Identification of Microbial Species and Analysis of Antimicrobial Resistance Patterns in Acute Cholangitis Patients with Malignant and Benign Biliary Obstructions: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patients and Sampling
2.3. Data Collection and Variables
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
3.2. Bacterial Identification
3.3. Antibiogram Study
4. Discussion
4.1. Current Findings and Published Data
4.2. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, E.C.; Mok, F.P.; Tan, E.S.; Lo, C.-M.; Fan, S.-T.; You, K.-T.; Wong, J. Endoscopic Biliary Drainage for Severe Acute Cholangitis. N. Engl. J. Med. 1992, 326, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Sokal, A.; Sauvanet, A.; Fantin, B.; de Lastours, V. Acute cholangitis: Diagnosis and management. J. Visc. Surg. 2019, 156, 515–525. [Google Scholar] [CrossRef]
- Andrew, D.J.; Johnson, S.E. Acute suppurative cholangitis, a medical and surgical emergency. A review of ten years experience emphasizing early recognition. Am. J. Gastroenterol. 1970, 54, 141–154. [Google Scholar] [PubMed]
- Du, L.; Cen, M.; Zheng, X.; Luo, L.; Siddiqui, A.; Kim, J.J. Timing of Performing Endoscopic Retrograde Cholangiopancreatography and Inpatient Mortality in Acute Cholangitis: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2020, 11, e00158. [Google Scholar] [CrossRef]
- Kimura, Y.; Takada, T.; Kawarada, Y.; Nimura, Y.; Hirata, K.; Sekimoto, M.; Yoshida, M.; Mayumi, T.; Wada, K.; Miura, F.; et al. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo Guidelines. J. Hepato-Biliary-Pancreat. Surg. 2007, 14, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, S.; Takada, T.; Strasberg, S.M.; Solomkin, J.; Mayumi, T.; Pitt, H.A.; Gouma, D.J.; Garden, O.J.; Büchler, M.W.; Yokoe, M.; et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2013, 20, 24–34. [Google Scholar] [CrossRef]
- Miura, F.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Pitt, H.A.; Gomi, H.; Solomkin, J.; Schlossberg, D.; Han, H.-S.; et al. Tokyo Guidelines 2018: Initial management of acute biliary infection and flowchart for acute cholangitis. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 31–40. [Google Scholar] [CrossRef]
- Charcot, M. De la fievre hepatique symptomatique—Comparison avec la fievre uroseptique. In Lecons sur les Maladies du Foie des Voies Biliares et des Reins; Bourneville et Sevestre: Paris, France, 1877; pp. 176–185. [Google Scholar]
- Rumsey, S.; Winders, J.; MacCormick, A.D. Diagnostic accuracy of Charcot’s triad: A systematic review. ANZ J. Surg. 2017, 87, 232–238. [Google Scholar] [CrossRef]
- Weiland, C.J.S.; Busch, C.B.E.; Bhalla, A.; Bruno, M.J.; Fockens, P.; Hooft, J.E.; Poen, A.C.; Timmerhuis, H.C.; Umans, D.S.; Venneman, N.G.; et al. Performance of diagnostic tools for acute cholangitis in patients with suspected biliary obstruction. J. Hepato-Biliary-Pancreat. Sci. 2021, 29, 479–486. [Google Scholar] [CrossRef]
- Wada, K.; Takada, T.; Kawarada, Y.; Nimura, Y.; Miura, F.; Yoshida, M.; Mayumi, T.; Strasberg, S.; Pitt, H.A.; Gadacz, T.R.; et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo Guidelines. J. Hepato-Biliary-Pancreat. Surg. 2007, 14, 52–58. [Google Scholar] [CrossRef]
- Yokoe, M.; Hata, J.; Takada, T.; Strasberg, S.M.; Bun, T.A.Y.; Wakabayashi, G.; Kozaka, K.; Endo, I.; DeZiel, D.J.; Miura, F.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Schepers, N.J.; Hallensleben, N.D.L.; Besselink, M.G.; Anten, M.-P.G.F.; Bollen, T.L.; da Costa, D.W.; van Delft, F.; van Dijk, S.M.; van Dullemen, H.M.; Dijkgraaf, M.G.W.; et al. Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (APEC): A multicentre randomised controlled trial. Lancet 2020, 396, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Mulki, R.; Shah, R.; Qayed, E. Early vs late endoscopic retrograde cholangiopancreatography in patients with acute cholangitis: A nationwide analysis. World J. Gastrointest. Endosc. 2019, 11, 41–53. [Google Scholar] [CrossRef]
- Gomi, H.; Solomkin, J.; Schlossberg, D.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Ukai, T.; Endo, I.; Iwashita, Y.; Hibi, T.; et al. Tokyo Guidelines 2018: Antimicrobial therapy for acute cholangitis and cholecystitis. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Tinusz, B.; Szapáry, L.; Paládi, B.; Tenk, J.; Rumbus, Z.; Pécsi, D.; Szakács, Z.; Varga, G.; Rakonczay, Z.; Szepes, Z.; et al. Short-Course Antibiotic Treatment Is Not Inferior to a Long-Course One in Acute Cholangitis: A Systematic Review. Dig. Dis. Sci. 2019, 64, 307–315. [Google Scholar] [CrossRef]
- Haal, S.; Böhmer, B.T.; Balkema, S.; Depla, A.C.; Fockens, P.; Jansen, J.M.; Kuiken, S.D.; Liberov, B.I.; Van Soest, E.; Hooft, J.E.; et al. Antimicrobial therapy of 3 days or less is sufficient after successful ERCP for acute cholangitis. United Eur. Gastroenterol. J. 2020, 8, 481–488. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y.; Yu, K.; Yang, Y.; Wang, X.; Yang, X.; Qian, J.; Liu, Z.-X.; Wu, B. Fatal Infections Among Cancer Patients: A Population-Based Study in the United States. Infect. Dis. Ther. 2021, 10, 871–895. [Google Scholar] [CrossRef]
- Rolston, K.V.I. Infections in Cancer Patients with Solid Tumors: A Review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef]
- Teillant, A.; Gandra, S.; Barter, D.; Morgan, D.J.; Laxminarayan, R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: A literature review and modelling study. Lancet Infect. Dis. 2015, 15, 1429–1437. [Google Scholar] [CrossRef]
- Gudiol, C.; Aguado, J.M.; Carratala, J. Bloodstream infections in patients with solid tumors. Virulence 2016, 7, 298–308. [Google Scholar] [CrossRef]
- Rupp, C.; Bode, K.; Weiss, K.H.; Rudolph, G.; Bergemann, J.; Kloeters-Plachky, P.; Chahoud, F.; Stremmel, W.; Gotthardt, D.N.; Sauer, P. Microbiological Assessment of Bile and Corresponding Antibiotic Treatment: A Strobe-Compliant Observational Study of 1401 Endoscopic Retrograde Cholangiographies. Medicine 2016, 95, e2390. [Google Scholar] [CrossRef]
- Reuken, P.A.; Torres, D.; Baier, M.; Löffler, B.; Lübbert, C.; Lippmann, N.; Stallmach, A.; Bruns, T. Risk Factors for Multi-Drug Resistant Pathogens and Failure of Empiric First-Line Therapy in Acute Cholangitis. PLoS ONE 2017, 12, e0169900. [Google Scholar] [CrossRef]
- Gromski, M.A.; Gutta, A.; Lehman, G.A.; Tong, Y.; Fogel, E.L.; Watkins, J.L.; Easler, J.J.; Bick, B.L.; McHenry, L.; Beeler, C.; et al. Microbiology of bile aspirates obtained at ERCP in patients with suspected acute cholangitis. Endoscopy 2022, 54, 1045–1052. [Google Scholar] [CrossRef]
- Otani, T.; Ichiba, T.; Seo, K.; Naito, H. Blood cultures should be collected for acute cholangitis regardless of severity. J. Infect. Chemother. 2022, 28, 181–186. [Google Scholar] [CrossRef]
- Chandra, S.; Klair, J.S.; Soota, K.; Livorsi, D.J.; Johlin, F.C. Endoscopic Retrograde Cholangio-Pancreatography-Obtained Bile Culture Can Guide Antibiotic Therapy in Acute Cholangitis. Dig. Dis. 2019, 37, 155–160. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, S.; Bai, X.; Song, J.; Fan, Q.; Chen, J. A Retrospective Study on Bile Culture and Antibiotic Susceptibility Patterns of Patients with Biliary Tract Infections. Evid.-Based Complement. Altern. Med. 2022, 2022, 9255444. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, R.; Fogel, E.L.; Kalayci, C.; Esber, E.; Lehman, G.A.; Sherman, S. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest. Endosc. 2002, 56, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Ligozzi, M.; Bernini, C.; Bonora, M.G.; de Fatima, M.; Zuliani, J.; Fontana, R. Evaluation of the VITEK 2 System for Identification and Antimicrobial Susceptibility Testing of Medically Relevant Gram-Positive Cocci. J. Clin. Microbiol. 2002, 40, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; CLSI Document M100-S22; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Rehman, S.; Ahmed, S.; Metry, M.; Canelo, R. Significance of Bile Culture and Biliary Tract Pathology in Determining Severity of Cholangitis; Review of Current Literature. Ann. Emerg. Surg. 2017, 2, 1009. [Google Scholar]
- Tanaka, A.; Takada, T.; Kawarada, Y.; Nimura, Y.; Yoshida, M.; Miura, F.; Hirota, M.; Wada, K.; Mayumi, T.; Gomi, H.; et al. Antimicrobial therapy for acute cholangitis: Tokyo Guidelines. J. Hepato-Biliary-Pancreat. Surg. 2007, 14, 59–67. [Google Scholar] [CrossRef]
- Melzer, M.; Toner, R.; Lacey, S.; Bettany, E.; Rait, G. Biliary tract infection and bacteraemia: Presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad. Med. J. 2007, 83, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Chang, I.-J.; Lai, Y.-C.; Chen, S.-Y.; Chen, S.-C. Epidemiology and Prognostic Determinants of Patients with Bacteremic Cholecystitis or Cholangitis. Am. J. Gastroenterol. 2007, 102, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Kwan, K.E.L.; Shelat, V.G.; Tan, C.H. Recurrent pyogenic cholangitis: A review of imaging findings and clinical management. Abdom. Radiol. 2017, 42, 46–56. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Teng, Y.; Ou, L.; Xi, Y.; Chen, S.; Duan, G. The resistance mechanism of Escherichia coli induced by ampicillin in laboratory. Infect. Drug Resist. 2019, 12, 2853–2863. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Sivori, F.; Marchesi, F.; Prignano, G.; Pimpinelli, F.; Sperduti, I.; Pelagalli, L.; Di Salvo, F.; Celesti, I.; et al. Biofilm Production by Carbapenem-Resistant Klebsiella pneumoniae Significantly Increases the Risk of Death in Oncological Patients. Front. Cell. Infect. Microbiol. 2020, 10, 561741. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215 (Suppl. 1), S28–S36. [Google Scholar] [CrossRef]
- Osama, D.; El-Mahallawy, H.; Mansour, M.T.; Hashem, A.; Attia, A.S. Molecular Characterization of Carbapenemase-Producing Klebsiella pneumoniae Isolated from Egyptian Pediatric Cancer Patients Including a Strain with a Rare Gene-Combination of β-Lactamases. Infect. Drug Resist. 2021, 14, 335–348. [Google Scholar] [CrossRef]
- Bouza, E.; Cercenado, E. Klebsiella and Enterobacter: Antibiotic resistance and treatment implications. Semin. Respir. Infect. 2002, 17, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Hakim, M.S.; Meliala, A.; Aman, A.T.; Nuryastuti, T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019, 13 (Suppl. 11), 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-J.; Chen, Y.-P.; Chen, Y.-H.; Liu, L.; Wang, N.-K.; Chao, A.-N.; Wu, W.-C.; Hwang, Y.-S.; Chou, H.-D.; Kang, E.Y.-C.; et al. Infection Sources and Klebsiella pneumoniae Antibiotic Susceptibilities in Endogenous Klebsiella Endophthalmitis. Antibiotics 2022, 11, 866. [Google Scholar] [CrossRef] [PubMed]
| Etiology | Total (n = 262) |
|---|---|
| Benign | Total 138 (52.7%) |
| Choledocholithiasis | 127 (48.5%) |
| Vaterian ampulloma | 5 (1.9%) |
| Benign coledochal stenosis | 3 (1.1%) |
| Mirizzi syndrome | 2 (0.8%) |
| Liver abscess | 1 (0.4%) |
| Malignant | Total 124 (47.3%) |
| Pancreatic cancer | 65 (24.8%) |
| Cholangiocarcinoma | 35 (13.4%) |
| Malignant vaterian ampulloma | 14 (5.3%) |
| Malignant extrinsic compression | 7 (2.7%) |
| Gallbladder cancer | 3 (1.1%) |
| Variables | Total (n = 262) | Malignant (n = 124) | Benign (n = 138) | Significance |
|---|---|---|---|---|
| Gender (male) | 128 (48.9%) | 61 (49.2%) | 67 (48.6) | 0.508 |
| Age, mean (SD) | 67.6 (14.1) | 68.5 (11.3) | 66.8 (16.2) | 0.330 |
| Age, median (IQR) | 70.0 (19.0) | 69.5 (16.8) | 70.0 (22.2) | 0.930 |
| Age category | 0.005 | |||
| Young adults (18–39 years) | 15 (5.7%) | 2 (1.6%) | 11 (8.0%) | |
| Middle age (40–65 years) | 84 (32.1%) | 50 (40.3%) | 34 (24.6%) | |
| Older adults (>65 years) | 159 (60.7%) | 72 (58.1%) | 87 (63.0%) | |
| Abdominal pain, yes | 183 (69.8) | 72 (58.1%) | 111 (80.4%) | <0.001 |
| Jaundice | 234 (89.3%) | 117 (94.4%) | 117 (84.8%) | 0.015 |
| Fever | 85 (32.4%) | 35 (28.2%) | 50 (36.2%) | 0.187 |
| Previous stent, yes | 48 (18.3%) | 41 (33.1%) | 7 (5.1%) | <0.001 |
| Cholecystectomy, yes | 50 (19.1%) | 19 (15.3%) | 31 (22.5%) | 0.158 |
| ERCP timing | 0.912 | |||
| Emergent (<48 h) | 176 (67.2%) | 83 (66.9%) | 93 (67.4%) | |
| Urgent (48–72 h) | 44 (16.8%) | 20 (16.1%) | 24 (17.4%) | |
| Late (>72 h) | 42 (16%) | 21 (16.9%) | 21 (15.2%) | |
| Hospitalization days | 7 (4–10) | 7 (5–10) | 6 (4–10) | 0.040 |
| Weekend admission, yes | 73 (27.9%) | 29 (23.4%) | 44 (31.9%) | 0.132 |
| Tokyo severity score, | 0.075 | |||
| Grade I | 103 (39.3%) | 43 (34.7%) | 60 (43.5%) | |
| Grade II | 95 (36.3%) | 43 (34.7%) | 52 (37.7%) | |
| Grade III | 64 (24.4%) | 38 (30.6%) | 26 (18.8%) |
| Tokyo Grade | Grade I (n = 103) | Grade II (n = 95) | Grade III (n = 64) | Significance |
|---|---|---|---|---|
| Sterile | 26 (10%) | 27 (10%) | 17 (6%) | 0.973 |
| 1 bacterium | 50 (19%) | 44 (17%) | 27 (10%) | |
| 2 bacteria | 24 (9%) | 21 (8%) | 17 (6%) | |
| 3 bacteria | 3 (1%) | 3 (1%) | 3 (1%) |
| Isolated Microorganisms from Bile Cultures No. of Patients (%) | Total (n = 192) | Malignant (n = 85) | Benign (n = 107) | Significance |
|---|---|---|---|---|
| Gram-negative organisms | ||||
| Escherichia coli | 92/192 (47.9%) | 32 (37.6%) | 60 (56.1%) | 0.003 |
| Klebsiella spp. | 51/192 (26.6%) | 25 (29.4%) | 26 (24.3%) | 0.876 |
| Pseudomonas spp. | 25/192 (13%) | 12 (14.1%) | 11 (10.3%) | 0.667 |
| Enterobacter spp. | 10/192 (5.2%) | 4 (4.7%) | 6 (5.6%) | 0.752 |
| Acinetobacter spp. | 6/192 (3.1%) | 5 (5.9%) | 1 (0.9%) | 0.104 |
| Citrobacter spp. | 11/192 (5.7%) | 6 (7.1%) | 5 (4.7%) | 0.760 |
| Gram-positive organisms | ||||
| Enterococcus spp. | 41/192 (21.6%) | 21 (24.7%) | 20 (18.7%) | 0.612 |
| Streptococcus spp. | 6/192 (3.1%) | 1 (1.2%) | 5 (4.8%) | 0.217 |
| Staphylococcus spp. | 3/192 (1.6%) | 2 (2.4%) | 1 (0.9%) | 0.604 |
| Isolated Microorganisms from Blood Cultures, n (%) | Malignant Disease (n = 16/127) | Benign Disease (n = 29/138) | Significance |
|---|---|---|---|
| Sterile | 109 (85.8%) | 111 (80.4%) | 0.242 |
| Gram-negative organisms | |||
| Escherichia coli | 5 (31.3%) | 16 (55.2%) | 0.123 |
| Klebsiella spp. | 4 (25.0%) | 5 (17.2%) | 0.533 |
| Pseudomonas spp. | 1 (6.3%) | 1 (3.4%) | 0.662 |
| Enterobacter spp. | 1 (6.3%) | 3 (10.3%) | 0.644 |
| Acinetobacter spp. | 1 (6.3%) | 0 (0.0%) | 0.173 |
| Citrobacter spp. | 0 (0.0%) | 1 (3.4%) | 0.452 |
| Gram-positive organisms | |||
| Enterococcus spp. | 1 (6.3%) | 1 (3.4%) | 0.662 |
| Staphylococcus spp. | 3 (18.8%) | 1 (3.4%) | 0.084 |
| Streptococcus spp. | 0 (0.0%) | 1 (3.4%) | 0.452 |
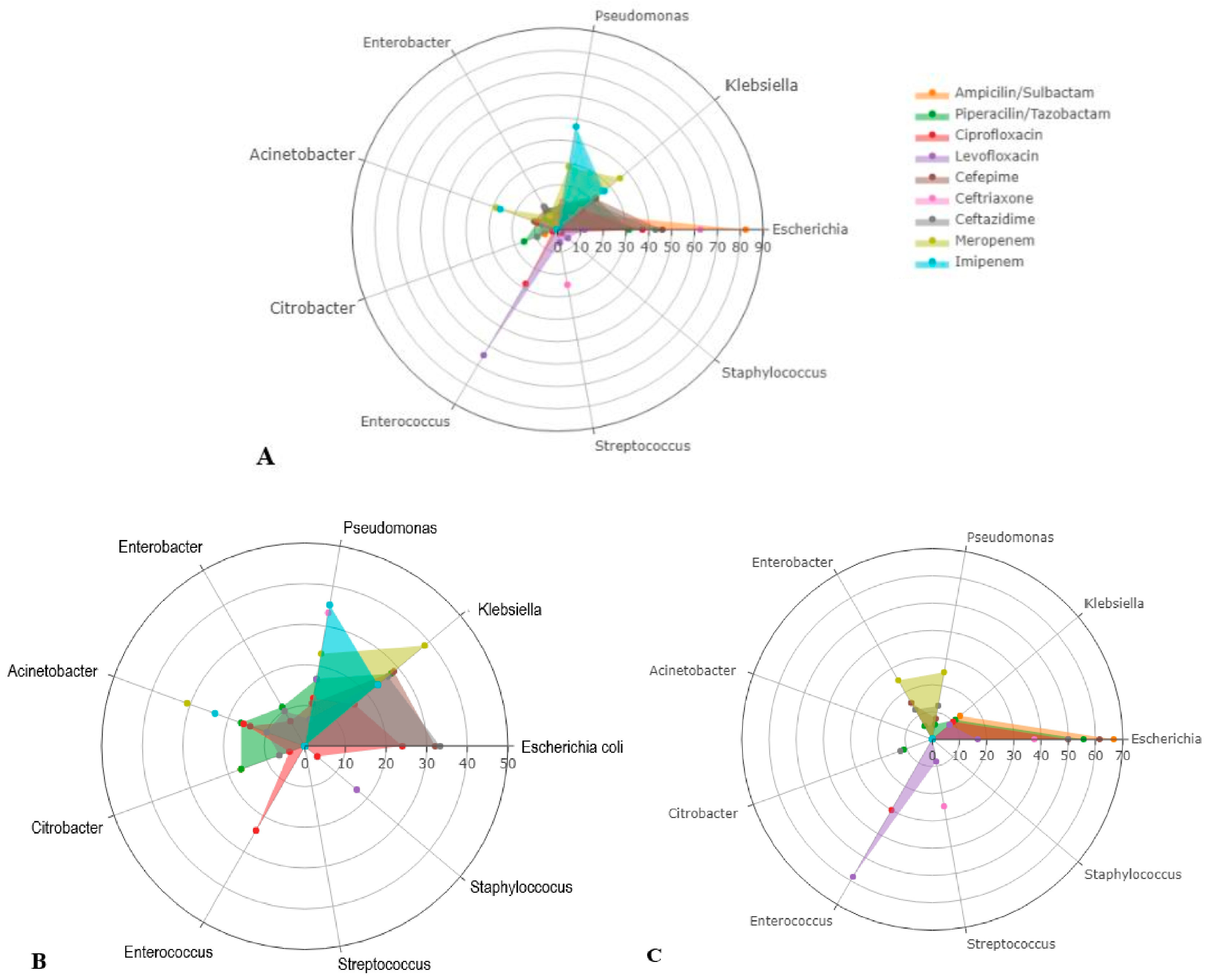
| Antibiotic Resistance n/Number of Antibiograms, % | Total (n = 266) | Malignant (n = 119) | Benign (n = 147) | Significance |
|---|---|---|---|---|
| Ampicillin/Sulbactam | 21/67 (31.3%) | 6/22 (27.2%) | 15/45 (33.3%) | 0.780 |
| Piperacillin/Tazobactam | 36/207 (17.3%) | 18/91 (19.7%) | 18/115 (15.6%) | 0.464 |
| Ciprofloxacin | 45/225 (20.0%) | 25/99 (25.2%) | 20/125 (16.0%) | 0.095 |
| Levofloxacin | 18/104 (17.3%) | 6/37 (16.2%) | 12/67 (17.9%) | 0.999 |
| Cefepime | 41/196 (20.9%) | 28/84 (33.3%) | 13/111 (11.7%) | 0.001 |
| Ceftriaxone | 11/73 (15.0%) | 3/22 (13.6%) | 8/51 (15.6%) | 0.999 |
| Ceftazidime | 46/192 (23.9%) | 30/82 (36.5%) | 16/110 (14.5%) | 0.001 |
| Meropenem | 17/194 (8.7%) | 13/84 (15.4%) | 4/110 (3.6%) | 0.004 |
| Imipenem | 20/197 (10.1%) | 17/84 (20.2%) | 3/113 (2.6%) | <0.001 |
| Isolated Microorganisms from Bile Cultures No. of Patients (%) | Escherichia coli n = 92/192 (47.9%) | Klebsiella spp. n = 52/192 (27.1%) | Pseudomonas spp. n = 23/192 (11.9%) | Enterococcus spp. n = 41/192 (21.4%) | ||||
|---|---|---|---|---|---|---|---|---|
| Malignant | Benign | Malignant | Benign | Malignant | Benign | Malignant | Benign | |
| 34.8% | 64.2% | 48.1% | 51.9% | 52.2% | 47.8% | 51.2% | 48.8% | |
| (32/92) | (60/92) | (25/52) | (27/52) | (12/23) | (11/23) | 21/41 | 20/41 | |
| Ampicillin/Sulbactam | 36.4% | 46.7% | 0% | 33.3% | 0% | 0% | 0% | 0% |
| (4/11) | (14/30) | (0/6) | (3/9) | (0/0) | (0/0) | (0/2) | (0/0) | |
| Piperacillin/Tazobactam | 9.4% | 25.9% | 20% | 18.5% | 33.3% | 22.2% | 0% | 0% |
| (3/32) | (15/58) | (5/25) | (5/27) | (4/12) | (2/9) | (0/0) | (0/0) | |
| Ciprofloxacin | 25% | 19.2% | 19% | 11.5 | 25 | 10% | 40% | 50% |
| (7/28) | (10/52) | (4/21) | (3/26) | (3/12) | (1/10) | (6/15) | (9/18) | |
| Levofloxacin | 0% | 8% | 0% | 11.1% | 20% | 0.5% | 26.7% | 50% |
| (0/7) | (2/25) | (0/2) | (1/9) | (1/5) | (0/4) | (4/15) | (9/18) | |
| Cefepime | 31% | 16.7% | 34.8% | 0% | 27.3% | 20% | 0% | 0% |
| (9/29) | (9/54) | (8/23) | (0/27) | (3/11) | (2/10) | (0/0) | (0/0) | |
| Ceftriaxone | 18.2% | 17.2% | 0% | 22.2% | 100% | 0% | 0% | 0% |
| (2/11) | (5/29) | (0/5) | (2/9) | (1/1) | (0/1) | (0/0) | (0/0) | |
| Ceftazidime | 33.3% | 22.6% | 34.8% | 7.7% | 20% | 30% | 0% | 0% |
| (10/30) | (12/53) | (8/23) | (2/26) | (2/10) | (3/10) | (0/0) | (0/0) | |
| Meropenem | 0% | 0% | 16.7% | 3.8% | 40% | 22.2% | 0% | 0% |
| (0/29) | (0/53) | (4/24) | (1/26) | (4/10) | (2/9) | (0/0) | (0/0) | |
| Imipenem | 0% | 1.8% | 17.4% | 3.7% | 60% | 20% | 0% | 0% |
| (0/30) | (1/55) | (4/23) | (1/27) | (6/10) | (2/10) | (0/0) | (0/0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miuțescu, B.; Vuletici, D.; Burciu, C.; Turcu-Stiolica, A.; Bende, F.; Rațiu, I.; Moga, T.; Sabuni, O.; Anjary, A.; Dalati, S.; et al. Identification of Microbial Species and Analysis of Antimicrobial Resistance Patterns in Acute Cholangitis Patients with Malignant and Benign Biliary Obstructions: A Comparative Study. Medicina 2023, 59, 721. https://doi.org/10.3390/medicina59040721
Miuțescu B, Vuletici D, Burciu C, Turcu-Stiolica A, Bende F, Rațiu I, Moga T, Sabuni O, Anjary A, Dalati S, et al. Identification of Microbial Species and Analysis of Antimicrobial Resistance Patterns in Acute Cholangitis Patients with Malignant and Benign Biliary Obstructions: A Comparative Study. Medicina. 2023; 59(4):721. https://doi.org/10.3390/medicina59040721
Chicago/Turabian StyleMiuțescu, Bogdan, Deiana Vuletici, Călin Burciu, Adina Turcu-Stiolica, Felix Bende, Iulia Rațiu, Tudor Moga, Omar Sabuni, Adnan Anjary, Sami Dalati, and et al. 2023. "Identification of Microbial Species and Analysis of Antimicrobial Resistance Patterns in Acute Cholangitis Patients with Malignant and Benign Biliary Obstructions: A Comparative Study" Medicina 59, no. 4: 721. https://doi.org/10.3390/medicina59040721
APA StyleMiuțescu, B., Vuletici, D., Burciu, C., Turcu-Stiolica, A., Bende, F., Rațiu, I., Moga, T., Sabuni, O., Anjary, A., Dalati, S., Ungureanu, B. S., Gadour, E., Horhat, F. G., & Popescu, A. (2023). Identification of Microbial Species and Analysis of Antimicrobial Resistance Patterns in Acute Cholangitis Patients with Malignant and Benign Biliary Obstructions: A Comparative Study. Medicina, 59(4), 721. https://doi.org/10.3390/medicina59040721










