The Effect of Remimazolam Compared to Sevoflurane on Postoperative Shivering in Patients Undergoing Laparoscopic Gynecologic Surgery under General Anesthesia: A Prospective Randomized Controlled Trial
Abstract
1. Introduction
2. Materials & Methods
2.1. Study Design and Setting
2.2. Anesthesia Protocol
2.3. Outcome Measurement
2.4. Statistical Analysis
3. Results
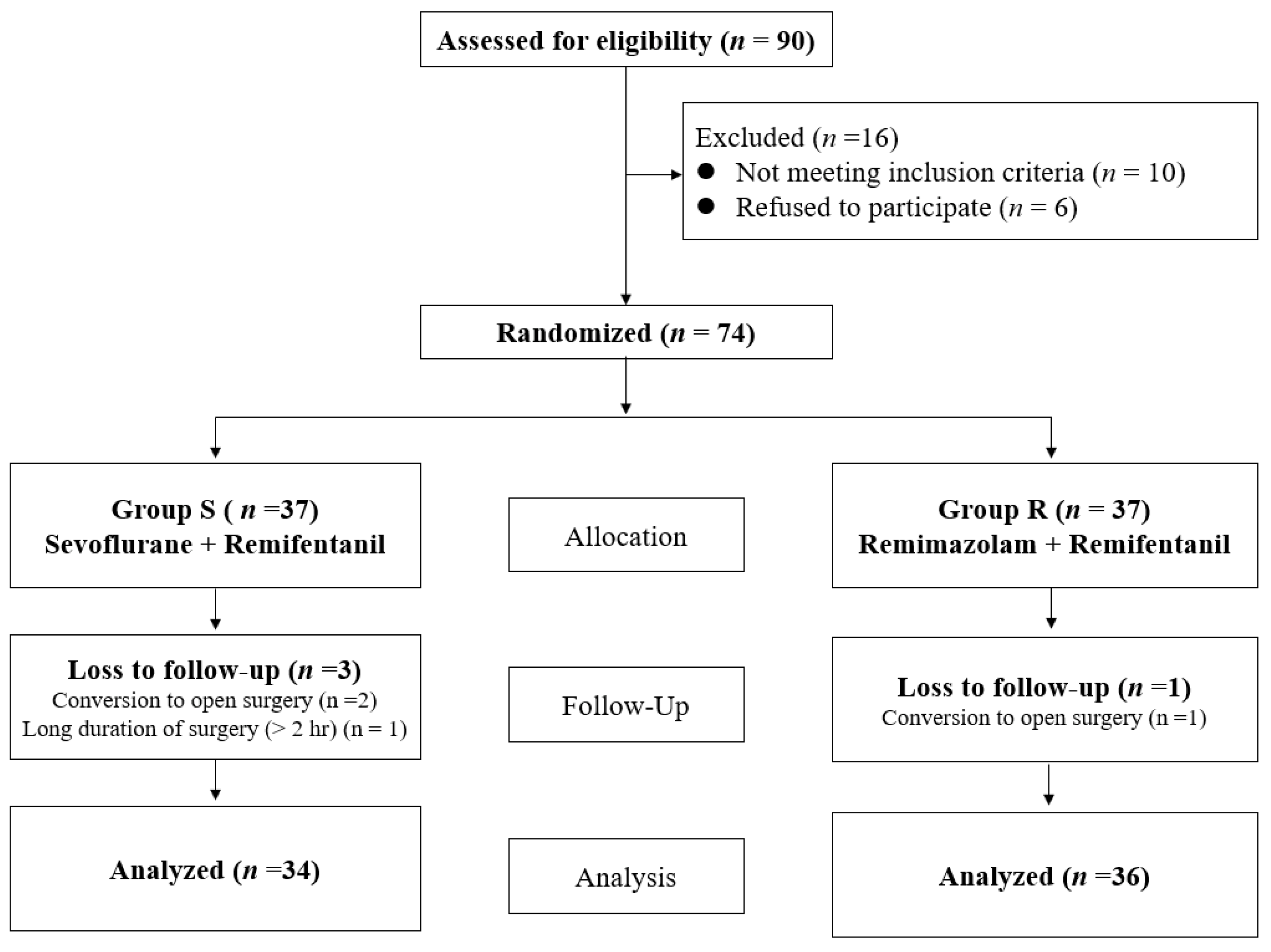
3.1. Study Population
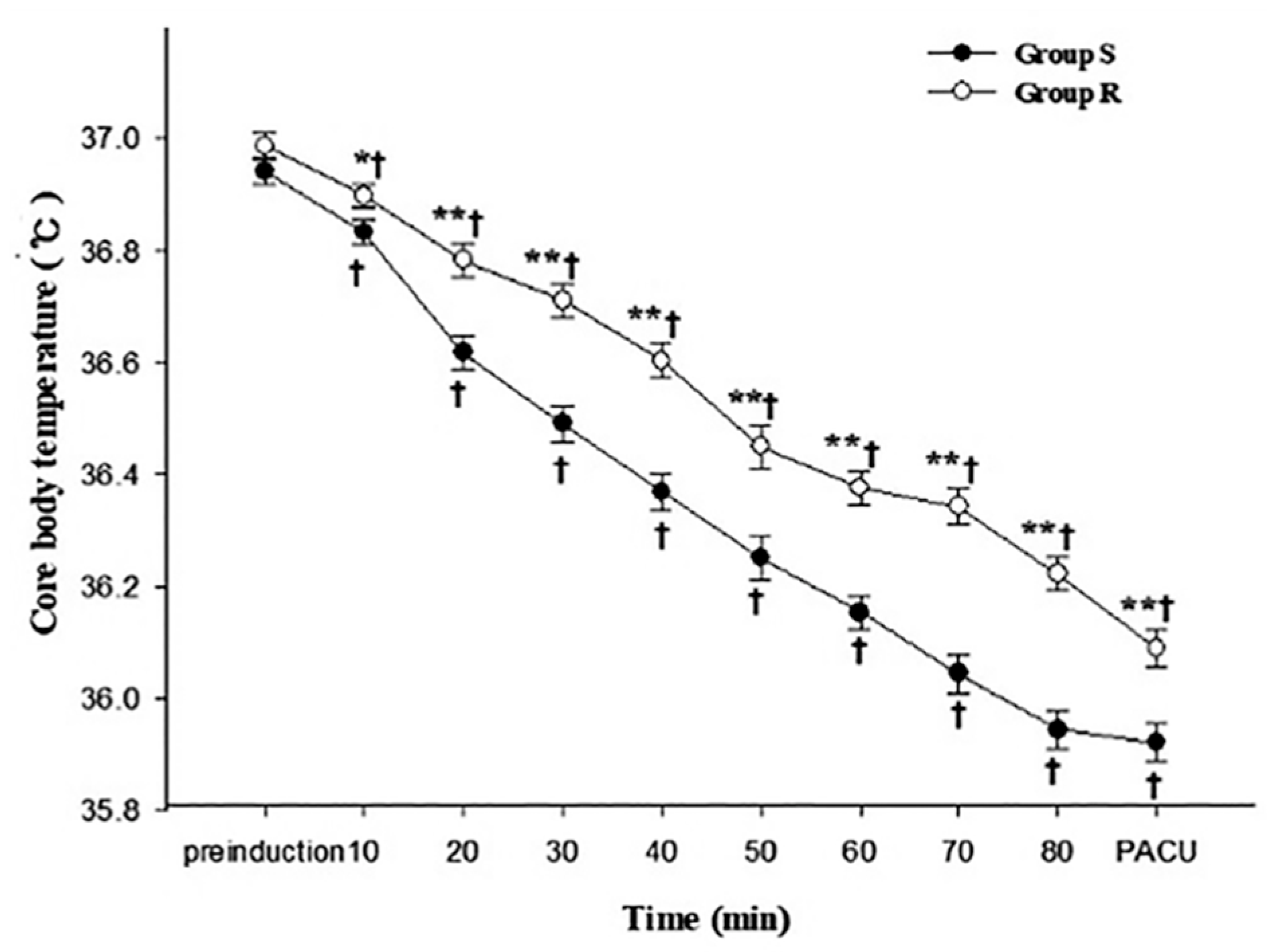
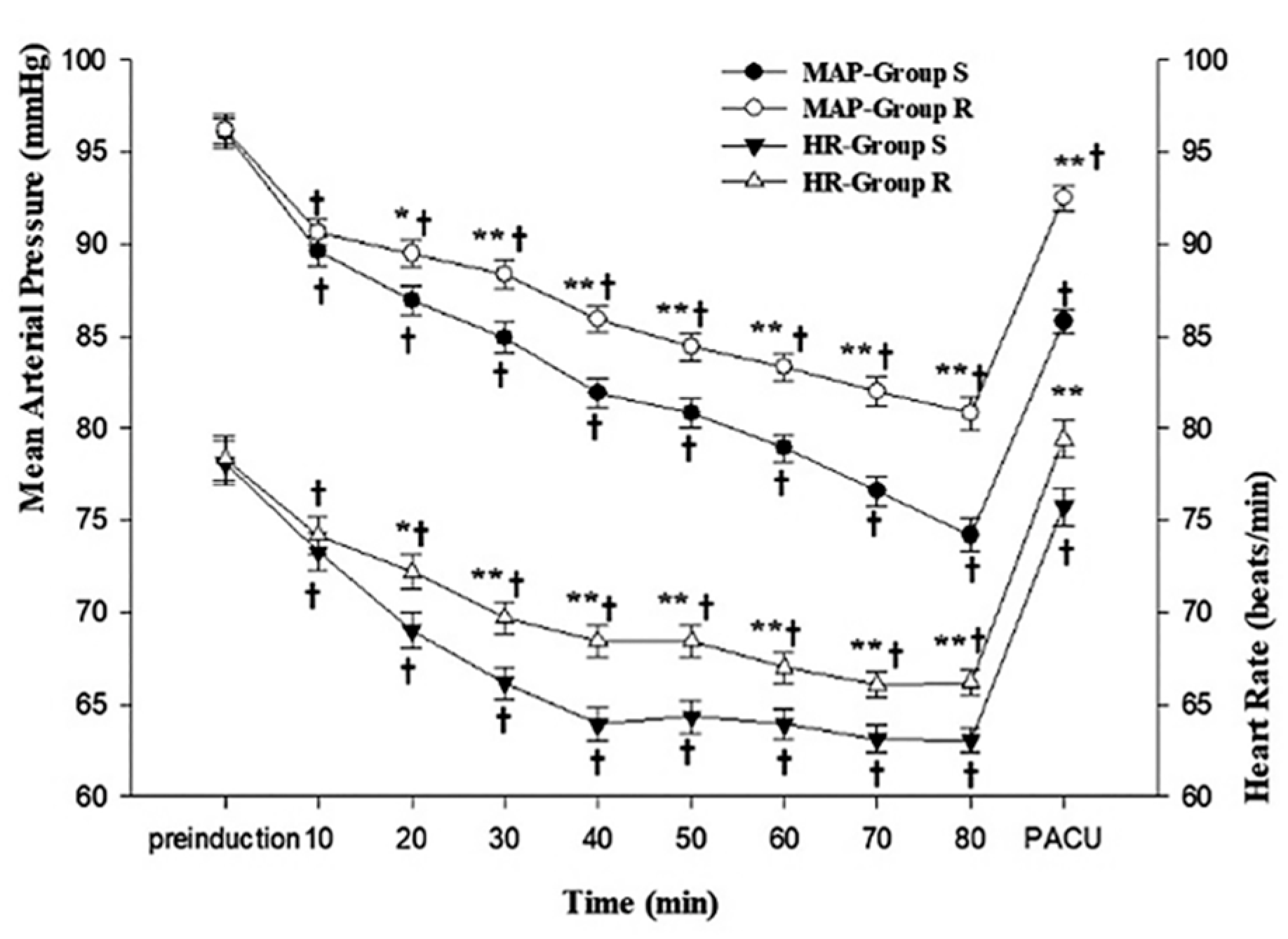
3.2. Primary and Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riley, C.; Andrzejowski, J. Inadvertent perioperative hypothermia. BJA Educ. 2018, 18, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sessler, D.I. Perioperative thermoregulation and heat balance. Lancet 2016, 387, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Annetta, M.G. Postoperative shivering: Prevention or treatment? Minerva Anestesiol. 2022, 88, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.B. Postanaesthetic shivering—From pathophysiology to prevention. Rom. J. Anaesth. Intensive Care 2018, 25, 73–81. [Google Scholar] [PubMed]
- Annadata, R.; Sessler, D.I.; Tayefeh, F.; Kurz, A.; Dechert, M. Desflurane slightly increases the sweating threshold but produces marked, nonlinear decreases in the vasoconstriction and shivering thresholds. Anesthesiology 1995, 83, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Talke, P.; Tayefeh, F.; Sessler, D.I.; Jeffrey, R.; Noursalehi, M.; Richardson, C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. J. Am. Soc. Anesthesiol. 1997, 87, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, C.; Lee, J.; Jang, G.; Kim, B.; Park, S. Comparison of core body temperatures in patients administered remimazolam or propofol during robotic-assisted and laparoscopic radical prostatectomy. Medicina 2022, 58, 690. [Google Scholar] [CrossRef] [PubMed]
- Badjatia, N.; Strongilis, E.; Gordon, E.; Prescutti, M.; Fernandez, L.; Fernandez, A.; Buitrago, M.; Schmidt, J.M.; Ostapkovich, N.D.; Mayer, S.A.; et al. Metabolic impact of shivering during therapeutic temperature modulation: The Bedside Shivering Assessment Scale. Stroke 2008, 39, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Goold, J.E. Postoperative spasticity and shivering. A review with personal observations of 500 patients. Anaesthesia 1984, 39, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Kiekkas, P.; Poulopoulou, M.; Papahatzi, A.; Souleles, P. Effects of hypothermia and shivering on standard PACU monitoring of patients. AANA J. 2005, 73, 47–53. [Google Scholar] [PubMed]
- Song, Y.K.; Lee, C.; Seo, D.H.; Park, S.N.; Moon, S.Y.; Park, C.H. Interaction between postoperative shivering and hyperalgesia caused by high-dose remifentanil. Korean J. Anesth. 2014, 66, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.P.; Sessler, D.I.; Standl, T.; Schroeder, F.; Bartz, H.J.; Beyer, J.C.; Esch, J.S.A. Non-thermoregulatory shivering in patients recovering from isoflurane or desflurane anesthesia. Anesthesiology 1998, 89, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Sessler, D.I.; Annadata, R.; Dechert, M.; Christensen, R.; Bjorksten, A.R. Midazolam minimally impairs thermoregulatory control. Anesth. Analg. 1995, 81, 393–398. [Google Scholar] [PubMed]
- Sund-Levander, M.; Grodzinsky, E.; Loyd, D.; Wahren, L.K. Errors in body temperature assessment related to individual variation, measuring technique and equipment. Int. J. Nurs. Pr. 2004, 10, 216–223. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, A.; Wyssusek, K.; Vivian, V. Verification of Nasopharyngeal Temperature Probes-They Are Not Always Where You Think They Are! Anesth. Analg. 2016, 123, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lim, H.; Son, K.G.; Ko, S. Optimal nasopharyngeal temperature probe placement. Anesth. Analg. 2014, 119, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, J.; Lee, G.; Park, S.; Lee, M.; Kim, H. Impact of the Hormonal Status in Women on Intraoperative Hypothermia during Laparoscopic Gynecologic Surgery when Considering the Fresh Gas Flow Rate: A Retrospective Study. Biomed Res. Int. 2022, 2022, 5305165. [Google Scholar] [CrossRef] [PubMed]
- Yanovich, R.; Ketko, I.; Charkoudian, N. Sex Differences in Human Thermoregulation: Relevance for 2020 and Beyond. Physiology 2020, 35, 177–184. [Google Scholar] [CrossRef] [PubMed]
| Group S (n = 34) | Group R (n = 36) | p-Value | SMD | |
|---|---|---|---|---|
| Age (yr) | 49.0 ± 8.1 | 49.7 ± 6.4 | 0.703 | −0.67 |
| ASA (I/II) | 15/19 (44.1/55.9) | 20/16 (55.4/44.4) | 0.339 | |
| Body mass index (kg/m2) | 24.0 ± 0.6 | 23.8 ± 0.9 | 0.705 | 0.14 |
| Total fluid administered (mL) | 698.5 ± 108.4 | 730.6 ± 98.0 | 0.199 | −32.0 |
| Duration of anesthesia (min) | 95.0 ± 8.3 | 96.9 ± 8.7 | 0.398 | −1.94 |
| Type of surgery | 0.94 | |||
| Laparoscopic subtotal hysterectomy | 12 (35.3) | 13 (36.1) | ||
| Laparoscopic-assisted vaginal hysterectomy | 22 (64.7) | 23 (63.9) | ||
| Duration of surgery (min) | 76.2 ± 9.2 | 76.9 ± 9.6 | 0.7485 | −0.77 |
| Hypotension | 2 (5.9) | 0 (0) | 0.14 | |
| Bradycardia | 0 (0) | 0 (0) |
| Group S (n = 34) | Group R (n = 36) | p-Value | |
|---|---|---|---|
| Incidence of hypothermia (<36 °C) | 20 (58.8) | 10 (27.8) | 0.009 |
| Incidence of PS | 14 (41.2) | 7 (19.4) | 0.047 |
| Severity of PS | 0.034 | ||
| Grade 0 | 20 (20.8) | 29 (46.7) | |
| Grade 1 | 3 (41.7) | 5 (40.0) | |
| Grade 2 | 8 (33.3) | 2 (13.3) | |
| Grade 3 | 3 (8.8) | 0 (0) | |
| Meperidine administered for the treatment of PS | 11 (32.4) | 2 (5.6) | 0.004 |
| No Hypothermia | Hypothermia | Cramer’s V (φc) Coefficient | p-Value | ||
|---|---|---|---|---|---|
| Group S | PS | 0.414 | 0.121 | ||
| Grade 0 | 11 (78.6) | 9 (45.0) | |||
| Grade 1 | 0 (0.0) | 3 (15.0) | |||
| Grade 2 | 3 (21.4) | 5 (25.0) | |||
| Grade 3 | 0 (0.0) | 3 (15.0) | |||
| Group R | PS | 0.418 | 0.043 | ||
| Grade 0 | 23 (88.5) | 6 (60.0) | |||
| Grade 1 | 3 (11.5) | 2 (20.0) | |||
| Grade 2 | 0 (0) | 2 (20.0) | |||
| Total | PS | 0.401 | 0.010 | ||
| Grade 0 | 34 (85.0) | 15 (50.0) | |||
| Grade 1 | 3 (7.5) | 5 (16.7) | |||
| Grade 2 | 3 (7.5) | 7 (23.3) | |||
| Grade 3 | 0 (0) | 3 (10.0) |
| No Hypothermia | Hypothermia | Cramer’s V (φc) Coefficient | p-Value | ||
|---|---|---|---|---|---|
| Group S | PS | 0.195 | 0.255 | ||
| ≤Grade 1 | 11 (78.6) | 12 (60.0) | |||
| ≥Grade 2 | 3 (21.4) | 8 (40.0) | |||
| Group R | PS | 0.391 | 0.019 | ||
| ≤Grade 1 | 26 (100) | 8 (80.0) | |||
| ≥Grade 2 | 0 | 2 (20.0) | |||
| Total | PS | 0.329 | 0.006 | ||
| ≤Grade 1 | 37 | 20 (66.7) | |||
| ≥Grade 2 | 3 | 10 (33.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Lee, C.; Lee, H.; Park, J.; Lim, J.; Kim, H. The Effect of Remimazolam Compared to Sevoflurane on Postoperative Shivering in Patients Undergoing Laparoscopic Gynecologic Surgery under General Anesthesia: A Prospective Randomized Controlled Trial. Medicina 2023, 59, 578. https://doi.org/10.3390/medicina59030578
Lee C, Lee C, Lee H, Park J, Lim J, Kim H. The Effect of Remimazolam Compared to Sevoflurane on Postoperative Shivering in Patients Undergoing Laparoscopic Gynecologic Surgery under General Anesthesia: A Prospective Randomized Controlled Trial. Medicina. 2023; 59(3):578. https://doi.org/10.3390/medicina59030578
Chicago/Turabian StyleLee, Cheol, Cheolhyeong Lee, Hayoung Lee, Jeongki Park, Junsung Lim, and Hyungtae Kim. 2023. "The Effect of Remimazolam Compared to Sevoflurane on Postoperative Shivering in Patients Undergoing Laparoscopic Gynecologic Surgery under General Anesthesia: A Prospective Randomized Controlled Trial" Medicina 59, no. 3: 578. https://doi.org/10.3390/medicina59030578
APA StyleLee, C., Lee, C., Lee, H., Park, J., Lim, J., & Kim, H. (2023). The Effect of Remimazolam Compared to Sevoflurane on Postoperative Shivering in Patients Undergoing Laparoscopic Gynecologic Surgery under General Anesthesia: A Prospective Randomized Controlled Trial. Medicina, 59(3), 578. https://doi.org/10.3390/medicina59030578






