How to Improve Non-Invasive Diagnosis of Endometriosis with Advanced Statistical Methods
Abstract
1. Introduction
2. Methods
2.1. Population
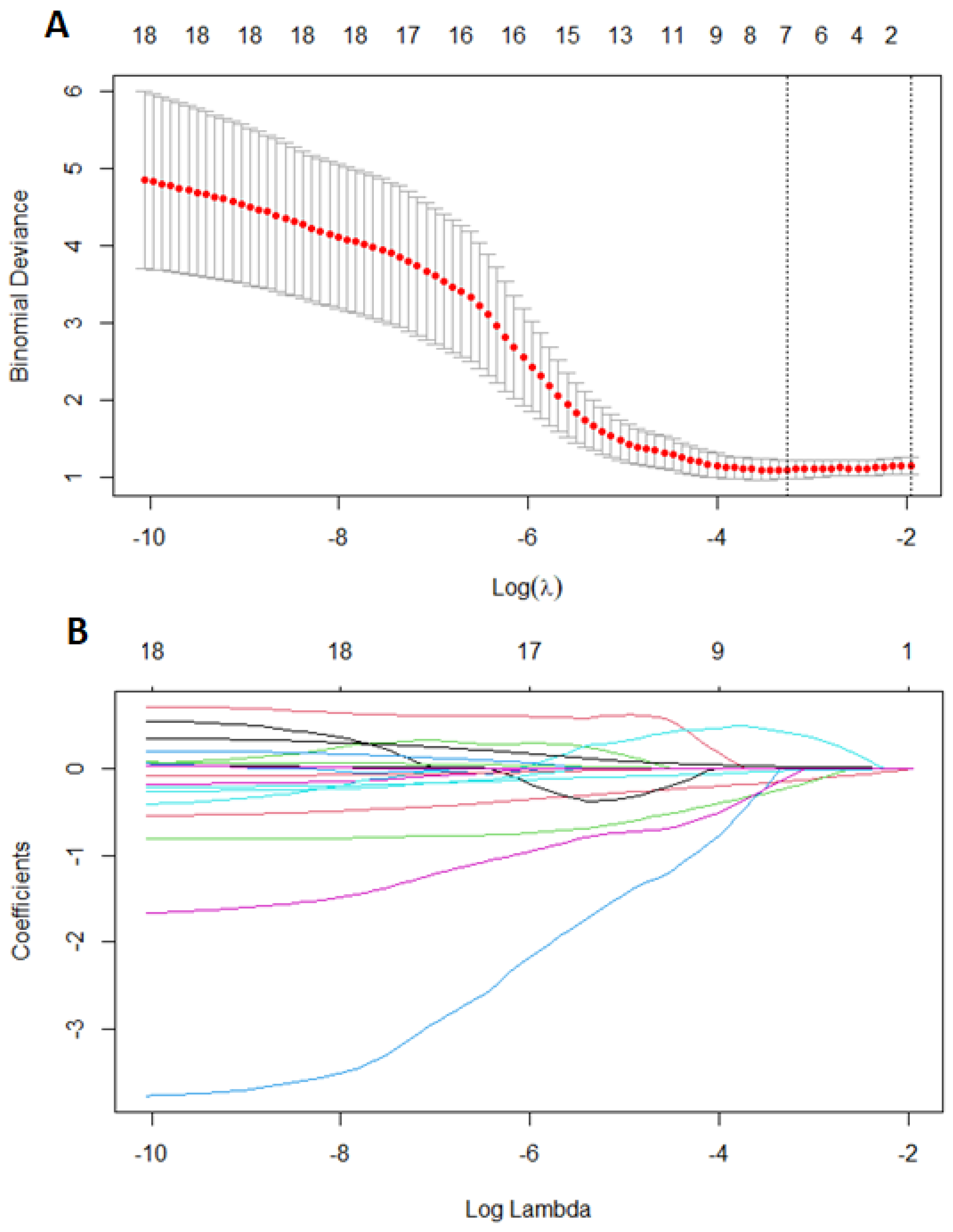
2.2. Statistical Analysis
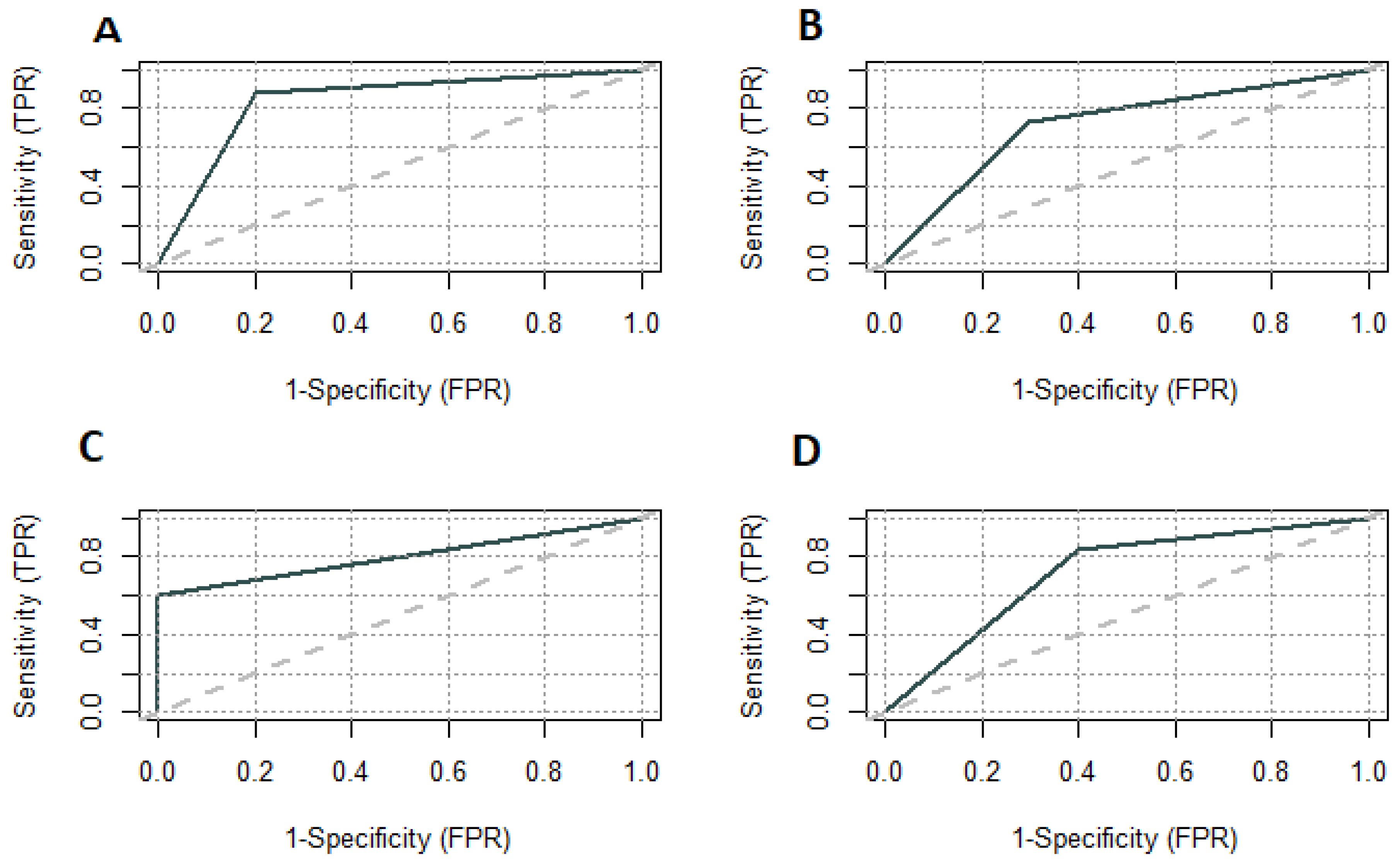
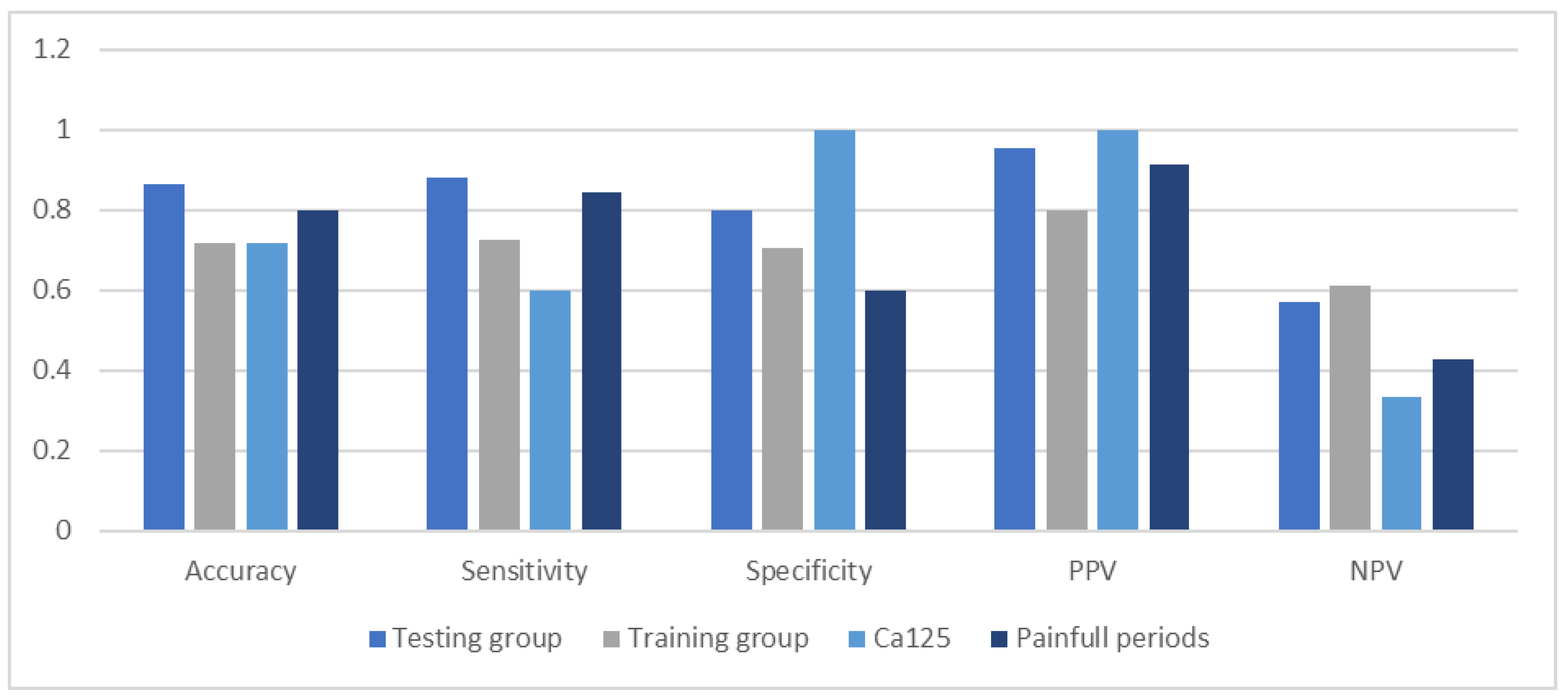
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guerriero, S.; Saba, L.; Pascual, M.A.; Ajossa, S.; Rodriguez, I.; Mais, V.; Alcazar, J.L. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 5, 586–595. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Van Schoubroeck, D. Ultrasound diagnosis of endometriosis and adenomyosis: State of the art. Best Pract Res. Clin. Obstet. Gynaecol. 2018, 51, 16–24. [Google Scholar] [CrossRef]
- Boyle, D.P.; McCluggage, W.G. Peritoneal stromal endometriosis: A detailed morphological analysis of a large series of cases of a common and under-recognised form of endometriosis. J. Clin. Pathol. 2009, 62, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Sachedina, A.; Todd, N. Dysmenorrhea, Endometriosis and Chronic Pelvic Pain in Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2020, 12 (Suppl. S1), 7–17. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Falcone, T. Laboratory Testing for Endometriosis. Clin. Chim. Acta 2004, 340, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.E.-D.R.; Hornung, D.; Al-Hendy, A. Biomarkers of Endometriosis. Expert Opin. Med. Diagn. 2008, 2, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Xavier, P.; Belo, L.; Beires, J.; Rebelo, I.; Martinez-de-Oliveira, J.; Lunet, N.; Barros, H. Serum Levels of VEGF and TNF-α and Their Association with C-Reactive Protein in Patients with Endometriosis. Arch. Gynecol. Obstet. 2006, 273, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.E.-D.R.; Hornung, D.; Salem, H.T.; Khalifa, E.A.; El-Metwally, T.H.; Al-Hendy, A. Serum Cytokines as Biomarkers for Nonsurgical Prediction of Endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 240–246. [Google Scholar] [CrossRef]
- Mihalyi, A.; Gevaert, O.; Kyama, C.M.; Simsa, P.; Pochet, N.; de Smet, F.; de Moor, B.; Meuleman, C.; Billen, J.; Blanckaert, N.; et al. Non-Invasive Diagnosis of Endometriosis Based on a Combined Analysis of Six Plasma Biomarkers. Hum. Reprod. 2010, 25, 654–664. [Google Scholar] [CrossRef]
- Liao, X.; Siu, M.K.; Au, C.W.; Chan, Q.K.; Chan, H.Y.; Wong, E.S.; Ip, P.P.; Ngan, H.Y.; Cheung, A.N. Aberrant Activation of Hedgehog Signaling Pathway Contributes to Endometrial Carcinogenesis through β-Catenin. Mod. Pathol. 2009, 22, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Uzan, C.; Cortez, A.; Dufournet, C.; Fauvet, R.; Siffroi, J.-P.; Daraï, E. Endometrium from Women with and without Endometriosis, and Peritoneal, Ovarian and Bowel Endometriosis, Show Different c-Kit Protein Expression. J. Reprod. Immunol. 2005, 65, 55–63. [Google Scholar] [CrossRef]
- Liu, D.; Yang, N.; Liang, Y.; Chen, M.; Yang, F.; Liu, L.; Yao, S. Increased Expression of Epithelial Cell Adhesion Molecule and Its Possible Role in Epithelial–Mesenchymal Transition in Endometriosis. J. Obstet. Gynaecol. Res. 2020, 46, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.R.; Meligy, F.Y.; Sayed, A.A.-R.; El-Mokhtar, M.A.; Refaiy, A.M. Stem Cell Markers Describe a Transition From Somatic to Pluripotent Cell States in a Rat Model of Endometriosis. Reprod. Sci. 2018, 25, 873–881. [Google Scholar] [CrossRef]
- Wolff, E.F.; Wolff, A.B.; Du, H.; Taylor, H.S. Demonstration of Multipotent Stem Cells in the Adult Human Endometrium by In Vitro Chondrogenesis. Reprod. Sci. 2007, 14, 524–533. [Google Scholar] [CrossRef]
- Guralp, O.; Kaya, B.; Tüten, N.; Kucur, M.; Malik, E.; Tüten, A. Non-Invasive Diagnosis of Endometriosis and Moderate-Severe Endometriosis with Serum CA125, Endocan, YKL-40, and Copeptin Quadruple Panel. J. Obstet. Gynaecol. 2021, 41, 927–932. [Google Scholar] [CrossRef]
- Dorien, O.F.; Fassbender, A.; van Bree, R.; Laenen, A.; Peterse, D.P.; Vanhie, A.; Waelkens, E.; D’Hooghe, T.M. Technical Verification and Assessment of Independent Validation of Biomarker Models for Endometriosis. Biomed. Res. Int. 2019, 2019, 3673060. [Google Scholar] [CrossRef]
- Vodolazkaia, A.; El-Aalamat, Y.; Popovic, D.; Mihalyi, A.; Bossuyt, X.; Kyama, C.M.; Fassbender, A.; Bokor, A.; Schols, D.; Huskens, D.; et al. Evaluation of a Panel of 28 Biomarkers for the Non-Invasive Diagnosis of Endometriosis. Hum. Reprod. 2012, 27, 2698–2711. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging Modalities for the Non-Invasive Diagnosis of Endometriosis. Cochrane Database Syst. Rev. 2016, 26, CD009591. [Google Scholar] [CrossRef]
- Kimber-Trojnar, Ż.; Pilszyk, A.; Niebrzydowska, M.; Pilszyk, Z.; Ruszała, M.; Leszczyńska-Gorzelak, B. The Potential of Non-Invasive Biomarkers for Early Diagnosis of Asymptomatic Patients with Endometriosis. J. Clin. Med. 2021, 10, 2762. [Google Scholar] [CrossRef]
- Das, S.; Batra, S.K. Understanding the Unique Attributes of MUC16 (CA125): Potential Implications in Targeted Therapy. Cancer Res. 2015, 75, 4669–4674. [Google Scholar] [CrossRef]
- Somigliana, E.; Viganò, P.; Tirelli, A.S.; Felicetta, I.; Torresani, E.; Vignali, M.; Di Blasio, A.M. Use of the concomitant serum dosage of CA 125, CA 19-9 and interleukin-6 to detect the presence of endometriosis. Results from a series of reproductive age women undergoing laparoscopic surgery for benign gynaecological conditions. Hum. Reprod. 2004, 19, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Mol, B.W.; Bayram, N.; Lijmer, J.G.; Wiegerinck, M.A.; Bongers, M.Y.; van der Veen, F.; Bossuyt, P.M. The performance of CA-125 measurement in the detection of endometriosis: A meta-analysis. Fertil. Steril. 1998, 70, 1101–1108. [Google Scholar] [CrossRef]
- Hirsch, M.; Duffy, J.M.N.; Deguara, C.S.; Davis, C.J.; Khan, K.S. Diagnostic accuracy of Cancer Antigen 125 (CA125) for endometriosis in symptomatic women: A multi-center study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Curchoe, C.L.; Malmsten, J.; Bormann, C.; Shafiee, H.; Flores-Saiffe Farias, A.; Mendizabal, G.; Chavez-Badiola, A.; Sigaras, A.; Alshubbar, H.; Chambost, J.; et al. Predictive Modeling in Reproductive Medicine: Where Will the Future of Artificial Intelligence Research Take Us? Fertil. Steril. 2020, 114, 934–940. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Chang, M.-Y.; Chiang, R.-D.; Hwang, L.-J.; Lee, C.-M.; Wang, Y.-H. Mining Medical Data: A Case Study of Endometriosis. J. Med. Syst. 2013, 37, 9899. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Hsieh, C.-L.; Shiau, C.-S.; Hsieh, T.-T.; Chiang, R.-D.; Chan, C.-H. Ultrasound-Guided Aspiration and Ethanol Sclerotherapy (EST) for Treatment of Cyst Recurrence in Patients after Previous Endometriosis Surgery: Analysis of Influencing Factors Using a Decision Tree. J. Minim. Invasive Gynecol. 2013, 20, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Xu, D.; Nagel, S.C.; Joshi, T. A Data Mining Approach for Biomarker Discovery Using Transcriptomics in Endometriosis. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; pp. 969–972. [Google Scholar]
- Bendifallah, S.; Puchar, A.; Suisse, S.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Touboul, C.; Dabi, Y.; Daraï, E. Machine Learning Algorithms as New Screening Approach for Patients with Endometriosis. Sci. Rep. 2022, 12, 639. [Google Scholar] [CrossRef]
- Szubert, M.; Suzin, J.; Duechler, M.; Szuławska, A.; Czyż, M.; Kowalczyk-Amico, K. Evaluation of Selected Angiogenic and Inflammatory Markers in Endometriosis before and after Danazol Treatment. Reprod. Fertil. Dev. 2014, 26, 414. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Alsuwaidi, S.; Amro, B.; Keckstein, J.; Adamyan, L.; Donnez, J.; Martin, M.C.; Wattiez, A. Reconsidering evidence-based management of endometriosis. Facts Views Vis. Obgyn. 2022, 14, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Warner, M.; Bonsignore, L.; Olive, D.; Samuels, S.; Vercellini, P. Validation Study of Nonsurgical Diagnosis of Endometriosis. Fertil. Steril. 2001, 76, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Gater, A.; Taylor, F.; Seitz, C.; Gerlinger, C.; Wichmann, K.; Haberland, C. Development and Content Validation of Two New Patient-Reported Outcome Measures for Endometriosis: The Endometriosis Symptom Diary (ESD) and Endometriosis Impact Scale (EIS). J. Patient Rep. Outcomes 2020, 4, 13. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood Biomarkers for the Non-Invasive Diagnosis of Endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef] [PubMed]
- Foster, W. Diagnosing endometriosis: CA125 rules in, but not out. BJOG 2016, 123, 1769. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.; Duffy, J.; Davis, C.J.; Nieves Plana, M.; Khan, K.S.; International Collaboration to Harmonise Outcomes and Measures for Endometriosis. Diagnostic accuracy of cancer antigen 125 for endometriosis: A systematic review and meta-analysis. BJOG 2016, 123, 1761–1768. [Google Scholar] [CrossRef]
- Maier, I.M.; Maier, A.C. MiRNAs and LncRNAs: Potential Non-Invasive Biomarkers for Endometriosis. Biomedicines 2021, 9, 1662. [Google Scholar] [CrossRef]
- Nikoo, S.; Ebtekar, M.; Jeddi-Tehrani, M.; Shervin, A.; Bozorgmehr, M.; Vafaei, S.; Kazemnejad, S.; Zarnani, A.-H. Menstrual Blood-Derived Stromal Stem Cells from Women with and without Endometriosis Reveal Different Phenotypic and Functional Characteristics. Mol. Hum. Reprod. 2014, 20, 905–918. [Google Scholar] [CrossRef]
- Bendifallah, S.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J. Clin. Med. 2022, 11, 612. [Google Scholar] [CrossRef]
- Ellis, K.; Munro, D.; Wood, R. The experiences of endometriosis patients with diagnosis and treatment in New Zealand. Front Glob. Womens Health. 2022, 3, 991045. [Google Scholar] [CrossRef]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, N.; DePari, M.; Vitonis, A.F.; Laufer, M.R.; Missmer, S.A.; Shafrir, A.L.; Terry, K.L. Evaluation of CA125 in relation to pain symptoms among adolescents and young adult women with and without surgically-confirmed endometriosis. PLoS ONE 2020, 15, e0238043. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.A.; Shih, A.; Renteira, S.M.; Seckin, T.; Blau, B.; Simpfendorfer, K.; Lee, A.; Metz, C.N.; Gregersen, P.K. Analysis of menstrual effluent: Diagnostic potential for endometriosis. Mol. Med. 2018, 24, 1. [Google Scholar] [CrossRef]
- Missmer, S.A. Why so null? Methodologic necessities to advance endometriosis discovery. Paediatr. Perinat. Epidemiol. 2019, 33, 26–27. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open. 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.D.; Gerancher, K.R. Dysmenorrhea and endometriosis in the adolescent. ACOG Committee Opinion No. 760. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2018, 132, e249–e258. Available online: https://www.acog.org/-/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2018/12/dysmenorrhea-and-endometriosis-in-the-adolescent.pdf (accessed on 20 February 2022).
- Ling, F.W. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet. Gynecol. 1999, 93, 51–58. [Google Scholar] [CrossRef]
- Grandi, G.; Barra, F.; Ferrero, S.; Sileo, F.G.; Bertucci, E.; Napolitano, A.; Facchinetti, F. Hormonal contraception in women with endometriosis: A systematic review. Eur. J. Contracept. Reprod. Health Care 2019, 24, 61–70. [Google Scholar] [CrossRef]
- Jensen, J.T.; Schlaff, W.; Gordon, K. Use of combined hormonal contraceptives for the treatment of endometriosis-related pain: A systematic review of the evidence. Fertil. Steril. 2018, 110, 137–152.e131. [Google Scholar] [CrossRef]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Luisi, S.; Petraglia, F. Is Stress a Cause or a Consequence of Endometriosis? Reprod. Sci. 2020, 27, 39–45. [Google Scholar] [CrossRef]
| Class | Training Group (n = 71) | Testing Group (n = 30) |
|---|---|---|
| Endometriosis | 44 | 25 |
| Non-endometriosis | 27 | 5 |
| All Patients (Mean) | Endometriosis | Non-Endometriosis | p Value | |
|---|---|---|---|---|
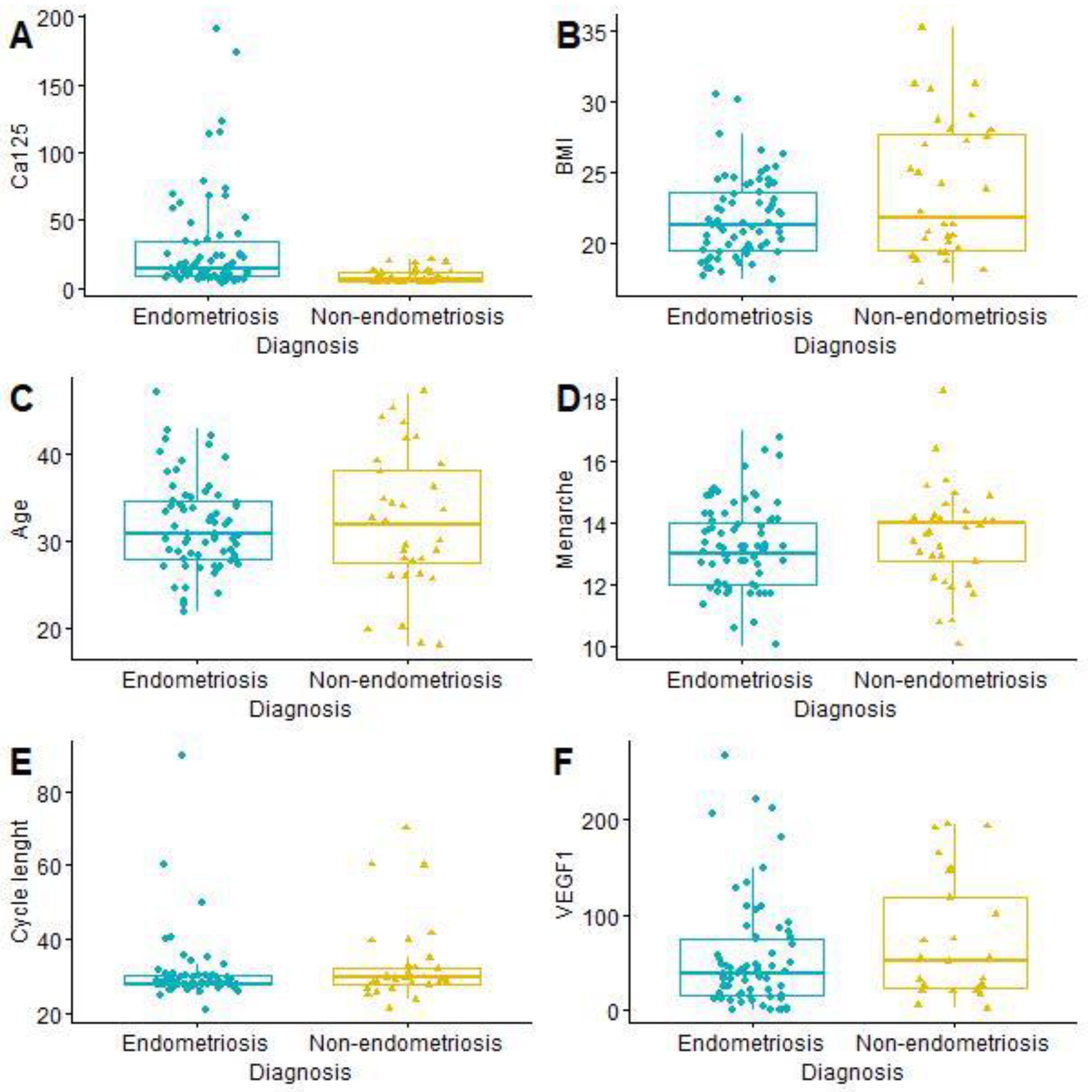
| Age (years) | 31.99 (SD = 6.09) | 31.87 (SD = 5.10) | 32.25 (SD = 7.93) | 0.8056 |
| Menarche (year) | 13.40 (SD = 1.41) | 13.35 (SD = 1.33) | 13.53 (SD = 1.59) | 0.5694 |
| BMI (kg/m2) | 22.39 (SD = 3.65) | 21.79 (SD = 2.82) | 23.70 (SD = 4.80) | 0.0422 |
| CA125 (U/mL) | 23.47 (SD = 32.79) | 29.97 (SD = 37.64) | 9.06 (SD = 5.16) | <0.0001 |
| Cycle length (days) | 31.29 (SD = 9.54) | 30.50 (SD = 8.83) | 33.06 (SD = 10.92) | 0.2480 |
| VEGF1 (pg/mL) | 59.24 (SD = 60.06) | 54.47 (SD = 57.94) | 72.40 (SD = 64.98) | 0.2315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szubert, M.; Rycerz, A.; Wilczyński, J.R. How to Improve Non-Invasive Diagnosis of Endometriosis with Advanced Statistical Methods. Medicina 2023, 59, 499. https://doi.org/10.3390/medicina59030499
Szubert M, Rycerz A, Wilczyński JR. How to Improve Non-Invasive Diagnosis of Endometriosis with Advanced Statistical Methods. Medicina. 2023; 59(3):499. https://doi.org/10.3390/medicina59030499
Chicago/Turabian StyleSzubert, Maria, Aleksander Rycerz, and Jacek R. Wilczyński. 2023. "How to Improve Non-Invasive Diagnosis of Endometriosis with Advanced Statistical Methods" Medicina 59, no. 3: 499. https://doi.org/10.3390/medicina59030499
APA StyleSzubert, M., Rycerz, A., & Wilczyński, J. R. (2023). How to Improve Non-Invasive Diagnosis of Endometriosis with Advanced Statistical Methods. Medicina, 59(3), 499. https://doi.org/10.3390/medicina59030499






