Association between Liver Stiffness and Liver-Related Events in HCV-Infected Patients after Successful Treatment with Direct-Acting Antivirals
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. HCV Treatment
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Cohort
3.2. Liver Stiffness at Baseline and at 24, 48, 72, and 96 Weeks after SVR
3.3. Liver Steatosis at Baseline and at 24, 48, 72, and 96 Weeks after SVR
3.4. FIB-4 Index at Baseline and 48 Weeks after SVR
3.5. Liver-Related Events after SVR with DAA Therapy
3.6. Factors Associated with Liver-Related Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 2011, 17, 107–115. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hepatitis C. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 7 July 2022).
- Khatun, M.; Ratna, B.R. Mechanisms Underlying Hepatitis C Virus-Associated Hepatic Fibrosis. Cells 2019, 8, 1249. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.L.; Perumpail, R.B.; Ahmed, A. Update on hepatitis C: Direct-acting antivirals. World J. Hepatol. 2015, 7, 2829–2833. [Google Scholar] [CrossRef] [PubMed]
- Baumert, T.F.; Berg, T.; Lim, J.K.; Nelson, D.R. Status of Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection and Remaining Challenges. Gastroenterology 2019, 156, 431–445. [Google Scholar] [CrossRef]
- Kang, Q.; Xu, J.; Luo, H.; Tan, N.; Chen, H.; Cheng, R.; Pan, J.; Han, Y.; Yang, Y.; Liu, D.; et al. Direct antiviral agent treatment leads to rapid and significant fibrosis regression after HCV eradication. J. Viral Hepat. 2021, 28, 1284–1292. [Google Scholar] [CrossRef]
- Kobayashi, N.; Iijima, H.; Tada, T.; Kumada, T.; Yoshida, M.; Aoki, T.; Nishimura, T.; Nakano, C.; Takata, R.; Yoh, K.; et al. Changes in liver stiffness and steatosis among patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. Eur. J. Gastroenterol. Hepatol. 2018, 30, 546–551. [Google Scholar] [CrossRef]
- Van der Meer, A.J.; Veldt, B.J.; Feld, J.J.; Wedemeyer, H. Association Between Sustained Virological Response and All-Cause Mortality Among Patients with Chronic Hepatitis C and Advanced Hepatic Fibrosis. J. Am. Med. Assoc. 2012, 308, 2584–2593. [Google Scholar] [CrossRef]
- Pietsch, V.; Deterding, K.; Attia, D.; Ringe, K.I.; Heidrich, B.; Cornberg, M.; Gebel, M.; Manns, M.P.; Wedemeyer, H.; Potthoff, A. Long-term changes in liver elasticity in hepatitis C virus-infected patients with sustained virologic response after treatment with direct-acting antivirals. United Eur. Gastroenterol. J. 2018, 6, 1188–1198. [Google Scholar] [CrossRef]
- Shiha, G.; Soliman, R.; Hassan, A.A.; Mikhail, N.N.H. Changes in hepatic fibrosis and incidence of HCC following direct-acting antiviral treatment of F3 chronic hepatitis c patients: A prospective observational study. Hepatoma Res. 2022, 8, 29. [Google Scholar] [CrossRef]
- Tachi, Y.; Hirai, T.; Miyata, A.; Ohara, K.; Iida, T.; Ishizu, Y.; Honda, T.; Kuzuya, T.; Hayashi, K.; Ishigami, M.; et al. Progressive fibrosis significantly correlates with hepatocellular carcinoma in patients with a sustained virological response. Hepatol. Res. 2015, 45, 238–246. [Google Scholar] [CrossRef]
- Pons, M.; Rodríguez-Tajes, S.; Esteban, J.I.; Mariño, Z.; Vargas, V.; Lens, S.; Buti, M.; Augustin, S.; Forns, X.; Mínguez, B.; et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J. Hepatol. 2020, 72, 472–480. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2018, 68, 25–32. [Google Scholar] [CrossRef]
- Bedossa, P.; Dargère, D.; Paradis, V. Sampling Variability of Liver Fibrosis in Chronic Hepatitis C. Hepatology 2003, 38, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Tse, Y.K.; Wong, G.L.H.; Ha, Y.; Lee, A.U.; Ngu, M.C.; Chan, H.L.-Y.; Wong, V.W.-S. Systematic review with meta-analysis: Non-invasive assessment of non-alcoholic fatty liver disease—The role of transient elastography and plasma cytokeratin-18 fragments. Aliment. Pharmacol. Ther. 2014, 39, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Morgan, T.R. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Castera, L.; Forns, X.; Alberti, A. Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 2008, 48, 835–847. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hu, T.H.; Hung, C.H.; Lu, S.N.; Chen, C.H.; Wang, J.H. The change in liver stiffness, controlled attenuation parameter and fibrosis-4 index for chronic hepatitis C patients with direct-acting antivirals. PLoS ONE 2019, 14, e0214323. [Google Scholar] [CrossRef]
- Singh, S.; Facciorusso, A.; Loomba, R.; Falck-Ytter, Y.T. Magnitude and Kinetics of Decrease in Liver Stiffness After Anti-viral Therapy in Patients With Chronic Hepatitis C: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 16, 27–38.e4. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, A.; Alem, S.A.; Fouad, R.; El Raziky, M.; El Akel, W.; Abdo, M.; Tantawi, O.; AbdAllah, M.; Bourliere, M.; Esmat, G. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J. Gastroenterol. Hepatol. 2017, 32, 1624–1630. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [PubMed]
- Aleman, S.; Rahbin, N.; Weiland, O.; Davidsdottir, L.; Hedenstierna, M.; Rose, N.; Verbaan, H.; Stal, P.; Carlsson, T.; Norrgren, H.; et al. A risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin. Infect. Dis. 2013, 57, 230–236. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Aghemo, A.; Fraquelli, M.; Rumi, M.G.; Donato, M.F.; Paradis, V.; Bedossa, P.; Colombo, M. The diagnostic accuracy of Fibroscan® for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J. Hepatol. 2013, 59, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mauro, E.; Crespo, G.; Montironi, C.; Londoño, M.C.; Hernández-Gea, V.; Ruiz, P.; Sastre, L.; Lombardo, J.; Mariño, Z.; Díaz, A.; et al. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology 2018, 67, 1683–1694. [Google Scholar] [CrossRef]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef]
- Ravaioli, F.; Conti, F.; Brillanti, S.; Andreone, P.; Mazzella, G.; Buonfiglioli, F.; Serio, I.; Verrucchi, G.; Reggiani, M.L.B.; Colli, A.; et al. Hepatocellular carcinoma risk assessment by the measurement of liver stiffness variations in HCV cirrhotics treated with direct acting antivirals. Dig. Liver Dis. 2018, 50, 573–579. [Google Scholar] [CrossRef]
- Degasperi, E.; Ambrosio, R.D.; Iavarone, M.; Sangiovanni, A.; Aghemo, A.; Soffredini, R.; Borghi, M.; Lunghi, G.; Colombo, M.; Lampertico, P. Factors Associated With Increased Risk of De Novo or Recurrent Hepatocellular Carcinoma in Patients With Cirrhosis Treated With Direct-Acting Antivirals for HCV Infection. Clin. Gastroenterol. Hepatol. 2019, 17, 1183–1191.e7. [Google Scholar] [CrossRef]
- Morisco, F.; Federico, A.; Marignani, M.; Cannavò, M.; Pontillo, G.; Guarino, M.; Dallio, M.; Begini, P.; Benigno, R.G.; Lambardo, F.L.; et al. Risk factors for liver decompensation and hcc in hcv-cirrhotic patients after daas: A multicenter prospective study. Cancers 2021, 13, 3810. [Google Scholar] [CrossRef] [PubMed]
- Noureddin, M.; Wong, M.M.; Todo, A.T.; Lu, S.C.; Sanyal, A.J.; Mena, E.A. Fatty liver in hepatitis C patients post-sustained virological response with direct-acting antivirals. World J. Gastroenterol. 2018, 24, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A. Small studies: Strengths and limitations. Eur. Respir. J. 2008, 32, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total n = 185 | Characteristics | Total n = 185 |
|---|---|---|---|
| Age (years) * | 56.1 ± 10.9 | HCV treatment status | |
| Male, n (%) | 115 (62.2%) | Naïve HCV treatment, n (%) | 130 (70.3%) |
| BMI (kg/m2) * | 24.5 ± 3.9 | Experienced treatment, n (%) | |
| Cirrhosis, n (%) | 80 (43.2%) | PEG-IFN/RBV | 52 (28.1%) |
| Comorbidity, n (%) | SOF-based regimen | 3 (1.6%) | |
| None | 88 (47.6%) | HCV-related variables | |
| Hypertension | 41 (22.2%) | HCV RNA (logIU/mL) * | 5.9 ± 0.9 |
| Dyslipidemia | 13 (7.0%) | HCV genotype, n (%) | |
| Diabetes mellitus | 35 (18.9%) | Genotype 1 | 100 (54.0%) |
| Chronic kidney disease | 6 (3.2%) | Genotype 3 | 63 (34.0%) |
| Cardiovascular disease | 7 (3.8%) | Genotype 6 | 22 (11.9%) |
| Cerebrovascular disease | 3 (1.6%) | DAA regimen, n (%) † | |
| Chronic respiratory disease | 2 (1.1%) | SOF/DAC | 35 (18.9%) |
| Others | 44 (23.8%) | SOF/DAC/RBV | 53 (28.7%) |
| Baseline laboratory results | SOF/LED | 37 (20.0%) | |
| Hemoglobin (g/dL) * | 13.5 ± 1.9 | SOF/LED/RBV | 26 (14.1%) |
| White cell count (cell/mm3) * | 6297 ± 2193 | SOF/VEL | 12 (6.5%) |
| Platelet count (×100/mm3) * | 193 ± 87 | SOF/VEL/RBV | 6 (3.2%) |
| INR * | 1.10 ± 0.15 | SOF/IFN/RBV | 11 (6.0%) |
| Alanine aminotransferase | 70 (43–115) | Elbasvir/Grazoprevir | 4 (2.2%) |
| (IU/L), median (IQR) | Duration of DAA regimen, n (%) | ||
| Aspartate aminotransferase | 56 (37–97) | 12 weeks | 175 (94.6%) |
| (IU/L), median (IQR) † | 16 weeks | 1 (0.5%) | |
| Serum albumin (g/dL) * | 4.0 ± 0.4 | 24 weeks | 9 (4.9%) |
| Total bilirubin (mg/dL) * | 1.0 ± 0.7 | Baseline LS (kPa), median (IQR) | 11.8 (7.4–21.2) |
| Serum creatinine (mg/dL) * | 0.8 ± 0.4 | Baseline CAP (dB/m), median (IQR) | 230 (204–259) |
| Baseline FIB-4 index, median (IQR) † | 2.08 (1.21–4.0) |
| Time Point | Liver Stiffness Measurement | Liver Steatosis Measurement | ||||
|---|---|---|---|---|---|---|
| n | Value (kPa), Median (IQR) | p * | n | Value (dB/m), Median (IQR) | p * | |
| Baseline vs. 24 weeks after SVR | 142 | 12 (7.8–21.5) vs. 8.5 (5.4–14.5) | <0.001 | 136 | 229 (200–259) vs. 239 (201–267) | 0.410 |
| Baseline vs. 48 weeks after SVR | 34 | 8.5 (7.0–15.4) vs. 5.8 (4.8–10.5) | <0.001 | 32 | 222 (208–260) vs. 240 (202–266) | 0.396 |
| Baseline vs. 72 weeks after SVR | 41 | 10.6 (7.3–21.3) vs. 6.1 (5.2–11.1) | <0.001 | 36 | 227 (215–267) vs. 244 (211–275) | 0.818 |
| Baseline vs. 96 weeks after SVR | 48 | 10.8 (7.3–18.1) vs. 6.6 (4.8–12.0) | <0.001 | 36 | 224 (199–253) vs. 228 (206–254) | 0.424 |
| Outcome | Number of Events | Observational Period in Person-Years | Rate per 100 Person-Years |
|---|---|---|---|
| Any event * | 12 | 508 | 2.36 |
| Hepatocellular carcinoma | 6 | 515 | 1.17 |
| Portal hypertension-related liver decompensation (ascites, encephalopathy) | 2 | 519 | 0.39 |
| Listed for liver transplantation | 1 | 528 | 0.19 |
| Liver-related mortality | 0 | 528 | 0.00 |
| All-cause mortality | 5 | 528 | 0.95 |
| Number | Age | Sex | Presence of Cirrhosis before Treatment | Liver Stiffness at Baseline (kPa) | Liver Stiffness at 48 Weeks after SVR (kPa) | Time to Develop HCC after SVR |
|---|---|---|---|---|---|---|
| 1 | 69 | Female | Yes | 21.8 | 19.4 | 10 months |
| 2 | 66 | Female | No | 18.8 | 7.6 | 19 months |
| 3 | 44 | Male | Yes | 17.5 | 15.4 | 29 months |
| 4 | 57 | Female | Yes | 34.3 | 23.5 | 24 months |
| 5 | 52 | Male | Yes | 35.3 | 21.8 | 20 months |
| 6 | 83 | Male | Yes | 10.2 | 14.1 | 17 months |
| Variables | HR (95%CI) | p | |
|---|---|---|---|
| Age | 1.08 (1.02–1.15) | 0.008 | |
| Male sex | 0.90 (0.28–2.83) | 0.085 | |
| Body mass index | 1.04 (0.90–1.21) | 0.559 | |
| HCV Genotype | Genotype 3 Non-Genotype 3 | 0.96 (0.29–3.19) reference | 0.944 |
| History of HCV treatment | Experienced Naïve treatment | 1.24 (0.39–3.91) reference | 0.715 |
| Cirrhosis status before treatment | Yes No | 1.76 (0.56–5.56) reference | 0.332 |
| DAA treatment | RBV-containing regimen RBV-free regimen | 0.45 (0.48–5.29) reference | 0.452 |
| Liver stiffness at baseline | ≥16 kPa <16 kPa | 1.71 (0.55–5.30) reference | 0.353 |
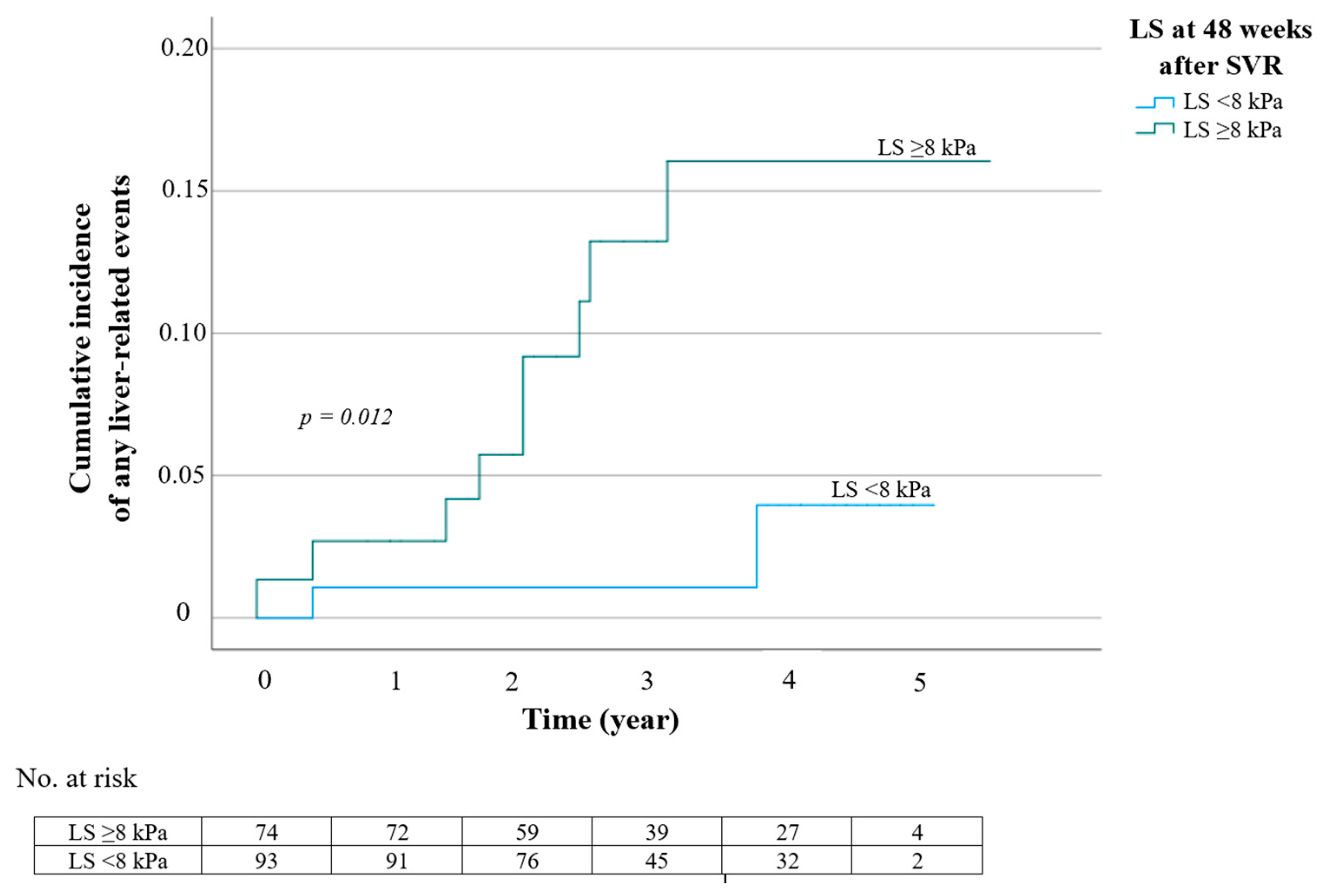
| Liver stiffness at 48 weeks after SVR * | ≥8 kPa <8 kPa | 5.68 (1.23–26.31) reference | 0.026 |
| Decreased liver stiffness value from baseline to 48 weeks after SVR | ≤3 kPa >3 kPa | 2.98 (0.79–11.24) reference | 0.107 |
| Baseline FIB-4 index at baseline | ≥3.50 <3.50 | 2.46 (0.76–7.99) reference | 0.134 |
| FIB-4 index at 48 weeks after SVR | ≥1.35 <1.35 | 5.18 (1.10–24.32) reference | 0.037 |
| Decreased FIB-4 index from baseline to 48 weeks after SVR | ≤1.30 >1.30 | 1.88 (0.58–6.09) reference | 0.291 |
| Baseline CAP | ≥230 dB/m <230 dB/m | 0.33 (0.09–1.21) reference | 0.095 |
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Adjusted HR (95%CI) | p | Adjusted HR (95%CI) | p | Adjusted HR (95%CI) | p | |
| Age | 1.07 (1.00–1.14) | 0.025 | 1.06 (0.99–1.13) | 0.110 | 1.06 (0.99–1.13) | 0.120 |
| Male sex | 1.32 (0.39–4.53) | 0.655 | 1.23 (0.36–4.22) | 0.74 | 1.47 (0.39–5.60) | 0.569 |
| Liver stiffness at 48 weeks after SVR ≥8 kPa <8 kPa | 5.06 (1.09–23.42) reference | 0.038 | 5.04 (1.01–25.26) reference | 0.049 | ||
| FIB-4 index at 48 weeks after SVR≥1.35 <1.35 | 3.08 (0.56–16.97) reference | 0.198 | 1.91 (0.33–11.10) reference | 0.468 | ||
| Study, Year | Enrollment Year (N) | Treatment and Duration | Baseline TE, Median (IQR), kPa | TE post-SVR, Median (IQR), kPa | Follow-up Duration | Incidence of HCC Post-SVR |
|---|---|---|---|---|---|---|
| Conti, 2016 [29] | 2015 (344) | SOF/SMV, SOF/RBV SOF/DAC, SOF/LED DAC/SIM, 3D Duration—NA | 23.6 ± 10.4 (mean ± SEM) | mean TE ± SEM was 23.1 ± 0.8 vs. 28.1 ± 2.5 in patients without vs. with HCC | 24 weeks | 17/344 (4.94%) Follow-up LS > 21.3 kPa was associated with 4.2-fold increased HCC risk |
| Kanwal, 2017 [25] | 2015 (22, 500) | SOF/LED, SIM PAR/Ritonavir, DAC Duration—NA | NA | NA | NA | 0.90/100 patient-years |
| Ravaioli, 2018 [30] | 2015–2016 (139) | SOF, SOF/DAC SOF/SMV, SOF/LDV PAR/r/OBV+DAS PAR/r/OBV 12 or 24 weeks | 18.6 (15–26.3) | EOT: 13.8 (10.4–20.4) | 15 months | 20/139 (14.4%) |
| Degasperi, 2019 [31] | 2014–2016 (565) | SOF/LED, SOF/RBV SOF/DAC, SOF/SIM O/P/R/DAS, O/P/R 12 weeks (56%), 24 weeks (36%), 16–20 weeks (8%) | 19.1 (12.0–75.0) | NA | 25 months (range 3–39) | The three-year cumulative incidence of HCC was 20% vs. 5% in patients with baseline LS > 30 kPa vs. ≤ 30 kPa |
| Pons, 2019 [12] | 2015–2016 (572) | SOF/SIM, SOF/LED SOF/DAC, PTV/r/O/D Others 12 weeks | 20.2 ± 10.4 (mean ± SD) | SVR48: 13.9 ± 9.2 (mean ± SD) | 2.8 years | 1.5/100 patient-years |
| Morisco, 2021 [32] | 2015–2017 (706) | SOF, SOF/other, 3D, 2D Duration—NA | NA | NA | 28 months ± 5 (mean ± SD) | 1.6/100 patient-years |
| Shiha, 2022 [10] | 2015–2019 (1517) | Types of DAA - NA 12 or 24 weeks | 12.4 (11.1–14.2) | EOT: 8.8 (6.6–12.3) | 28 months (range 24–32) | 0.91/100 patient-years |
| The present study | 2016–2020 (185) | SOF/DAC, SOF/DAC/RBV, SOF/LED SOF/LED/RBV, SOF/VEL SOF/VEL/RBV, SOF/IFN/RBV; 12, 16, or 24 weeks | 11.8 (7.4–21.2) | SVR24: 8.5 (5.4–14.) SVR48: 5.8 (4.8–10.5) SVR72: 6.1 (5.2–11.1) SVR96: 6.6 (4.8–12.0) | 41.6 months | 6/185 (3.24%) Follow-up at 48 weeks after SVR LS ≥ 8kPa was associated with liver-related events (HR = 5.68) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodprasert, N.; Hongboontry, T.; Cherdchoochart, C.; Chaiteerakij, R. Association between Liver Stiffness and Liver-Related Events in HCV-Infected Patients after Successful Treatment with Direct-Acting Antivirals. Medicina 2023, 59, 602. https://doi.org/10.3390/medicina59030602
Rodprasert N, Hongboontry T, Cherdchoochart C, Chaiteerakij R. Association between Liver Stiffness and Liver-Related Events in HCV-Infected Patients after Successful Treatment with Direct-Acting Antivirals. Medicina. 2023; 59(3):602. https://doi.org/10.3390/medicina59030602
Chicago/Turabian StyleRodprasert, Napas, Tinn Hongboontry, Chitchanok Cherdchoochart, and Roongruedee Chaiteerakij. 2023. "Association between Liver Stiffness and Liver-Related Events in HCV-Infected Patients after Successful Treatment with Direct-Acting Antivirals" Medicina 59, no. 3: 602. https://doi.org/10.3390/medicina59030602
APA StyleRodprasert, N., Hongboontry, T., Cherdchoochart, C., & Chaiteerakij, R. (2023). Association between Liver Stiffness and Liver-Related Events in HCV-Infected Patients after Successful Treatment with Direct-Acting Antivirals. Medicina, 59(3), 602. https://doi.org/10.3390/medicina59030602





