Abstract
Background and Objectives: The study of clinical pharmacokinetics of inhaled antivirals is particularly important as it helps one to understand the therapeutic efficacy of these drugs and how best to use them in the treatment of respiratory viral infections such as influenza and the current COVID-19 pandemic. The article presents a systematic review of the available pharmacokinetic data of inhaled antivirals in humans, which could be beneficial for clinicians in adjusting doses for diseased populations. Materials and Methods: This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines. A comprehensive literature search was conducted using multiple databases, and studies were screened by two independent reviewers to assess their eligibility. Data were extracted from the eligible studies and assessed for quality using appropriate tools. Results: This systematic review evaluated the pharmacokinetic parameters of inhaled antiviral drugs. The review analyzed 17 studies, which included Zanamivir, Laninamivir, and Ribavirin with 901 participants, and found that the non-compartmental approach was used in most studies for the pharmacokinetic analysis. The outcomes of most studies were to assess clinical pharmacokinetic parameters such as the Cmax, AUC, and t1/2 of inhaled antivirals. Conclusions: Overall, the studies found that the inhaled antiviral drugs were well tolerated and exhibited favorable pharmacokinetic profiles. The review provides valuable information on the use of these drugs for the treatment of influenza and other viral respiratory infections.
1. Introduction
Clinical pharmacokinetics studies are crucial in the context of inhaled antivirals. They clarify the therapeutic efficacy of these medications and the optimal ways to administer them to treat respiratory viral diseases such as influenza and the current COVID-19 [1,2]. Inhaled antivirals refer to a class of drugs that are delivered directly to the respiratory tract through inhalation, as opposed to being taken orally or intravenously [3]. This method of administration offers several advantages over other routes, including a faster onset of action, more direct and localized exposure to the site of infection, and lower systemic drug exposure and associated side effects [4]. Although there are certain advantages to inhaled medication administration, this route also has significant drawbacks, particularly when it comes to achieving accurate and repeatable doses [5]. The amount of medication that is delivered to the lungs and, consequently, the therapeutic effect can be influenced by a number of variables. These include the size of the drug particles, the shape of the inhaler device, patient technique, anatomy of the airway, pathophysiological effects of acute and chronic diseases, and environmental factors [6,7].
The pharmacokinetics of inhaled antivirals can be divided into three main phases: deposition, absorption, and elimination [8]. Deposition refers to the amount of drug that is delivered to the respiratory tract and deposited in the lungs. The optimal drug deposition site after inhalation varies based on the indication and the physical and chemical characteristics of the drug [9]. Absorption refers to the process by which the drug moves from the lungs into the site of action, where it can then exert its therapeutic effect [10]. The rapid absorption of small molecules within alveolar cells can make it challenging to measure plasma drug concentrations after inhalation. This sets the pharmacokinetic study of inhaled antimicrobials apart from traditional pharmacokinetic studies, as it requires a highly sensitive assay and frequent sampling over a short period to accurately determine the absorption rate [11]. The elimination of inhaled antivirals from the body can occur via multiple pathways, including mucociliary clearance, removal via alveolar macrophages, metabolism, excretion, penetration into systemic circulation, and exhalation. The specific pathways used for elimination may vary based on factors such as the specific antiviral, patient characteristics, and the disease state [12]. It is important to consider the pharmacokinetics of inhaled antivirals when determining the dosing regimen for these drugs. The goal is to achieve therapeutic concentrations of the drug in the respiratory tract, while minimizing systemic exposure and the associated side effects [13]. Understanding the interplay between drug delivery, absorption, and elimination is essential for optimizing dosing regimens and ensuring that patients receive the maximum therapeutic benefit from these drugs.
Inhaled antivirals offered several advantages over oral or intravenous formulations of these drugs during the COVID-19 pandemic [14]. First, inhaled drugs reach the site of infection more quickly, leading to a faster onset of action and improved therapeutic outcomes. Second, inhaled drugs are delivered directly to the respiratory tract, which minimizes systemic exposure and reduces the risk of side effects that may be associated with oral or intravenous formulations of these drugs [15]. In addition, inhaled antivirals have been shown to be effective in reducing the severity and duration of respiratory viral infections, as well as reducing the risk of hospitalization and death in some cases [16]. This is particularly important for individuals who are at high risk of complications from these infections, such as the elderly, young children, and individuals with underlying medical conditions [17]. Dry powder inhalers (DPIs), including the Diskhaler and Rotahaler, are significant drug delivery devices for inhaled antiviral drugs such as Zanamivir, Laninamivir, and Ribavirin. DPIs are particularly useful for delivering drugs to the lungs, where respiratory viral infections are most prevalent.
The main objective of this systematic review was to fill the gap in knowledge regarding the pharmacokinetics of inhaled antiviral drugs. While there is a significant amount of literature on the pharmacokinetics of systemically administered antivirals, there is a lack of comprehensive reviews on the pharmacokinetics of inhaled antivirals. The authors therefore aimed to systematically review the existing literature to provide a comprehensive overview of the pharmacokinetic aspects of inhaled antivirals in humans. To date, no systematic review has been conducted to determine the PK parameters of inhaled antivirals. This review endeavors to systematically gather, summarize, and analyze all of the available PK data of inhaled antivirals in the human population, which could be beneficial for clinicians in adjusting the doses in both healthy and diseased populations.
2. Materials and Methods
The methodology used for conducting this systematic review on the pharmacokinetics of inhaled antivirals in humans was the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.1. Identification of the Research Question
The research question was identified, which was clear, specific, and focused on the pharmacokinetics of inhaled antivirals in humans following the inhalation route of administration.
2.2. Literature Search
A comprehensive literature search was conducted using multiple databases, such as PubMed, Google Scholar, Science Direct, Embase, and the Cochrane Library, to identify relevant studies. The search was performed using relevant keywords and Medical Subject Headings (MeSH) terms. These databases were searched with the following key terms: ‘Inhaled antivirals’, ‘antivirals’, ‘Inhaled antimicrobial’, ‘Inhaled antibiotic’, ‘Respiratory Infections’, ‘clinical pharmacokinetic’, and ‘pharmacokinetic’ with “AND” or “OR”. An overview of the final search strategy with MeSH terms and text words for each of the four domains is described in Table 1.

Table 1.
Overview of final search strategy with MeSH terms and text words for each of the four domains (pharmacokinetics, antivirals, characteristics of patient, and type of population).
2.3. Inclusion and Exclusion Criteria
The inclusion criteria were clearly defined and included studies that examined the PK parameters of inhaled antivirals in humans following inhalation. The studies were limited to those conducted in healthy and diseased populations. Only peer-reviewed articles in the English language and at least one reported PK parameter that specified an inhaled antiviral were included. Both randomized control trials (RCTs) and observational studies were included. Moreover, there was no limitation on the publication year, whereas the studies that did not meet the inclusion criteria or those that did not provide PK data of inhaled antivirals were excluded.
2.4. Study Selection
The studies identified through the literature search were screened by two independent reviewers to assess their eligibility. Any disagreements were resolved through consensus or by a third reviewer. After evaluating all of the databases, studies were screened for duplication detection, which were then deleted, respectively. The articles were excluded after screening title and abstract. Review articles and book chapters were also excluded.
2.5. Data Extraction
Data were extracted from the eligible studies using a standardized data extraction form. The data included information on study reference, design, research objectives, outcome measures, spray device, model structure, population characteristics, sample size, drug name, dosing practice, and pharmacokinetic parameters, including plasma concentration time curve (AUC), peak concentration of antivirals in plasma (Cmax), half-life (T1/2), volume of distribution (Vd), and clearance (Cl). The author’s name with publication year and the number were also mentioned.
2.6. Quality Assessment
The quality of the eligible studies was assessed using appropriate tools, such as the Cochrane Risk of Bias tool for randomized controlled trials and the Newcastle–Ottawa Scale (NOS) for cohort studies.
2.7. Data Synthesis and Reporting
The extracted data were synthesized and analyzed to provide a comprehensive overview of the PK aspects of inhaled antivirals in humans following inhalation.
3. Results
3.1. Literature Search
A total of 1102 relevant published articles were identified from the gray literature and databases such as PubMED, ScienceDirect, Web of Science, and EMBASE. Of 1695 articles, 341 articles were screened after the exclusion of duplicates. On the basis of the exclusion and inclusion criteria, 1050 articles were excluded after the screening of the titles and abstracts of the articles. Seventy-eight of the included articles that did not contain any information regarding the pharmacokinetics of inhaled antiviral agents were excluded. After the screening of abstracts and full-text articles, 142 articles were excluded for the following reasons: non-English (N = 22), articles on other modes of administration (N = 45), inappropriate interventions (N = 34), review articles (N = 5), and animal studies (N = 36). Data extraction was performed for 17 full-text articles with data on the PKs of inhaled antivirals (Figure 1).
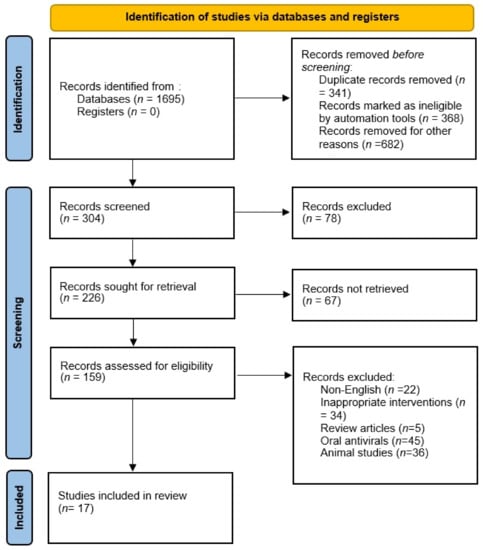
Figure 1.
Flow chart of included studies.
3.2. Characteristics of Studies
Seventeen articles met the inclusion criteria for this systematic review [8,10,13,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. The characteristics of selected articles are listed in Table 2. The majority of the articles studied the pharmacokinetic parameters of Zanamivir and Laninamivir. Of 17 articles, 11 studies were randomized controlled trials (RCTs) [8,13,18,19,20,21,22,23,24,25,26], followed by preliminary studies [27,28], open-label studies [10,29], prospective studies [30], and non-randomized studies [31]. The non-compartmental approach was used in 12 studies for the pharmacokinetic analysis. The outcomes of most of the studies were to assess the clinical pharmacokinetics parameters such as maximum concentration (Cmax), area under curve (AUC), and half-life (t1/2) of inhaled antivirals. A total of 901 patients participated in the studies. The majority of the studies used a Rotahaler and Diskhaler for the administration of the drug [13,19,25,30,31].

Table 2.
Main characteristics of studies on inhaled antiviral drugs.
3.3. Quality Assessment of the Studies
The quality of the studies was assessed and summarized in the Supplementary File (Tables S1 and S2). On the basis of the NOS, five articles were rated as 7 and one article scored 6. Overall, the quality score for prospective and preliminary studies was seven. For RCT, the Cochrane bias tool assessed that most of the domains were at low risk.
Pharmacokinetic parameters of inhaled antiviral drugs.
3.4. Zanamivir
In most of the studies, the Zanamivir was administered via Diskhaler (Table 3) [13,19,30,31]. Doses utilized in the studies ranged from 4 mg to 16 mg and were administered in either a single dose or multiple doses. However, Weller and his colleagues support the use of Rotahaler/Rotacap especially in an influenza pandemic [13]. The clearance of this drug was 49 L/h via Rotahaler while the clearance was 54 L/h via Diskhaler. Overall, the half-life ranged from 2–3 h when administered via Diskhaler [13,19,30]. In another study, single doses of 4, 8, and 16 mg and multiple doses of 16 mg BID on Day 1 followed by QID for 6 days of Zanamivir were well tolerated when administered via nebulizer and dry powder inhaler [18].

Table 3.
Pharmacokinetic parameters of inhaled antiviral drugs.
A dose of 10 mg of Zanamivir was well tolerated and safe in pediatric patients [8,30]. Zenamivir was administered twice daily for 5 days intranasally and via being inhaled orally, and the maximum concentration was achieved by 1.5 h after dosing [30]. However, another study reported that the systemic absorption of Zanamivir following oral inhalation or intranasal administration was low [8].
3.5. Laninamivir
The majority of the studies reported that the dose of 40 mg of prodrug CS-8958 was well tolerated and exhibited a PK profile, suggesting the potential for the parameters of Laninamivir and its prodrug CS-8958 in healthy participants and patients with comorbidities (Table 3) [20,21]. The maximum concentration of CS-8958 ranged from 12.8 to 433 ng/mL. Ishizuka and her colleagues reported that CS-8958 was well tolerated in patients with renal impairment [20]. The PK parameters such as AUC0-inf, Cmax, and time to Cmax of CS-8958 did not change with the degree of renal impairment; however, the t1/2 of CS-8958 gradually increased with increased renal insufficiency [20].
A study conducted in adults and pediatric patients reported that the volume of distribution of Laninamivir Octanoate (LO) and metabolic clearance of LO were altered with body weight [23]. For a single inhaled dose of 40 mg of LO, the Laninamivir amount was evaluated to be approximately 0.46 mg in the respiratory tract compartment at 1-week post-dose [29].
The concentrations of Laninamivir in the epithelial lining fluid (ELF) and bronchoalveolar lavage (BAL) fluid samples were assessed by Ishizuka and her colleagues to study the drug distribution in airways [10]. The ELF concentration profiles of Laninamivir showed the potential long-lasting effect for the treatment of patients with influenza virus infection [10].
3.6. Ribavirin
The studies reported that the utilization of aerosolized Ribavirin was well tolerated in healthy volunteers and the patient with other conditions (Table 3) [24,25,27,28]. Dumont and his colleagues developed dry powder particles using PRINT technology. The doses of 60 mg and 120 mg followed by 30 mg twice daily for 14 days were administered via Rotahaler to healthy volunteers and to those with chronic obstructive pulmonary disease (COPD). They concluded that PRINT formulation was an efficient and convenient mode of administration of the drug to the lungs while minimizing systemic exposure [25]. Moreover, a randomized, placebo-controlled study was performed to evaluate the safety and pharmacokinetics of inhaled Ribavirin [24]. The participants were recruited in four groups where they received different doses. Cohort 1 received 50 mg/mL Ribavirin/placebo (10 mL total volume); Cohort 2 received 50 mg/mL Ribavirin/placebo (20 mL total volume); Cohort 3 received 100 mg/mL Ribavirin/placebo (10 mL total volume); and Cohort 4 received 100 mg/mL Ribavirin/placebo (20 mL total volume). The mean maximum observed concentration (Cmax) and area under the curve (AUC) values were higher in Cohort 4, whereas Cohorts 2 and 3 showed similar PK values. The data support the development of Ribavirin as an empirical treatment option in patients with coronavirus.
In pediatric patients with suspected respiratory syncytial virus infection, patients received aerosolized Ribavirin of 60 mg/mL for 2 h periods TID for 3 days [27]. After the first dose, the mean peak Ribavirin level ranged from 1725 to 2179 mol/L in secretions and 3.8 mol/L in plasma. Ribavirin was rapidly cleared with a mean t1/2 of 1.9 h.
3.7. Rimantadine
The safety and pharmacokinetics of rimantadine were assessed by administering it via small-particle aerosol and oral inhalation in healthy volunteers and volunteers with acute influenza virus infection [26]. Rimantadine was delivered at a concentration of 20 µg/L every 4–12 h and 40 µg/L every 15 min to 4 h of air. The clearance of rimantadine ranged from 25.3 to 29.9 L/h and Vd ranged from 904 to 906 L. Some of the participants experienced nasal burning and irritation. This study concluded that a concentration of 20 µg/liter of air was well tolerated for up to 12 h by normal volunteers as well as in those with acute influenza virus infection.
4. Discussion
In this systematic review, we have investigated the pharmacokinetic parameters of inhaled antivirals. The non-compartmental model was used in the majority of the studies. Non-compartmental modeling is commonly used to study the pharmacokinetic (PK) parameters of drugs because it provides a simple and efficient way to analyze drug concentration–time data without making any assumptions about the underlying biological system [32]. This makes it particularly useful when the pharmacokinetics of a drug are not well understood or when the data are limited. The studies utilized dry powder inhalers (DPI) such as Diskhaler and Rotahaler for drug delivery. DPIs are used to deliver the drug directly to the lungs and are preferred for different types of drugs and inspiratory flow rates [33]. They are easy to use and have a lower environmental impact compared to other types of inhalers. Overall, drug powder inhalers can have a significant impact on the pharmacokinetic parameters of a drug, which can affect its overall effectiveness and safety. It is important to carefully consider the particle size and density of the drug powder when designing inhaler devices to ensure that medications are delivered effectively and efficiently to the lungs.
While emerging respiratory infectious diseases and associated morbidity and mortality have abated, they remain substantial threats to the public as well as scientific/medical communities [34,35]. Among highly contagious respiratory infections, influenza is responsible for significant morbidity and mortality worldwide [36]. Neuraminidase inhibitors such as oseltamivir, Zanamivir, and Laninamivir have been the mainstay of influenza antiviral treatment over the past few decades [37,38]. Zanamivir is a widely used drug as a therapeutic and prophylactic and is currently available in dry powder inhalation and IV formulation. The recommended dose for prophylaxis is 10 mg once daily for up to 28 days in adults and for pediatric patients aged above 5 years treatment is 10 mg twice daily for 5 days [39]. In a study, it was reported that about 12% of the dose is absorbed systemically after inhaled administration and reached Cmax within 2 h [19]. Approximately 78% and 13% of the drug Zanamivir was deposited in the oropharynx and the lungs when administered intranasally [18,40]. In these studies, Zanamivir was considered to be a well-tolerated antiviral drug administered intranasally by healthy volunteers and patients with influenza.
Furthermore, Laninamivir, a substituted compound of Zanamivir, is a therapeutic agent for the prophylaxis and treatment of viral infections [41,42]. Among the prodrugs of Laninamivir, Laninamivir Octanoate (LO), also known as CS-8958, is the most potent drug for the treatment of influenza available in only orally inhaled formulation, which delivers the drug directly to the respiratory tract [40]. Multisite studies have reported that LO has prophylactic as well as therapeutic efficacy against highly pathogenic H5N1 influenza viruses [43,44]. A single inhaled dose of 20 mg in pediatric patients <10 years old or 40 mg in adults and children >10 years old of LO was found to be more effective and safe over oseltamivir regimens concerning mean time to illness alleviation [45]. The pharmacokinetic profile of LO has been assessed in healthy adults, patients with renal insufficiency, elderly subjects, and patients with influenza infection [20,21,23]. Linear PKs of Laninamivir and its prodrug was observed across a wide range of doses (5–120 mg) by Yoshihara et al. [23]. The peak of plasma concentration was achieved after dosing and then declined with a half-life of approximately 2 h, whereas the peak of plasma Laninamivir concentration was achieved at approximately 4 h post-dose and declined with a half-life of approximately 3 days [21,23]. The findings documented that the single inhaled dose of LO was sufficient to treat influenza virus infection; moreover, it may show better compliance in patients with influenza infection and this feature may be favorable for prophylactic use.
Besides influenza virus infection, respiratory syncytial virus (RSV) is the most important cause of serious lower respiratory tract infections (LRTIs), especially in pediatric patients [46,47]. Ribavirin, an inosine monophosphate dehydrogenase inhibitor, is currently recommended for the treatment of LRTIs in hospitalized patients with RSV [48]. Aerosolized Ribavirin has shown significant clinical improvement in patients with RSV [49,50]. The PK results are promising, specifically in the context of coronavirus and intensive care settings where patients’ ability to swallow is compromised, and the delivery of a drug to the site of infection (respiratory tract) provides advantages over oral and intravenous formulations [51]. Ribavirin exhibits two rapid phases, i.e., absorption and distribution and a long terminal clearance phase [52,53].
Aerosolization provides the direct delivery of antiviral drugs to the site via the respiratory tract in a desirable amount to remove pathogenic organisms [54]. This method of drug administration usually results in a higher concentration of the drug at the site of infection than systematic administration does, and may minimize systemic toxicities [55]. Rimantadine has better antiviral activity against influenza A virus strains than amantadine. Hayden et al. have previously reported that the use of rimantadine delivered via an ultrasonic nebulizer in subjects with influenza A virus is well tolerated except for minor complaints, i.e., unpleasant smell or taste [56]. However, Atmar et al. reported that nasal burning or irritation was the most common side effect associated with rimantadine SPA [26]. About 45.6% of the dose of rimantadine reached the systemic circulation after SPA administration, and the mean peak levels in serum were 8.6-fold lower [57]. The studies found that Ribavirin was rapidly cleared, with a t1/2 of 1.9 h. SPA delivery of antiviral drugs has been shown to be effective in the treatment of other respiratory viral infections [26,58].
The novelty of this systematic review lies in its comprehensive and systematic approach to synthesizing the available literature on the clinical pharmacokinetics of inhaled antiviral drugs. The review provides a detailed and up-to-date summary of the pharmacokinetic parameters of various inhaled antiviral drugs, which can help to inform clinical decision-making and optimize the use of these drugs in the treatment of respiratory viral infections. Additionally, the review highlights the gaps in current knowledge and identifies areas for further research, which can guide future studies in this field. Overall, this systematic review tried to provide details of the inhaled antiviral agents, and offered a few inhaled antiviral drugs for the treatment of respiratory viral infections. A limited amount of data on the clinical pharmacokinetics of inhaled antiviral drugs is still a matter of concern. Several factors such as inspiratory flow rate, tidal volume, and presence and degree of airway obstruction can affect the concentration of antimicrobials [40]. However, the data on these factors are limited. Few studies are performed during the preclinical and clinical stages. It is hereby recommended that more studies are required for the further development of a novel aerosolized drug.
5. Conclusions
The clinical pharmacokinetics (PKs) of inhaled antivirals have been studied using non-compartmental models. Despite the aforementioned factors, clinical PK studies of inhaled antivirals have shown that they result in high concentrations in the respiratory tract, with relatively low systemic exposure, and reduce the risk of toxicity, which leads to the development of such advanced formulations and aids in modifying advanced aerosolization devices. Moreover, limited data were available on the pharmacokinetic parameters of existing inhaled drugs. Therefore, further studies, especially randomized controlled trials, are required to obtain a PK profile of inhaled antiviral drugs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/medicina59040642/s1, Table S1: Quality assessment of cohort studies; Table S2: Risk of bias assessment for randomized controlled trials.
Author Contributions
M.K.A., M.S.A., M.D.A., W.A.A., S.A.A., R.R.A., Z.H.A.K., T.J.A., A.S.A., H.S.A.D., A.F.A., F.N.A. and A.A.A. significantly contributed to this systematic review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stockmann, C.; Roberts, J.K.; Yellepeddi, V.K.; Sherwin, C.M.T. Clinical Pharmacokinetics of Inhaled Antimicrobials. Clin. Pharmacokinet. 2015, 54, 473–492. [Google Scholar] [CrossRef]
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011, 377, 1032–1045. [Google Scholar] [CrossRef]
- Hickey, A.J. Emerging trends in inhaled drug delivery. Adv. Drug Deliv. Rev. 2020, 157, 63–70. [Google Scholar] [CrossRef]
- Anderson, S.; Atkins, P.; Bäckman, P.; Cipolla, D.; Clark, A.; Daviskas, E.; Disse, B.; Entcheva-Dimitrov, P.; Fuller, R.; Gonda, I.; et al. Inhaled Medicines: Past, Present, and Future. Pharmacol. Rev. 2022, 74, 48–118. [Google Scholar] [CrossRef]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- Ibrahim, M.; Verma, R.; Garcia-Contreras, L. Inhalation drug delivery devices: Technology update. Med. Devices 2015, 8, 131–139. [Google Scholar]
- Heyder, J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc. Am. Thorac. Soc. 2004, 1, 315–320. [Google Scholar] [CrossRef]
- Peng, A.W.; Hussey, E.K.; Moore, K.H.P.; Peng, A.W. A Population Pharmacokinetic Analysis of Zanamivir in Subjects with Experimental and Naturally Occurring Influenza: Effects of Formulation and Route of Administration. J. Clin. Pharmacol. 2000, 40, 242–249. [Google Scholar] [CrossRef]
- Groneberg, D.; Witt, C.; Wagner, U.; Chung, K.; Fischer, A. Fundamentals of pulmonary drug delivery. Respir. Med. 2003, 97, 382–387. [Google Scholar] [CrossRef]
- Ishizuka, H.; Toyama, K.; Yoshiba, S.; Okabe, H.; Furuie, H. Intrapulmonary Distribution and Pharmacokinetics of Laninamivir, a Neuraminidase Inhibitor, after a Single Inhaled Administration of Its Prodrug, Laninamivir Octanoate, in Healthy Volunteers. Antimicrob. Agents Chemother. 2012, 56, 3873–3878. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 600–612. [Google Scholar] [CrossRef]
- Ibrahim, M.; Garcia-Contreras, L. Mechanisms of absorption and elimination of drugs administered by inhalation. Ther. Deliv. 2013, 4, 1027–1045. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.; Jones, L.S.; Lou, Y.; Piscitelli, S.; Peppercorn, A.; Ng-Cashin, J. Safety, Tolerability and Pharmacokinetics of Orally Inhaled Zanamivir: A Randomized Study Comparing Rotacap/Rotahaler and Rotadisk/Diskhaler in Healthy Adults; SAGE Publications: London, UK, 2013. [Google Scholar]
- Sahin, G.; Akbal-Dagistan, O.; Culha, M.; Erturk, A.; Basarir, N.S.; Sancar, S.; Yildiz-Pekoz, A. Antivirals and the Potential Benefits of Orally Inhaled Drug Administration in COVID-19 Treatment. J. Pharm. Sci. 2022, 111, 2652–2661. [Google Scholar] [CrossRef]
- Bodier-Montagutelli, E.; Mayor, A.; Vecellio, L.; Respaud, R.; Heuzé-Vourc’h, N. Designing inhaled protein therapeutics for topical lung delivery: What are the next steps? Expert Opin. Drug Deliv. 2018, 15, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yuan, S.; Cao, J.; Tang, K.; Qiu, Y.; Seow, H.C.; Man, R.C.H.; Shao, Z.; Huang, Y.; Liang, R.; et al. Inhaled Dry Powder Formulation of Tamibarotene, a Broad-Spectrum Antiviral against Respiratory Viruses Including SARS-CoV-2 and Influenza Virus. Adv. Ther. 2021, 4, 2100059. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Tawfeek, H.M.; Abdelfattah, A.; Batiha, G.E.-S.; Hetta, H.F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: Nanostructured and targeting systems. J. Drug Deliv. Sci. Technol. 2021, 63, 102435. [Google Scholar] [CrossRef] [PubMed]
- Cass, L.M.; Efthymiopoulos, C.; Bye, A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin. Pharmacokinet. 1999, 36, 1–11. [Google Scholar] [CrossRef]
- Cass, L.M.R.; Brown, J.; Pickford, M.; Fayinka, S.; Newman, S.P.; Johansson, C.J.; Bye, A. Pharmacoscintigraphic Evaluation of Lung Deposition of Inhaled Zanamivir in Healthy Volunteers. Clin. Pharmacokinet. 1999, 36, 21–31. [Google Scholar] [CrossRef]
- Ishizuka, H.; Yoshiba, S.; Yoshihara, K.; Okabe, H. Assessment of the Effects of Renal Impairment on the Pharmacokinetic Profile of Laninamivir, a Novel Neuraminidase Inhibitor, After a Single Inhaled Dose of Its Prodrug, CS-8958. J. Clin. Pharmacol. 2011, 51, 243–251. [Google Scholar] [CrossRef]
- Ishizuka, H.; Yoshiba, S.; Okabe, H.; Yoshihara, K. Clinical Pharmacokinetics of Laninamivir, a Novel Long-Acting Neuraminidase Inhibitor, After Single and Multiple Inhaled Doses of Its Prodrug, CS-8958, in Healthy Male Volunteers. J. Clin. Pharmacol. 2010, 50, 1319–1329. [Google Scholar] [CrossRef]
- Yoshiba, S.; Okabe, H.; Ishizuka, H. Pharmacokinetics of laninamivir after a single administration of its prodrug, laninamivir octanoate, a long-acting neuraminidase inhibitor, using an easy-to-use inhaler in healthy volunteers. J. Bioequiv. Availab. 2011, 3, 001–004. [Google Scholar] [CrossRef]
- Yoshihara, K.; Ishizuka, H.; Kubo, Y. Population Pharmacokinetics of Laninamivir and Its Prodrug Laninamivir Octanoate in Healthy Subjects and in Adult and Pediatric Patients with Influenza Virus Infection. Drug Metab. Pharmacokinet. 2013, 28, 416–426. [Google Scholar] [CrossRef]
- Couroux, P.; Brkovic, A.; Vittitow, J.L.; Israel, R.J.; Pamidi, C.; Patel, J.; Barakat, M. A randomized, placebo-controlled study to evaluate safety and pharmacokinetics of inhaled ribavirin. Clin. Transl. Sci. 2022, 15, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.F.; Oliver, A.J.; Ioannou, C.; Billiard, J.; Dennison, J.; Van Den Berg, F.; Yang, S.; Chandrasekaran, V.; Young, G.C.; Lahiry, A.; et al. A Novel Inhaled Dry-Powder Formulation of Ribavirin Allows for Efficient Lung Delivery in Healthy Participants and Those with Chronic Obstructive Pulmonary Disease in a Phase 1 Study. Antimicrob. Agents Chemother. 2020, 64, e02267-19. [Google Scholar] [CrossRef]
- Atmar, R.L.; Greenberg, S.B.; Quarles, J.M.; Wilson, S.Z.; Tyler, B.; Feldman, S.; Couch, R.B. Safety and pharmacokinetics of rimantadine small-particle aerosol. Antimicrob. Agents Chemother. 1990, 34, 2228–2233. [Google Scholar] [CrossRef]
- Englund, J.A.; Piedra, P.A.; Jefferson, L.S.; Wilson, S.Z.; Taber, L.H.; Gilbert, B.E. High-dose, short-duration ribavirin aerosol therapy in children with suspected respiratory syncytial virus infection. J. Pediatr. 1990, 117, 313–320. [Google Scholar] [CrossRef]
- Linn, W.S.; Gong, H.; Anderson, K.R.; Clark, K.W.; Shamoo, D.A. Exposures of Health-Care Workers to Ribavirin Aerosol: A Pharmacokinetic Study. Arch. Environ. Health Int. J. 1995, 50, 445–451. [Google Scholar] [CrossRef]
- Toyama, K.; Furuie, H.; Ishizuka, H. Safety and Pharmacokinetics of Nebulized Laninamivir Octanoate, A Long Acting Neuraminidase Inhibitor, In Healthy Subjects. Clin. Ther. 2017, 39, e25–e26. [Google Scholar] [CrossRef]
- Peng, A.W.; Hussey, E.K.; Rosolowski, B.; Blumer, J.L. Pharmacokinetics and tolerability of a single inhaled dose of zanamivir in children. Curr. Ther. Res. 2000, 61, 36–46. [Google Scholar] [CrossRef]
- Shelton, M.J.; Lovern, M.; Ng-Cashin, J.; Jones, L.; Gould, E.; Gauvin, J.; Rodvold, K.A. Zanamivir Pharmacokinetics and Pulmonary Penetration into Epithelial Lining Fluid following Intravenous or Oral Inhaled Administration to Healthy Adult Subjects. Antimicrob. Agents Chemother. 2011, 55, 5178–5184. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-H.; Cao, Y.-X. A method to determine pharmacokinetic parameters based on andante constant-rate intravenous infusion. Sci. Rep. 2017, 7, 13279. [Google Scholar] [CrossRef] [PubMed]
- Berkenfeld, K.; Lamprecht, A.; McConville, J.T. Devices for Dry Powder Drug Delivery to the Lung. AAPS PharmSciTech 2015, 16, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.T.; Bryan, A. Emerging respiratory infections: The infectious disease pathology of SARS, MERS, pandemic influenza, and Legionella. Semin. Diagn. Pathol. 2019, 36, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Clayville, L.R. Influenza update: A review of currently available vaccines. P T A Peer-Rev. J. Formul. Manag. 2011, 36, 659–684. [Google Scholar]
- Wang, K.; Shun-Shin, M.; Gill, P.; Perera, R.; Harnden, A. Neuraminidase inhibitors for preventing and treating influenza in children (published trials only). Cochrane Database Syst. Rev. 2012, 2012, CD002744. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, E.J.; Hayden, F.G.; Hurt, A.C. Antivirals targeting the polymerase complex of influenza viruses. Antivir. Res. 2019, 169, 104545. [Google Scholar] [CrossRef]
- Świerczyńska, M.; Mirowska-Guzel, D.M.; Pindelska, E. Antiviral Drugs in Influenza. Int. J. Environ. Res. Public Health 2022, 19, 3018. [Google Scholar] [CrossRef]
- Chairat, K.; Tarning, J.; White, N.J.; Lindegardh, N. Pharmacokinetic Properties ofAnti-Influenza Neuraminidase Inhibitors. J. Clin. Pharmacol. 2013, 53, 119–139. [Google Scholar] [CrossRef]
- Feng, E.; Ye, D.; Li, J.; Zhang, D.; Wang, J.; Zhao, F.; Hilgenfeld, R.; Zheng, M.; Jiang, H.; Liu, H. Recent Advances in Neuraminidase Inhibitor Development as Anti-influenza Drugs. Chemmedchem 2012, 7, 1527–1536. [Google Scholar] [CrossRef]
- Yamashita, M. Laninamivir and its Prodrug, CS-8958: Long-Acting Neuraminidase Inhibitors for the Treatment of Influenza. Antivir. Chem. Chemother. 2010, 21, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Sriwilaijaroen, N.; Vavricka, C.J.; Kiyota, H.; Suzuki, Y. Influenza A Virus Neuraminidase Inhibitors. In Glycovirology: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 321–353. [Google Scholar]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef]
- Zaraket, H.; Saito, R. Japanese Surveillance Systems and Treatment for Influenza. Curr. Treat. Options Infect. Dis. 2016, 8, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Pavia, A.T. Viral Infections of the Lower Respiratory Tract: Old Viruses, New Viruses, and the Role of Diagnosis. Clin. Infect. Dis. 2011, 52 (Suppl. S4), S284–S289. [Google Scholar] [CrossRef] [PubMed]
- Azzari, C.; Baraldi, E.; Bonanni, P.; Bozzola, E.; Coscia, A.; Lanari, M.; Manzoni, P.; Mazzone, T.; Sandri, F.; Lisi, G.C.; et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital. J. Pediatr. 2021, 47, 198. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.L.; Kopp, B.T.; Paul, G.; Landgrave, L.C.; Hayes, N.; Thompson, R. Respiratory syncytial virus: Current and emerging treatment options. Clin. Outcomes Res. 2014, 6, 217–225. [Google Scholar] [CrossRef]
- Rodriguez, W.J.; Hall, C.B.; Welliver, R.; Simoes, E.A.; Ryan, M.E.; Stutman, H.; Johnson, G.; Van Dyke, R.; Groothuis, J.R.; Arrobio, J.; et al. Efficacy and safety of aerosolized ribavirin in young children hospitalized with influenza: A double-blind, multicenter, placebo-controlled trial. J. Pediatr. 1994, 125, 129–135. [Google Scholar] [CrossRef]
- Englund, J.A. Antiviral therapy of influenza. Semin. Pediatr. Infect. Dis. 2002, 13, 120–128. [Google Scholar] [CrossRef]
- Venisse, N.; Peytavin, G.; Bouchet, S.; Gagnieu, M.C.; Garraffo, R.; Guilhaumou, R.; Solas, C.; Monitoring, S.T.D. ANRS-AC43 Clinical Pharmacology Committee Concerns about pharmacokinetic (PK) and pharmacokinetic-pharmacodynamic (PK-PD) studies in the new therapeutic area of COVID-19 infection. Antivir. Res. 2020, 181, 104866. [Google Scholar] [CrossRef]
- Nyström, K.; Waldenström, J.; Tang, K.-W.; Lagging, M. Ribavirin: Pharmacology, multiple modes of action and possible future perspectives. Future Virol. 2019, 14, 153–160. [Google Scholar] [CrossRef]
- Glue, P.; Schenker, S.; Gupta, S.; Clement, R.P.; Zambas, D.; Salfi, M. The single dose pharmacokinetics of ribavirin in subjects with chronic liver disease. Br. J. Clin. Pharmacol. 2000, 49, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Nainwal, N. Treatment of respiratory viral infections through inhalation therapeutics: Challenges and opportunities. Pulm. Pharmacol. Ther. 2022, 77, 102170. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Keyt, H.; Reyes, L.F. Aerosolized Antibiotics. Respir. Care 2015, 60, 762–773. [Google Scholar] [CrossRef]
- Hayden, F.G.; Zylidnikov, D.; Iljenko, V.; Padolka, Y. Comparative therapeutic effect of aerosolized and oral rimantadine HC1 in experimental human influenza A virus infection. Antivir. Res. 1982, 2, 147–153. [Google Scholar] [CrossRef]
- Hayden, F.G.; Minocha, A.; A Spyker, D.; E Hoffman, H. Comparative single-dose pharmacokinetics of amantadine hydrochloride and rimantadine hydrochloride in young and elderly adults. Antimicrob. Agents Chemother. 1985, 28, 216–221. [Google Scholar] [CrossRef]
- Velkov, T.; Abdul Rahim, N.; Zhou, Q.T.; Chan, H.K.; Li, J. Inhaled anti-infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead. Adv. Drug Deliv. Rev. 2014, 85, 65–82. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).