Spectrum of Autoimmune Diseases—Chronic Lymphocytic Inflammation with Pontine Perivascular Enhancement Responsive to Steroids (CLIPPERS)—Clinical Case
Abstract
1. Introduction
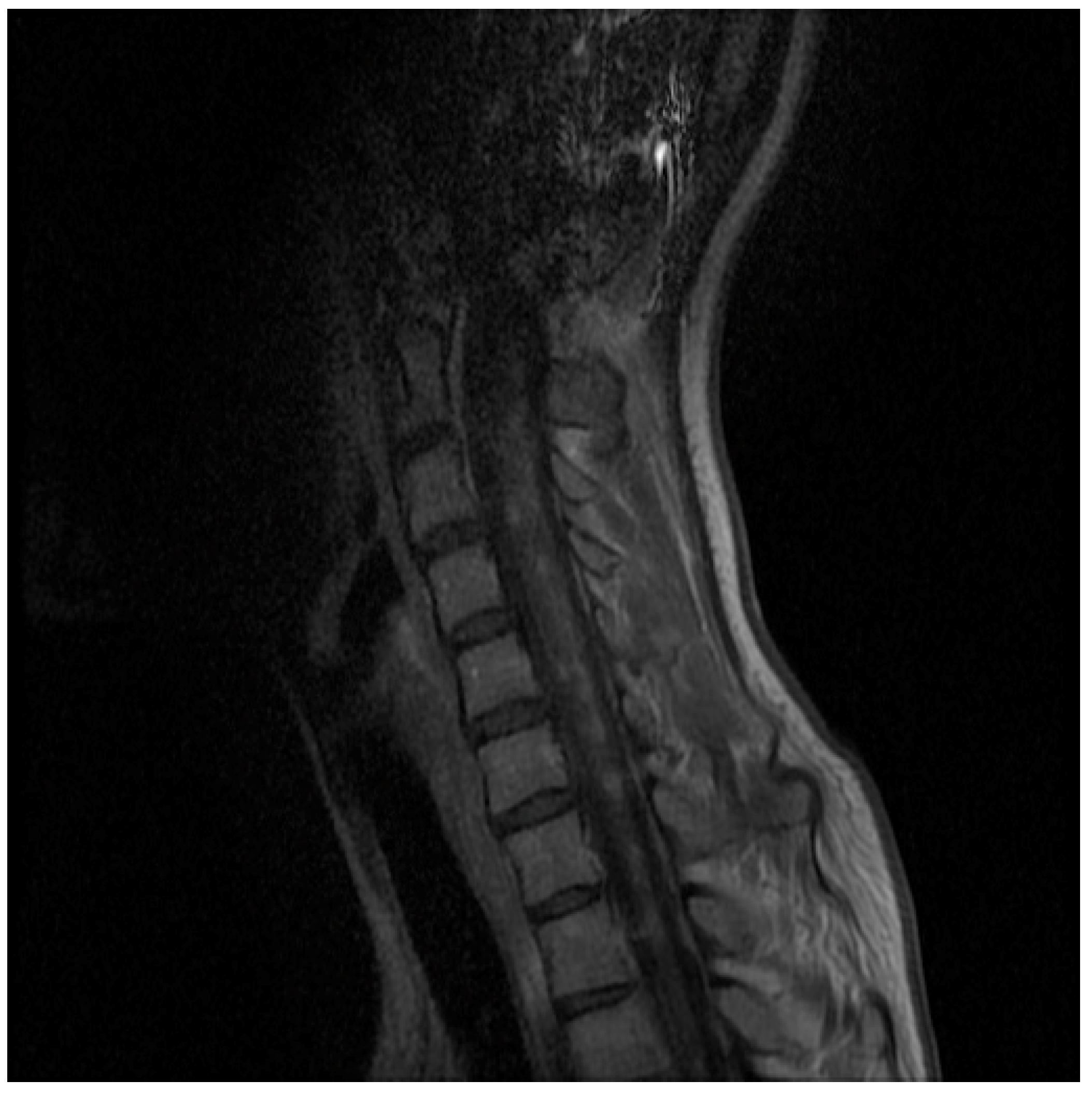
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sean, J.; Pittock, J.D.; Krecke, K.N.; Giannini, C.; van den Ameele, J.; De Herdt, V.; McKeon, A.; Fealey, R.D.; Weinshenker, B.G.; Aksamit, A.J.; et al. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain 2010, 133, 2626–2634. [Google Scholar] [CrossRef]
- Dudesek, A.; Rimmele, F.; Tesar, S.; Kolbaske, S.; Rommer, P.S.; Benecke, R.; Zettl, U.K. CLIPPERS: Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Review of an increasingly recognized entity within the spectrum of inflammatory central nervous system disorders. Clin. Exp. Immunolactions 2014, 175, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Axelerad, A.D.; Stroe, A.Z.; Mihai, C.; Frecus, C.; Jianu, D.C.; Axelerad, D.D.; Gogu, A.E. CLIPPERS, chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids: A challenge in neurological practice, clinical landmarks. Exp. Ther. Med. 2021, 22, 1191. [Google Scholar] [CrossRef] [PubMed]
- Abkur, T.M.; Kearney, H.; Hennessy, M.J. CLIPPERS and the need for long-term immunosuppression. Scott. Med. J. 2017, 62, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Taieb, G.; Duflos, C.; Renard, D.; Audoin, B.; Kaphan, E.; Pelletier, J.; Limousin, N.; Trancant, C.; Kremer, S.; de Sèze, J.; et al. Long-term outcomes of CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) in a consecutive series of 12 patients. Arch. Neurol. 2012, 69, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Ambia, A.R.; AlZahrani, N.; Almakadma, A.H.; Elgazzar, T.A.; Almustanyir, S. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids: An acute presentation. Cureus 2022, 14, e21382. [Google Scholar] [CrossRef] [PubMed]
- Kerrn-Jespersen, B.M.; Lindelof, M.; Illes, Z.; Blaabjerg, M.; Lund, E.L.; Klausen, C.; Christiansen, I.; Sellebjerg, F.; Kondziella, D. CLIPPERS among patients diagnosed with non-specific CNS neuroinflammatory diseases. J. Neurol. Sci. 2014, 343, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Gabilondo, I.; Saiz, A.; Graus, F.; Villoslada, P. Response to immunotherapy in CLIP- PERS syndrome. J. Neurol. 2011, 258, 2090–2092. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, D.; Huang, X.; Zhang, J.; Ran, Y.; Lou, X.; Gui, Q.; Yu, S. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS): A lymphocytic reactive response of the central nervous system? A case report. J. Neuroimmunol. 2017, 305, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Salvarani, C.; Hunder, G.; Brown, R.; Parisi, J.E.; Christianson, T.J.; Giannini, C. Biopsy findings in primary angiitis of the central nervous system. Am. J. Surg. Pathol. 2009, 33, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Tobin, W.; Guo, Y.; Krecke, K.; Parisi, J.E.; Lucchinetti, C.F.; Pittock, S.J.; Mandrekar, J.; Dubey, D.; Debruyne, J.; Keegan, B.M. Diagnostic criteria for chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain 2017, 140, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Ueno, Y.; Ando, J.; Matsuda, H.; Masuda, A.; Iiduka, K.; Shingai, N.; Takanashi, M.; Yokoyama, K.; Komatsu, N.; et al. Clinical and radiological CLIPPERS features after complete remission of peripheral T-cell lymphoma, not otherwise specified. J. Neurol. Sci. 2016, 364, 6–8. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meller, A.; Pawlukowska, W.; Machowska-Sempruch, K.; Marta, M. Spectrum of Autoimmune Diseases—Chronic Lymphocytic Inflammation with Pontine Perivascular Enhancement Responsive to Steroids (CLIPPERS)—Clinical Case. Medicina 2023, 59, 549. https://doi.org/10.3390/medicina59030549
Meller A, Pawlukowska W, Machowska-Sempruch K, Marta M. Spectrum of Autoimmune Diseases—Chronic Lymphocytic Inflammation with Pontine Perivascular Enhancement Responsive to Steroids (CLIPPERS)—Clinical Case. Medicina. 2023; 59(3):549. https://doi.org/10.3390/medicina59030549
Chicago/Turabian StyleMeller, Agnieszka, Wioletta Pawlukowska, Karolina Machowska-Sempruch, and Masztalewicz Marta. 2023. "Spectrum of Autoimmune Diseases—Chronic Lymphocytic Inflammation with Pontine Perivascular Enhancement Responsive to Steroids (CLIPPERS)—Clinical Case" Medicina 59, no. 3: 549. https://doi.org/10.3390/medicina59030549
APA StyleMeller, A., Pawlukowska, W., Machowska-Sempruch, K., & Marta, M. (2023). Spectrum of Autoimmune Diseases—Chronic Lymphocytic Inflammation with Pontine Perivascular Enhancement Responsive to Steroids (CLIPPERS)—Clinical Case. Medicina, 59(3), 549. https://doi.org/10.3390/medicina59030549






