The Effect of Biological Treatment on Stress Parameters Determined in Saliva in Patients with Severe Psoriasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Treatment
2.4. Procedures
2.4.1. Observation
2.4.2. Assessment of Disease Severity
2.4.3. Laboratory Tests
Measurement of α-Amylase Activity
Estimation of Chromogranin A Levels
Measurement of Salivary Secretory Immunoglobulin A
2.5. Statistical Methods
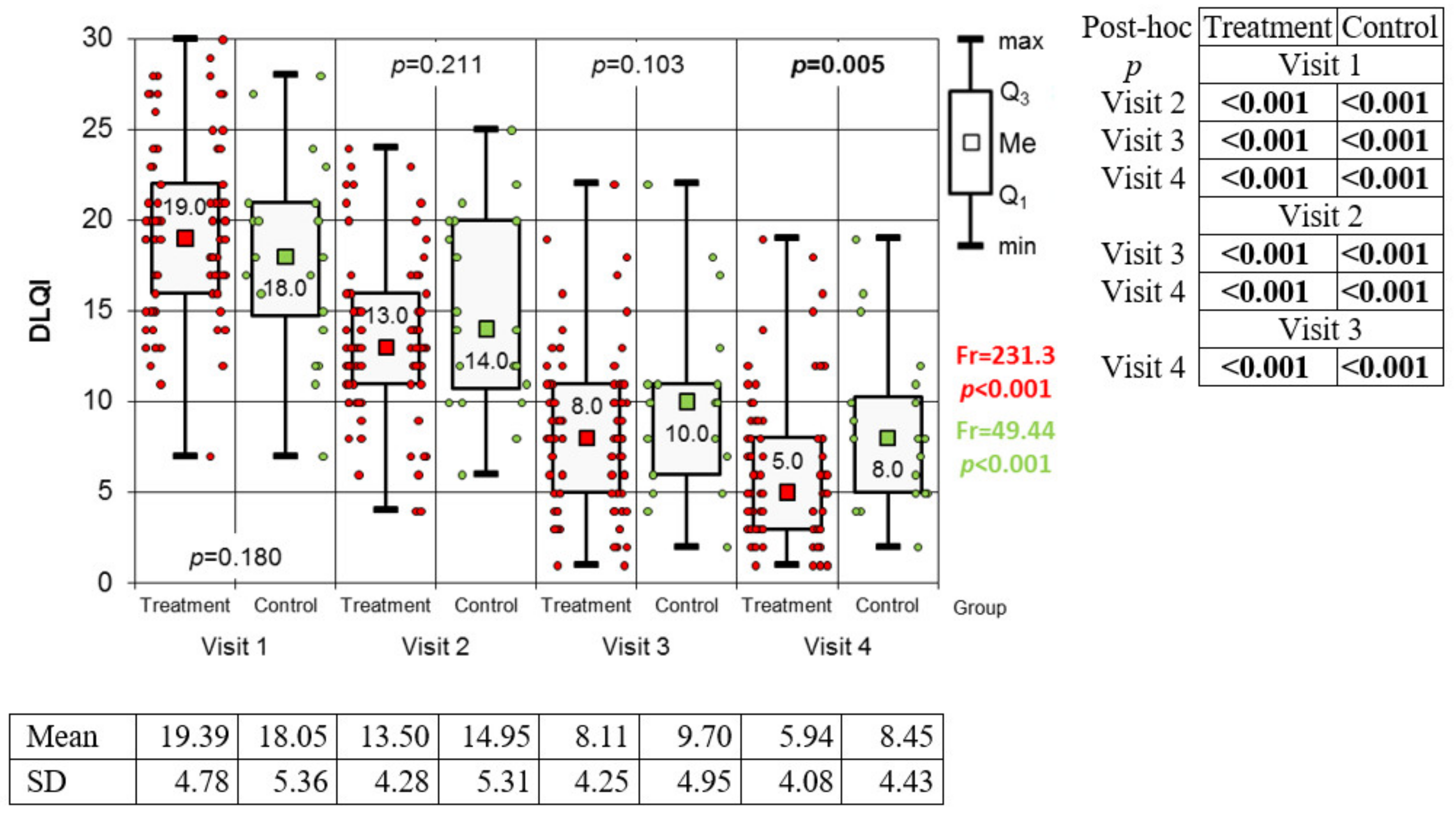
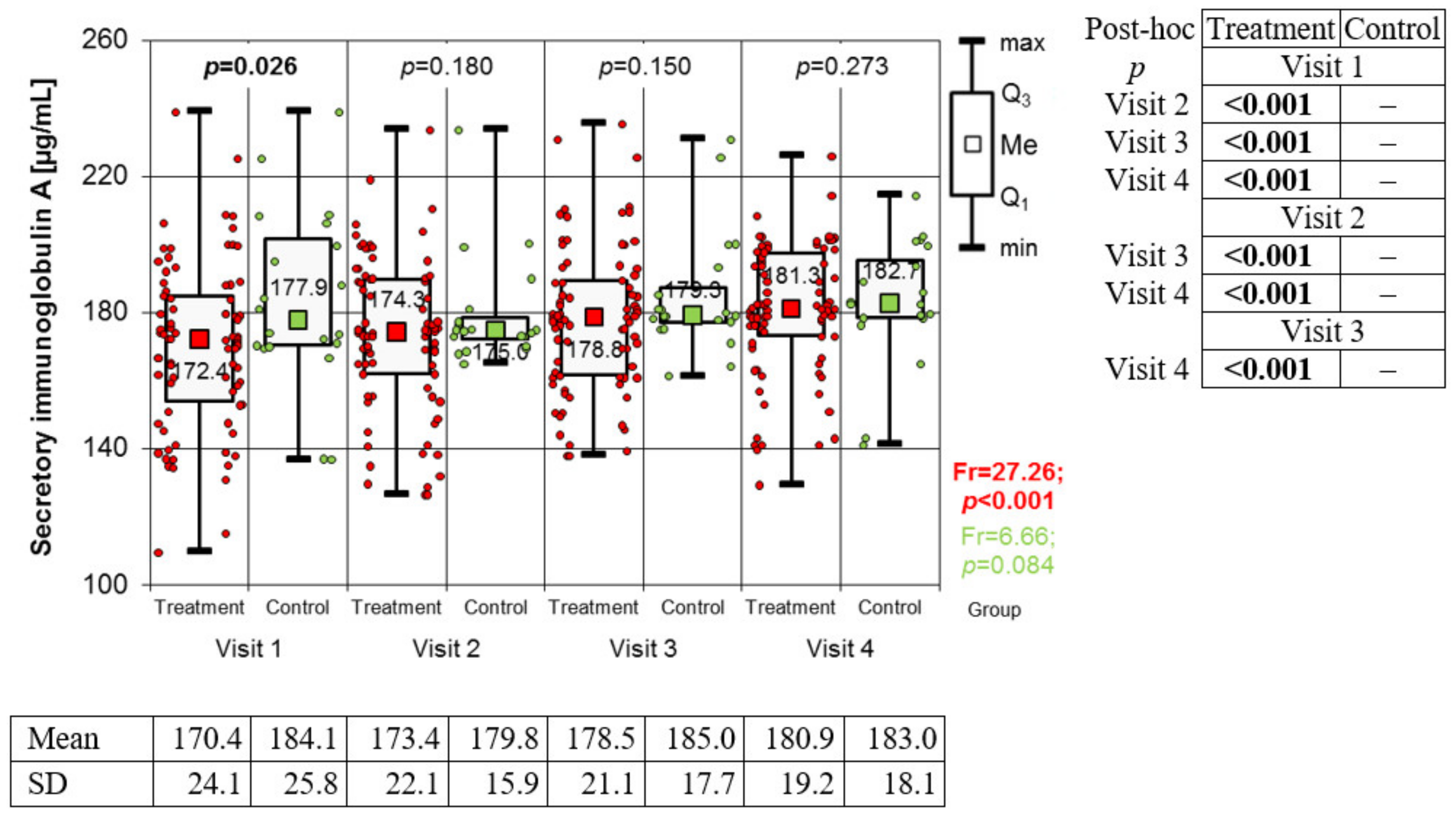
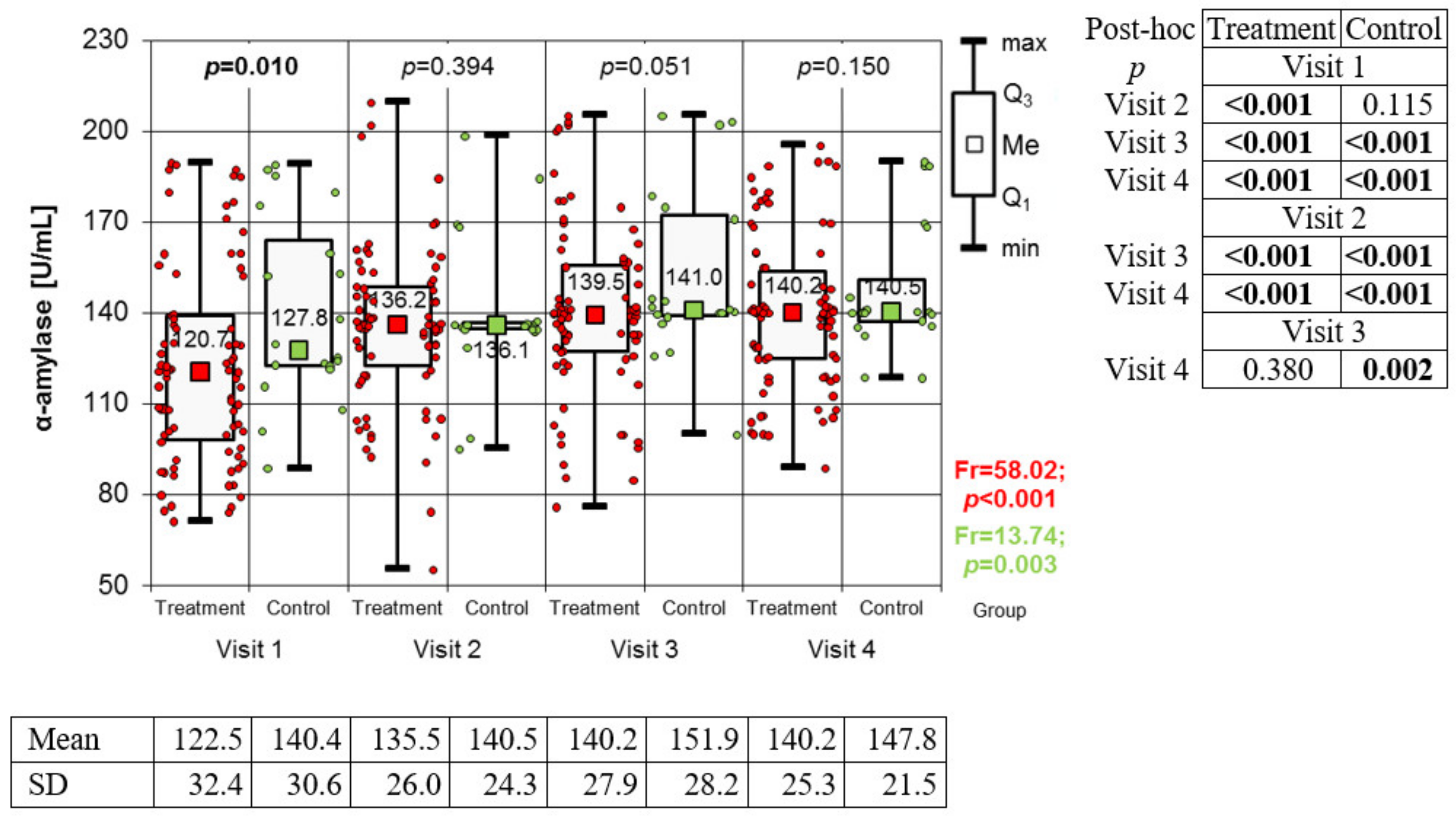
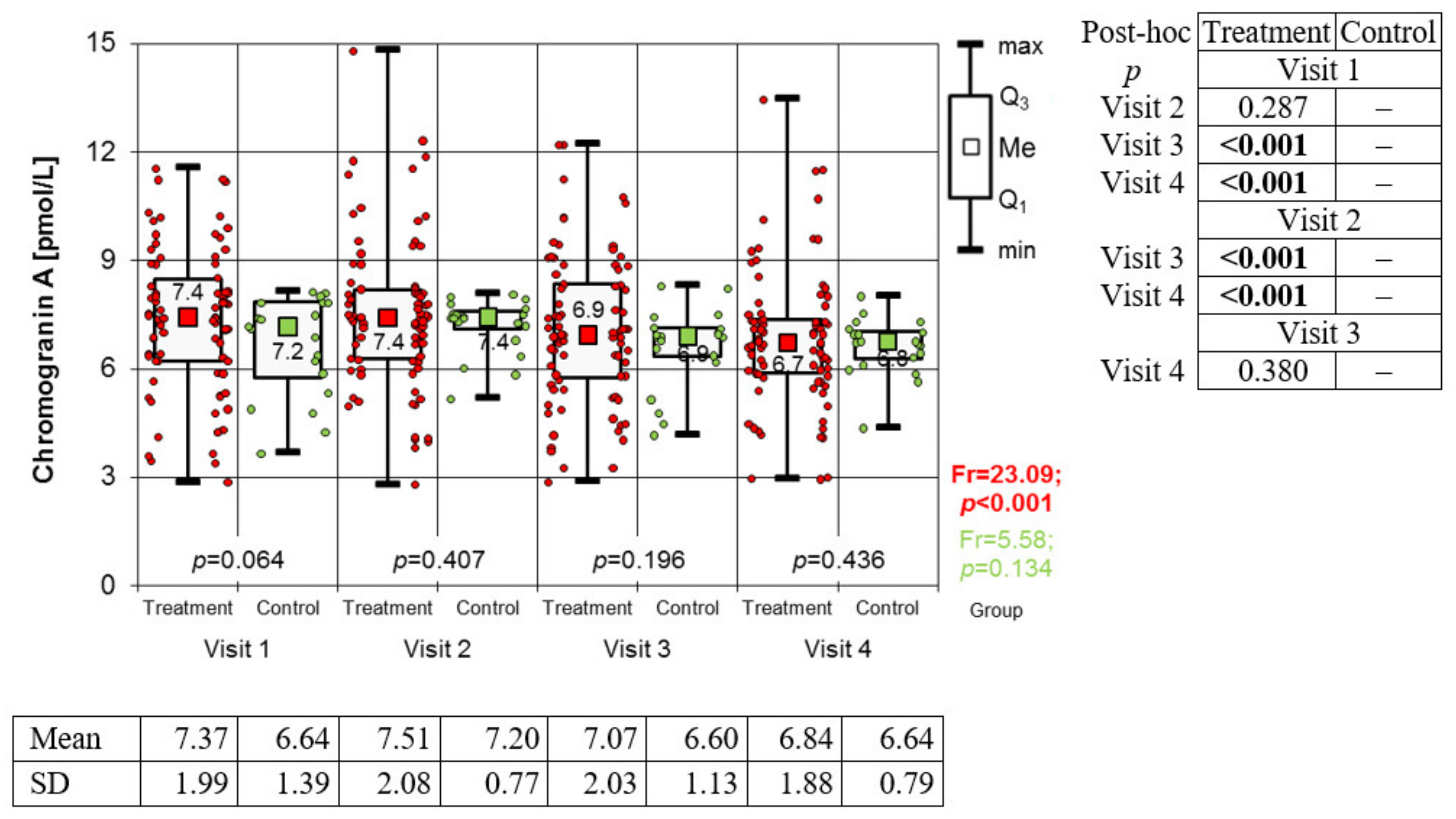
3. Results
3.1. Efficacy of Therapy
3.2. Variation of Biomarkers during Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zachariae, R.; Zachariae, H.; Blomqvist, K.; Davidsson, S.; Molin, L.; Mørk, C.; Sigurgeirsson, B. Self-reported stress reactivity and psoriasis-related stress of Nordic psoriasis sufferers. J. Eur. Acad. Derm. Venereol. 2004, 18, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulos, D.; Gregoriou, S.; Katrinaki, A.; Korfitis, C.; Larios, G.; Stamou, C.; Mourellou, O.; Petridis, A.; Rallis, E.; Sotiriadis, D.; et al. Characteristics of psoriasis in Greece: An epidemiological study of a population in a sunny mediterranean climate. Eur. J. Dermatol. 2010, 20, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Torales, J.; Echeverria, C.; Barrios, I.; García, O.; O’Higgins, M.; Castaldelli-Maia, J.M.; Jafferany, M. Psychodermatological mechanisms of psoriasis. Dermatol. Ther. 2020, 33, 13827. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.L.; Burke, L.B.; Powers, J.H.; Scott, J.A.; Rock, E.P.; Dawisha, S.; Kennedy, D.L. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health 2007, 10, s125–s137. [Google Scholar] [CrossRef] [PubMed]
- Both, H.; Essink-Bot, M.L.; Busschbach, J.; Nijsten, T. Critical review of generic and dermatology-specific health-related quality of life instruments. J. Investig. Dermatol. 2007, 127, 2726–2739. [Google Scholar] [CrossRef]
- Le Cleach, L.; Chassany, O.; Levy, A.; Wolkenstein, P.; Chosidow, O. Poor reporting of quality of life outcomes in dermatology randomized controlled clinical trials. Dermatology 2008, 216, 46–55. [Google Scholar] [CrossRef]
- Basra, M.K.; Chowdhury, M.M.; Smith, E.V.; Freemantle, N.; Piguet, V. A review of the use of the dermatology life quality index as a criterion in clinical guidelines and health technology assessments in psoriasis and chronic hand eczema. Dermatol. Clin. 2012, 30, 237–244. [Google Scholar] [CrossRef]
- Reich, K.; Griffiths, C.E.M. The relationship between quality of life and skin clearance in moderate-to-severe psoriasis: Lessons learnt from clinical trials with infliximab. Arch. Dermatol. Res. 2008, 300, 537–544. [Google Scholar] [CrossRef]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Rizzuto, F.; Dastoli, S.; Patruno, C.; Bianchi, L.; Nisticò, S.P. A novel vehicle for the treatment of psoriasis. Dermatol. Ther. 2020, 33, e13185. [Google Scholar] [CrossRef]
- Dastoli, S.; Nisticò, S.P.; Morrone, P.; Patruno, C.; Leo, A.; Citraro, R.; Gallelli, L.; Russo, E.; De Sarro, G.; Bennardo, L. Colchicine in Managing Skin Conditions: A Systematic Review. Pharmaceutics 2022, 14, 294. [Google Scholar] [CrossRef]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef]
- Al-Janabi, A.; Yiu, Z.Z.N. Biologics in Psoriasis: Updated Perspectives on Long-Term Safety and Risk Management. Psoriasis 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Nemati, H.; Sadeghi, M. Salivary biomarkers in patients with psoriasis—A meta-analysis. Dermatol. Rev./Przegląd Dermatol. 2021, 108, 105–116. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, J. Influence of Stress on the Development of Psoriasis. Clin. Exp. Dermatol. 2020, 45, 284–288. [Google Scholar] [CrossRef]
- Woźniak, E.; Owczarczyk-Saczonek, A.; Placek, W. Psychological Stress, Mast Cells, and Psoriasis—Is There Any Relationship? Int. J. Mol. Sci. 2021, 22, 13252. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Romanelli, P.; Volpe, E.; Borsellino, G.; Romanelli, M. Scanning the Immunopathogenesis of Psoriasis. Int. J. Mol. Sci. 2018, 19, 179. [Google Scholar] [CrossRef]
- Surace, A.E.A.; Hedrich, C.M. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef]
- Nijakowski, K.; Rutkowski, R.; Eder, P.; Simon, M.; Korybalska, K.; Witowski, J.; Surdacka, A. Potential Salivary Markers for Differential Diagnosis of Crohn’s Disease and Ulcerative Colitis. Life 2021, 11, 943. [Google Scholar] [CrossRef]
- Kułak-Bejda, A.; Waszkiewicz, N.; Bejda, G.; Zalewska, A.; Maciejczyk, M. Diagnostic Value of Salivary Markers in Neuropsychiatric Disorders. Dis. Mark. 2019, 2019, 4360612. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; Manzano-Moreno, F.J.; Ruiz, C.; Illescas-Montes, R. Salivary Biomarkers and Their Application in the Diagnosis and Monitoring of the Most Common Oral Pathologies. Int. J. Mol. Sci. 2020, 21, 5173. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.; Zawilska, A.; Trzcionka, A.; Tanasiewicz, M.; Mazurek, H.; Świętochowska, E. Estimation of Proinflammatory Factors in the Saliva of Adult Patients with Cystic Fibrosis and Dental Caries. Medicina 2020, 56, 612. [Google Scholar] [CrossRef] [PubMed]
- Farnaud, S.J.; Kosti, O.; Getting, S.J.; Renshaw, D. Saliva: Physiology and diagnostic potential in health and disease. Sci. World J. 2010, 10, 434–456. [Google Scholar] [CrossRef]
- Malamud, D. Saliva as a diagnostic fluid. Dent. Clin. N. Am. 2011, 55, 159–178. [Google Scholar] [CrossRef]
- Jiménez, C.; Bordagaray, M.J.; Villarroel, J.L.; Flores, T.; Benadof, D.; Fernández, A.; Valenzuela, F. Biomarkers in Oral Fluids as Diagnostic Tool for Psoriasis. Life 2022, 12, 501. [Google Scholar] [CrossRef]
- Asa’ad, F.; Fiore, M.; Alfieri, A.; Pigatto, P.D.M.; Franchi, C.; Berti, E.; Damiani, G. Saliva as a Future Field in Psoriasis Research. BioMed Res. Int. 2018, 2018, 7290913. [Google Scholar] [CrossRef]
- Cohen, S.; Miller, G.E.; Rabin, B.S. Psychological stress and antibody response to immunization: A critical review of the human literature. Psychosom. Med. 2001, 63, 7–18. [Google Scholar] [CrossRef]
- Shete, M.D.; Patil, D.B.; Karade, P.; Chopade, R.; Gandhi, N.; Alane, U. Assessment of age-related changes of salivary immunoglobulin a levels among healthy individuals. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. 1), S461. [Google Scholar] [CrossRef]
- Paszynska, U.E.; Dmitrzak-Weglarz, M.; Tyszkiewicz-Nwafor, M.; Slopien, A. Salivary alpha-amylase, secretory Iga and free cortisol as neurobiological components of the stress response in the acute phase of anorexia nervosa. World J. Biol. Psychiatry 2016, 17 (Suppl. 4), 266–273. [Google Scholar] [CrossRef]
- Nomura, S.; Handri, S.; Honda, H. Development of a bionanodevice for detecting stress levels. IOP Conf. Ser. Mater. Sci. Eng. 2011, 21, 012029. [Google Scholar] [CrossRef]
- Svobodová, I.; Chaloupková, H.; Koncel, R.; Bartos, L.; Hradecká, L.; Jebavý, L. Cortisol and secretory immunoglobulin a response to stress in german shepherd dogs. PLoS ONE 2014, 9, 90820. [Google Scholar] [CrossRef]
- Engeland, C.G.; Hugo, F.N.; Hilgert, J.B.; Nascimento, G.G.; Celeste, R.K.; Lim, H.-J.; Marucha, P.T.; Bosch, J.A. Psychological distress and salivary secretory immunity. Brain Behav. Immun. 2016, 52, 11–17. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Torres, G.; Levine, M.J. Salivary α-Amylase: Role in Dental Plaque and Caries Formation. Crit. Rev. Oral Biol. Med. 1993, 4, 301–307. [Google Scholar] [CrossRef]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of physiological stress on salivary cortisol and amylase levels in healthy young adults. Arch. Oral Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kammerer, M.; O’Reilly, R.; Taylor, A.; Glover, V. Salivary α-amylase stability, diurnal profile and lack of response to the cold hand test in young women. Stress 2009, 12, 549–554. [Google Scholar] [CrossRef]
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Otsuki, M.; Tagami, J. Age-related changes in salivary biomarkers. J. Dent. Sci. 2014, 9 (Suppl. 1), 85–90. [Google Scholar] [CrossRef]
- Strahler, J.; Mueller, A.; Rosenloecher, F.; Kirschbaum, C.; Rohleder, N. Salivary alpha-amylase stress reactivity across different age groups. Psychophysiology 2010, 47, 587–595. [Google Scholar] [CrossRef]
- Koh, D.; Ng, V.; Naing, L. Alpha Amylase as a Salivary Biomarker of Acute Stress of Venepuncture from Periodic Medical Examinations. Front. Public Health 2014, 2, 121. [Google Scholar] [CrossRef]
- Jafari, A.; Pouramir, M.; Shirzad, A.; Motallebnejad, M.; Bijani, A.; Moudi, S.; Abolghasem-Zade, F.; Dastan, Z. Evaluation of Salivary Alpha Amylase as a Biomarker for Dental Anxiety. Iran. J. Psychiatr. Behav. Sci. 2018, 12, 9350. [Google Scholar] [CrossRef]
- Skutnik-Radziszewska, A.; Maciejczyk, M.; Flisiak, I.; Krahel, J.; Kołodziej, U.; Kotowska-Rodziewicz, A.; Klimiuk, A.; Zalewska, A. Enhanced Inflammation and Nitrosative Stress in the Saliva and Plasma of Patients with Plaque Psoriasis. J. Clin. Med. 2020, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, S.M. Chemical analysis of parotid saliva and lacrimal fluid in psoriatics. Arch. Dermatol. Res. 1983, 275, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Soudan, R.A.; Daoud, S.A.; Mashlah, A.M. Study of some salivary changes in cutaneous psoriatic patients. Saudi Med. J. 2011, 32, 386–389. [Google Scholar] [PubMed]
- de Brouwer, S.J.; van Middendorp, H.; Stormink, C.; Kraaimaat, F.W.; Sweep, F.C.; de Jong, E.M.; Evers, A.W.M. The psychophysiological stress response in psoriasis and rheumatoid arthritis. Br. J. Dermatol. 2014, 170, 824–831. [Google Scholar] [CrossRef]
- Winkler, H.; Fischer-Colbrie, R. The chromogranins A and B: The first 25 years and future perspectives. Neuroscience 1992, 49, 497–528. [Google Scholar] [CrossRef]
- Kanno, T.; Asada, N.; Yanase, H.; Inagawa, T.; Yanaihara, N. Salivary secretion of chromogranin A. Control by autonomic nervous system. Adv. Exp. Med. Biol. 2000, 482, 143–151. [Google Scholar] [CrossRef]
- Nakane, H.; Asami, O.; Yamada, Y.; Harada, T.; Matsui, N.; Kanno, T.; Yanaihara, N. Salivary chromogranin A as an index of psychosomatic stress response. Biomed. Res. 1998, 19, 401–406. [Google Scholar] [CrossRef]
- Jantaratnotai, N.; Rungnapapaisarn, K.; Ratanachamnong, P.; Pachimsawat, P. Comparison of salivary cortisol, amylase, and chromogranin A diurnal profiles in healthy volunteers. Arch. Oral Biol. 2022, 142, 105516. [Google Scholar] [CrossRef]
- Nijakowski, K.; Rutkowski, R.; Eder, P.; Korybalska, K.; Witowski, J.; Surdacka, A. Changes in Salivary Parameters of Oral Immunity after Biologic Therapy for Inflammatory Bowel Disease. Life 2021, 11, 1409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foks-Ciekalska, A.; Jarząb, J.; Hadas, E.; Świętochowska, E.; Gumieniak, K.; Ciekalski, W.; Bożek, A. The Effect of Biological Treatment on Stress Parameters Determined in Saliva in Patients with Severe Psoriasis. Medicina 2023, 59, 692. https://doi.org/10.3390/medicina59040692
Foks-Ciekalska A, Jarząb J, Hadas E, Świętochowska E, Gumieniak K, Ciekalski W, Bożek A. The Effect of Biological Treatment on Stress Parameters Determined in Saliva in Patients with Severe Psoriasis. Medicina. 2023; 59(4):692. https://doi.org/10.3390/medicina59040692
Chicago/Turabian StyleFoks-Ciekalska, Aleksandra, Jerzy Jarząb, Ewa Hadas, Elżbieta Świętochowska, Kamila Gumieniak, Wiktor Ciekalski, and Andrzej Bożek. 2023. "The Effect of Biological Treatment on Stress Parameters Determined in Saliva in Patients with Severe Psoriasis" Medicina 59, no. 4: 692. https://doi.org/10.3390/medicina59040692
APA StyleFoks-Ciekalska, A., Jarząb, J., Hadas, E., Świętochowska, E., Gumieniak, K., Ciekalski, W., & Bożek, A. (2023). The Effect of Biological Treatment on Stress Parameters Determined in Saliva in Patients with Severe Psoriasis. Medicina, 59(4), 692. https://doi.org/10.3390/medicina59040692





