Bachus, E.; Di Somma, S.; et al. Bioactive adrenomedullin, proenkephalin A and clinical outcomes in an acute heart failure setting. Open Heart 2019, 6, e001048. [Google Scholar] [CrossRef]
- Chang, K.Y.; Duval, S.; Badesch, D.B.; Bull, T.M.; Chakinala, M.M.; De Marco, T.; Frantz, R.P.; Hemnes, A.; Mathai, S.C.; Rosenzweig, E.B.; et al. PHAR Investigators. Mortality in pulmonary arterial hypertension in the modern era: Early insights from the Pulmonary Hypertension Association Registry. J. Am. Heart Assoc. 2022, 11, e024969. [Google Scholar] [CrossRef]
- Kolditz, M.; Seyfarth, H.J.; Wilkens, H.; Ewert, R.; Bollmann, T.; Dinter, C.; Hertel, S.; Klose, H.; Opitz, C.; Grünig, E.; et al. MR-proADM predicts exercise capacity and survival superior to other biomarkers in PH. Lung 2015, 193, 901–910. [Google Scholar] [CrossRef]
- Bouzina, H.; Rådegran, G. Plasma adrenomedullin peptides and precursor levels in pulmonary arterial hypertension disease severity and risk stratification. Pulm. Circ. 2020, 10, 2045894020931317. [Google Scholar] [CrossRef]
- Nagaya, N.; Kyotani, S.; Uematsu, M.; Ueno, K.; Oya, H.; Nakanishi, N.; Shirai, M.; Mori, H.; Miyatake, K.; Kangawa, K. Effects of adrenomedullin inhalation on hemodynamics and exercise capacity in patients with idiopathic pulmonary arterial hypertension. Circulation 2004, 109, 351–356. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P. ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Dinarti, L.K.; Anggrahini, D.W.; Lilyasari, O.; Siswanto, B.B.; Hartopo, A.B. Pulmonary arterial hypertension in Indonesia: Current status and local application of international guidelines. Glob. Heart 2021, 16, 23. [Google Scholar] [CrossRef]
- Dinarti, L.K.; Hartopo, A.B.; Kusuma, A.D.; Satwiko, M.G.; Hadwiono, M.R.; Pradana, A.D.; Anggrahini, D.W. The COngenital HeARt Disease in adult and Pulmonary Hypertension (COHARD-PH) registry: A descriptive study from single-center hospital registry of adult congenital heart disease and pulmonary hypertension in Indonesia. BMC Cardiovasc. Disord. 2020, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Kitamura, K. Translational studies of adrenomedullin and related peptides regarding cardiovascular diseases. Hypertens. Res. 2022, 45, 389–400. [Google Scholar] [CrossRef]
- Kita, T.; Suzuki, Y.; Kitamura, K. Hemodynamic and hormonal effects of exogenous adrenomedullin administration in humans and relationship to insulin resistance. Hypertens. Res. 2010, 33, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Nishikimi, T.; Uematsu, M.; Satoh, T.; Oya, H.; Kyotani, S.; Sakamaki, F.; Ueno, K.; Nakanishi, N.; Miyatake, K.; et al. Haemodynamic and hormonal effects of adrenomedullin in patients with pulmonary hypertension. Heart 2000, 84, 653–658. [Google Scholar] [CrossRef]
- Nashat, H.; Montanaro, C.; Li, W.; Kempny, A.; Wort, S.J.; Dimopoulos, K.; Gatzoulis, M.A.; Babu-Narayan, S.V. Atrial septal defects and pulmonary arterial hypertension. J. Thorac. Dis. 2018, 10 (Suppl. S24), S2953–S2965. [Google Scholar] [CrossRef]
- Hartopo, A.B.; Anggrahini, D.W.; Satwiko, M.G.; Damarkusuma, A.; Pritazahra, A.; Hadwiono, M.R.; Dewanto, V.C.; Di Somma, S.; Emoto, N.; Dinarti, L.K. Usefulness of combining NT-proBNP level and right atrial diameter for simple and early noninvasive detection of pulmonary hypertension among adult patients with atrial septal defect. Acta Med. Indones. 2022, 54, 556–566. [Google Scholar]
- Hagner, S.; Haberberger, R.; Hay, D.L.; Facer, P.; Reiners, K.; Voigt, K.; McGregor, G.P. Immunohistochemical detection of the calcitonin receptor-like receptor protein in the microvasculature of rat endothelium. Eur. J. Pharmacol. 2003, 481, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Dschietzig, T.; Azad, H.A.; Asswad, L.; Böhme, C.; Bartsch, C.; Baumann, G.; Stangl, K. The adrenomedullin receptor acts as clearance receptor in pulmonary circulation. Biochem. Biophys. Res. Commun. 2002, 294, 315–318. [Google Scholar] [CrossRef]
- Harel, F.; Langleben, D.; Provencher, S.; Fournier, A.; Finnerty, V.; Nguyen, Q.T.; Letourneau, M.; Levac, X.; Abikhzer, G.; Guimond, J.; et al. Molecular imaging of the human pulmonary vascular endothelium in pulmonary hypertension: A phase II safety and proof of principle trial. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Dinarti, L.K.; Hartopo, A.B.; Anggrahini, D.W.; Sadewa, A.H.; Setianto, B.Y.; Wahab, A.S. Profile of endothelin-1, nitric oxide, and prostacyclin levels in pulmonary arterial hypertension related to uncorrected atrial septal defect: Results from a single center study in Indonesia. Cardiol. Res. Pract. 2020, 2020, 7526508. [Google Scholar] [CrossRef]
- Karakas, M.; Jarczak, D.; Becker, M.; Roedl, K.; Addo, M.M.; Hein, F.; Bergmann, A.; Zimmermann, J.; Simon, T.P.; Marx, G.; et al. Targeting endothelial dysfunction in eight extreme-critically ill patients with COVID-19 using the anti-adrenomedullin antibody adrecizumab (HAM8101). Biomolecules 2020, 10, 1171. [Google Scholar] [CrossRef]
- Laterre, P.F.; Pickkers, P.; Marx, G.; Wittebole, X.; Meziani, F.; Dugernier, T.; Huberlant, V.; Schuerholz, T.; François, B.; Lascarrou, J.B.; et al. AdrenOSS-2 study participants. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: The AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. 2021, 47, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Karakas, M.; Akin, I.; Burdelski, C.; Clemmensen, P.; Grahn, H.; Jarczak, D.; Keßler, M.; Kirchhof, P.; Landmesser, U.; Lezius, S.; et al. Single-dose of adrecizumab versus placebo in acute cardiogenic shock (ACCOST-HH): An investigator-initiated, randomised, double-blinded, placebo-controlled, multicentre trial. Lancet Respir. Med. 2022, 10, 247–254. [Google Scholar] [CrossRef]
- Deniau, B.; Takagi, K.; Asakage, A.; Mebazaa, A. Adrecizumab: An investigational agent for the biomarker-guided treatment of sepsis. Expert. Opin. Investig. Drugs 2021, 30, 95–102. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control Group (n = 20) | ASD-PAH (n = 85) | I/H PAH (n = 15) | p Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 30.70 ± 7.63 | 36.45 ± 11.60 | 39.33 ± 10.80 | 0.049 |
| Sex | ||||
| Female, n(%) (n = 99 (82.5%) | 18 (90.0) | 69 (81.2) | 12 (80.0) | 0.623 |
| Male, n(%) (n = 21 (17.5%) | 2 (10.0) | 16 (18.8) | 3 (20.0) | |
| LV EF, % (mean ± SD) | 69.70 ± 6.63 | 71.52 ± 9.07 | NA | 0.313 |
| TAPSE, mm (mean ± SD) | 27.80 ± 3.65 | 23.59 ± 5.89 | 17.50 ± 7.05 (n = 12) | <0.001 |
| TV gradient, mmHg (mean ± SD) | 28.11 ± 9.40 | 84.90 ± 31.09 | 85.00 ± 18.38 (n = 2) | <0.001 |
| Hemoglobin, g/dL (mean ± SD) | 12.84 ± 1.21 | 14.49 ± 2.30 | 13.93 ± 1.81 | 0.008 |
| Hematocrit, % (mean ± SD) | 38.93 ± 3.03 | 43.68 ± 6.37 | 42.40 ± 4.77 | 0.005 |
| NT-proBNP, pg/mL (mean ± SD) | 107.45 ± 95.39 (n = 13) | 2256.32 ± 3497.64 (n = 69) | 1566.36 ± 1143.37 (n = 14) | 0.063 |
| mPAP, mmHg (mean ± SD) | 17.40 ± 2.21 | 56.93 ± 17.57 | 64.27 ± 17.13 | <0.001 |
| PVR index, WU/m2 (mean ± SD) | 1.46 ± 0.75 | 16.31 ± 16.55 | 31.32 ± 10.89 | <0.001 |
| mRAP, mmHg (mean ± SD) | 5.72 ± 3.34 (n = 18) | 11.83 ± 6.28 | 13.73 ± 7.74 | <0.001 |
| SaO2 Aorta, % (mean ± SD) | 96.23 ± 1.72 | 88.97 ± 6.32 (n = 80) | 90.35 ± 6.39 | <0.001 |
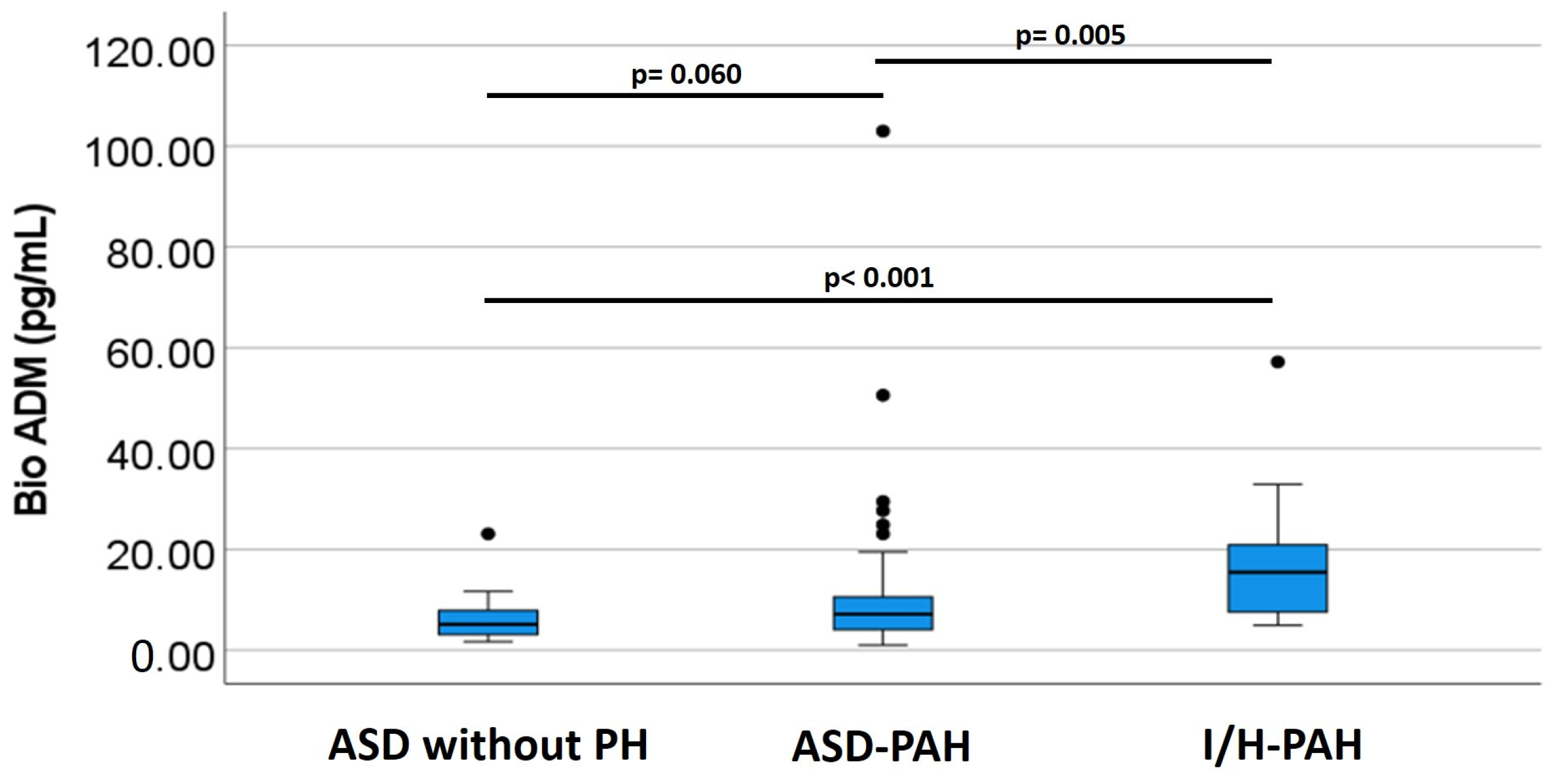
| Bio-ADM, pg/mL | Control Group (n = 20) | ASD-PAH (n = 85) | I/H PAH (n = 15) |
|---|---|---|---|
| Mean ± SD * | 6.33 ± 4.79 | 10.55 ± 12.82 | 17.65 ± 13.62 |
| Median (IQR) | 5.15 (3.0–7.95) | 7.30 (4.10–13.50) | 15.50 (7.50–24.10) |
| Parameters | Mortality (+) (n = 21) | Mortality (−) (n = 99) | p Value |
|---|---|---|---|
| Age, years (mean ± SD) | 34.43 ± 11.49 | 36.15 ± 11.13 | 0.261 |
| Sex | |||
| Female, n (%) | 21 (100) | 78 (78.8) | 0.023 |
| Male, n (%) | 0 (0) | 21 (21.2) | |
| LV ejection fraction, % (mean ± SD) | 68.08 ± 9.16 (n = 13) | 71.61 ± 8.54 (n = 91) | 0.085 |
| TAPSE, mm (mean ± SD) | 21.10 ± 6.65 (n = 20) | 24.23 ± 6.05 (n = 96) | 0.020 |
| TV gradient, mmHg (mean ± SD) | 76.58 ± 29.37 (n = 12) | 74.26 ± 36.58 (n = 92) | 0.417 |
| Hemoglobin, g/dL (mean ± SD) | 13.18 ± 1.49 (n = 20) | 14.34 ± 2.26 | 0.014 |
| Hematocrit, % (mean ± SD) | 40.51 ± 4.14 (n = 20) | 43.18 ± 6.23 | 0.035 |
| NT-proBNP, pg/mL (mean ± SD) | 2180.45 ± 3148.9 (n = 18) | 1791.84 ± 3079.41 (n = 78) | 0.316 |
| mPAP, mmHg (median, IQR) | 56.00 (45.00–67.00) | 50.50 (34.75–66.00) | 0.257 * |
| PVR index, WU/m2 (median, IQR) | 15.30 (5.30–24.40) (n = 19) | 7.93 (2.76–25.08) (n = 98) | 0.185 * |
| mRAP, mmHg (mean ± SD) | 13.52 ± 7.81 | 10.61 ± 6.16 (n = 96) | 0.032 |
| SaO2 Aorta, % (mean ± SD) | 90.48 ± 6.10 (n = 19) | 90.32 ± 6.48 (n = 94) | 0.460 |
| PAH, n (%) | 21 (100.0) | 79 (79.8) | 0.022 |
| Diagnosis | |||
| ASD without PH | 0 (0) | 20 (20.2) | <0.001 |
| ASD-PAH | 13 (61.9) | 72 (72.7) | |
| I/H-PAH | 8 (38.1) | 7 (7.1) |
| All Subjects (n = 120) | Mortality (+) (n = 21) | Mortality (−) (n = 99) | p Value * |
| Mean ± SD, pg/mL | 12.63 ± 8.11 | 10.33 ± 12.99 | 0.031 |
| Median (IQR), pg/mL | 11.70 (7.20–16.40) | 6.90 (4.10–10.20) | |
| ASD-PAH (n = 85) | Mortality (+) (n = 13) | Mortality (−) (n = 72) | |
| Mean ± SD, pg/mL | 10.69 ± 13.69 | 9.81 ± 6.45 | 0.580 |
| Median (IQR), pg/mL | 10.00 (4.45–13.55) | 7.15 (4.10–13.45) | |
| I/H-PAH (n = 15) | Mortality (+) (n = 8) | Mortality (−) (n = 7) | |
| Mean ± SD, pg/mL | 17.21 ± 3.12 | 18.14 ± 18.48 | 0.487 |
| Median (IQR), pg/mL | 16.45 (8.90–24.23) | 9.80 (6.50–24.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartopo, A.B.; Anggrahini, D.W.; Dinarti, L.K.; Schäfer, A.-K.; Bergmann, A.; Fachiroh, J.; Somma, S.D. Prognostic Value for Mortality of Plasma Bioactive Adrenomedullin in Patients with Pulmonary Arterial Hypertension: A Sub Analysis of the Biomarker Study in the COHARD-PH Registry. Medicina 2023, 59, 748. https://doi.org/10.3390/medicina59040748
Hartopo AB, Anggrahini DW, Dinarti LK, Schäfer A-K, Bergmann A, Fachiroh J, Somma SD. Prognostic Value for Mortality of Plasma Bioactive Adrenomedullin in Patients with Pulmonary Arterial Hypertension: A Sub Analysis of the Biomarker Study in the COHARD-PH Registry. Medicina. 2023; 59(4):748. https://doi.org/10.3390/medicina59040748
Chicago/Turabian StyleHartopo, Anggoro Budi, Dyah Wulan Anggrahini, Lucia Kris Dinarti, Anne-Kathrin Schäfer, Andreas Bergmann, Jajah Fachiroh, and Salvatore Di Somma. 2023. "Prognostic Value for Mortality of Plasma Bioactive Adrenomedullin in Patients with Pulmonary Arterial Hypertension: A Sub Analysis of the Biomarker Study in the COHARD-PH Registry" Medicina 59, no. 4: 748. https://doi.org/10.3390/medicina59040748
APA StyleHartopo, A. B., Anggrahini, D. W., Dinarti, L. K., Schäfer, A.-K., Bergmann, A., Fachiroh, J., & Somma, S. D. (2023). Prognostic Value for Mortality of Plasma Bioactive Adrenomedullin in Patients with Pulmonary Arterial Hypertension: A Sub Analysis of the Biomarker Study in the COHARD-PH Registry. Medicina, 59(4), 748. https://doi.org/10.3390/medicina59040748








