Ablative Techniques for Sarcoma Metastatic Disease: Current Role and Clinical Applications
Abstract
1. Introduction
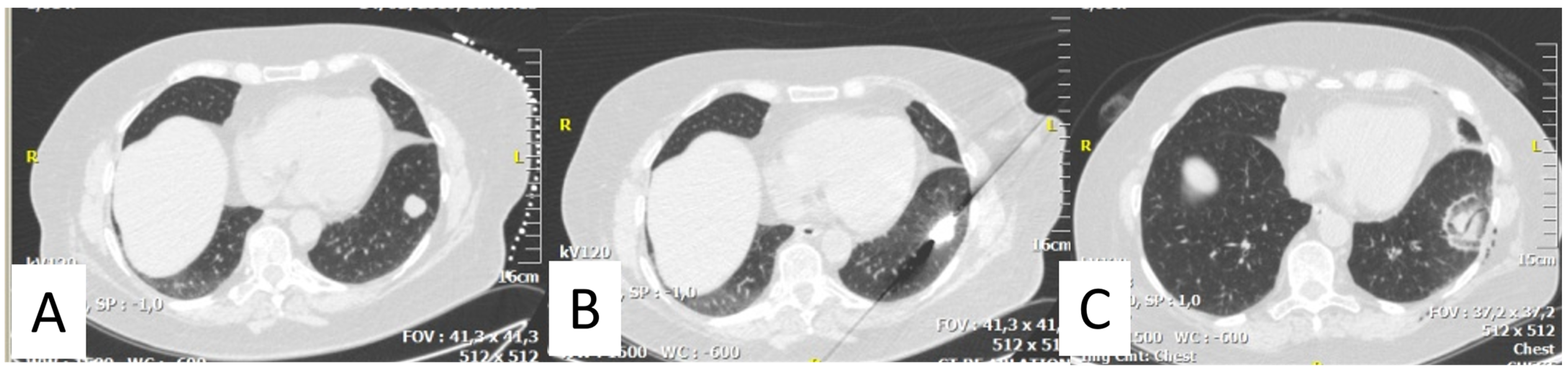
2. Thermal Ablation of Lung Metastasis
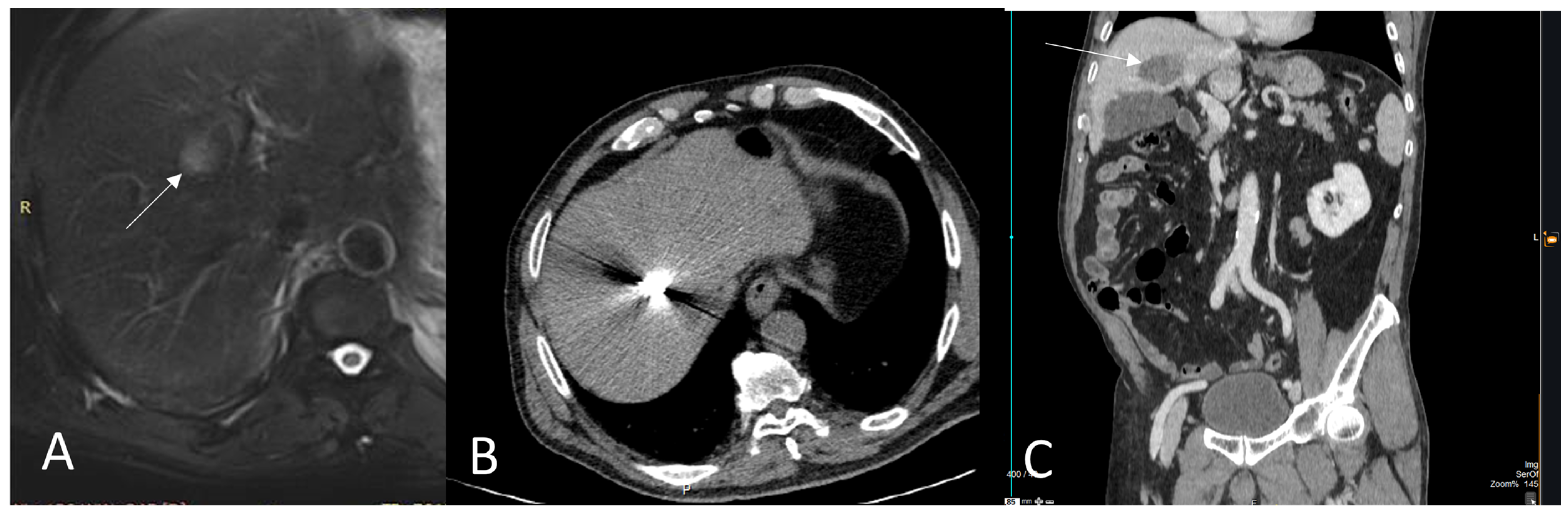
3. Thermal Ablation of Liver Metastasis
4. Thermal Ablation of Bone Metastasis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of tumors of soft tissue: Selected changes and new entities. Adv. Anat. Pathol. 2021, 28, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brodowicz, T.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv268–iv269. [Google Scholar] [CrossRef] [PubMed]
- Bourcier, K.; Le Cesne, A.; Tselikas, L.; Adam, J.; Mir, O.; Honore, C.; de Baere, T. Basic knowledge in soft tissue sarcoma. Cardiovasc. Interv. Radiol. 2019, 42, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v174–v177. [Google Scholar]
- von Mehren, M.; Kane, J.M.; Bui, M.M.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; et al. NCCN guidelines insights: Soft tissue sarcoma, version 1.2021. J. Natl. Compr. Cancer Netw. 2020, 18, 1604–1612. [Google Scholar] [CrossRef]
- Odri, G.A.; Tchicaya-Bouanga, J.; Yoon, D.J.Y.; Modrowski, D. Metastatic progression of osteosarcomas: A review of current knowledge of environmental versus oncogenic drivers. Cancers 2022, 14, 360. [Google Scholar] [CrossRef]
- Dangoor, A.; Seddon, B.; Gerrand, C.; Grimer, R.; Whelan, J.; Judson, I. UK guidelines for the management of soft tissue sarcomas. Clin. Sarcoma Res. 2016, 6, 20. [Google Scholar] [CrossRef]
- Gerrand, C.; Athanasou, N.; Brennan, B.; Grimer, R.; Judson, I.; Morland, B.; Peake, D.; Seddon, B.; Whelan, J.; on behalf of the British Sarcoma Group. UK guidelines for the management of bone sarcomas. Clin. Sarcoma Res. 2016, 6, 7. [Google Scholar] [CrossRef]
- Farooqi, A.; Mitra, D.; Guadagnolo, B.A.; Bishop, A.J. The Evolving Role of Radiation Therapy in Patients with Metastatic Soft Tissue Sarcoma. Curr. Oncol. Rep. 2020, 22, 79. [Google Scholar] [CrossRef]
- Naghavi, A.O.; Fernandez, D.C.; Mesko, N.; Juloori, A.; Martinez, A.; Scott, J.G.; Shah, C.; Harrison, L.B. American Brachytherapy Society consensus statement for soft tissue sarcoma brachytherapy. Brachytherapy 2017, 16, 466–489. [Google Scholar] [CrossRef]
- Falk, A.; Moureau-Zabotto, L.; Ouali, M.; Penel, N.; Italiano, A.; Bay, J.-O.; Olivier, T.; Sunyach, M.-P.; Boudou-Roquette, P.; Salas, S.; et al. Effect on survival of local ablative treatment of metastases from sarcomas: A study of the French sarcoma group. Clin. Oncol. (R. Coll. Radiol.) 2015, 27, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.M.; Schmitz, J.J.; Schmit, G.D.; Callstrom, M.R.; Kurup, A.N. Image-guided thermal ablative therapies in the treatment of sarcoma. Curr. Treat. Options Oncol. 2017, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Cariati, M.; Marra, P.; Masala, S.; Pereira, P.L.; Carrafiello, G. CIRSE standards of practice on thermal ablation of primary and secondary lung tumours. CardioVascular Interv. Radiol. 2020, 43, 667–683. [Google Scholar] [CrossRef] [PubMed]
- de Baère, T.; Aupérin, A.; Deschamps, F.; Chevallier, P.; Gaubert, Y.; Boige, V.; Fonck, M.; Escudier, B.; Palussiére, J. Radiofrequency ablation is a valid treatment option for lung metastases: Experience in 566 patients with 1037 metastases. Ann. Oncol. 2015, 26, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.; Belfiore, M.P.; Floridi, C.; Serra, N.; Belfiore, G.; Carmignani, L.; Grasso, R.F.; Mazza, E.; Pusceddu, C.; Brunese, L.; et al. Radiofrequency versus microwave ablation for treatment of the lung tumours: LUMIRA (lung microwave radiofrequency) randomized trial. Med. Oncol. 2017, 34, 96. [Google Scholar] [CrossRef]
- de Baere, T.; Tselikas, L.; Woodrum, D.; Abtin, F.; Littrup, P.; Deschamps, F.; Suh, R.; Aoun, H.D.; Callstrom, M. Evaluating cryoablation of metastatic lung tumors in patients—safety and efficacy: The ECLIPSE trial—interim analysis at 1 year. J. Thorac. Oncol. 2015, 10, 1468–1474. [Google Scholar] [CrossRef]
- Georgiades, C.S.; Marx, J.K. Cryoablation: Mechanism of action and devices. In Percutaneous Tumor Ablation Strategies and Tech-niques; Hong, K., Georgiades, C.S., Eds.; Thieme: New York, NY, USA, 2011; pp. 15–26. [Google Scholar]
- Sato, T.; Iguchi, T.; Hiraki, T.; Gobara, H.; Fujiwara, H.; Sakurai, J.; Matsui, Y.; Mitsuhashi, T.; Soh, J.; Toyooka, S.; et al. Radiofrequency ablation of pulmonary metastases from sarcoma: Single-center retrospective evaluation of 46 patients. JPN J. Radiol. 2017, 35, 61–67. [Google Scholar] [CrossRef]
- Palussière, J.; Italiano, A.; Descat, E.; Ferron, S.; Cornélis, F.; Avril, A.; Brouste, V.; Bui, B.N. Sarcoma lung metastases treated with percutaneous radiofrequency ablation: Results from 29 patients. Ann. Surg. Oncol. 2011, 18, 3771–3777. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumine, A.; Yamakado, K.; Matsubara, T.; Takaki, H.; Nakatsuka, A.; Takeda, K.; Abo, D.; Shimizu, T.; Uchida, A. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas [corrected]. Cancer 2009, 115, 4041. [Google Scholar] [CrossRef]
- Yevich, S.; Gaspar, N.; Tselikas, L.; Brugières, L.; Pacquement, H.; Schleiermacher, G.; Tabone, M.-D.; Pearson, E.; Canale, S.; Muret, J.; et al. Percutaneous computed tomography-guided thermal ablation of pulmonary osteosarcoma metastases in children. Ann. Surg. Oncol. 2016, 23, 1380–1386. [Google Scholar] [CrossRef]
- Bourgouin, P.P.; Wrobel, M.M.; Mercaldo, N.D.; Murphy, M.C.; Leppelmann, K.S.; Levesque, V.M.; Muniappan, A.; Silverman, S.G.; Shepard, J.O.; Shyn, P.B.; et al. Comparison of percutaneous image-guided microwave ablation and cryoablation for sarcoma lung metastases: A 10-year experience. AJR Am. J. Roentgenol. 2022, 218, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Predina, J.D.; Puc, M.M.; Bergey, M.R.; Sonnad, S.S.; Kucharczuk, J.C.; Staddon, A.; Kaiser, L.R.; Shrager, J.B. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J. Thorac. Oncol. 2011, 6, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Hornbech, K.; Ravn, J.; Steinbrüchel, D.A. Outcome after pulmonary metastasectomy: Analysis of 5 years consecutive surgical resections 2002–2006. J. Thorac. Oncol. 2011, 6, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- de Baere, T.; Tselikas, L.; Gravel, G.; Hakime, A.; Deschamps, F.; Honoré, C.; Mir, O.; Lecesne, A. Interventional radiology: Role in the treatment of sarcomas. Eur. J. Cancer 2018, 94, 148–155. [Google Scholar] [CrossRef]
- Koelblinger, C.; Strauss, S.; Gillams, A. Outcome after radiofrequency ablation of sarcoma lung metastases. Cardiovasc. Interv. Radiol. 2014, 37, 147–153. [Google Scholar] [CrossRef]
- Yashiro, H.; Nakatsuka, S.; Inoue, M.; Kawamura, M.; Tsukada, N.; Asakura, K.; Yamauchi, Y.; Hashimoto, K.; Kuribayashi, S. Factors affecting local progression after percutaneous cryoablation of lung tumors. J. Vasc. Interv. Radiol. 2013, 24, 813–821. [Google Scholar] [CrossRef]
- Jaques, D.P.; Coit, D.G.; Casper, E.S.; Brennan, M.F. Hepatic metastases from soft-tissue sarcoma. Ann. Surg. 1995, 221, 392–397. [Google Scholar] [CrossRef]
- Saumet, L.; Deschamps, F.; Marec-Berard, P.; Gaspar, N.; Corradini, N.; Petit, P.; Sirvent, N.; Brugières, L. Radiofrequency ablation of metastases from osteosarcoma in patients under 25 years: The SCFE experience. Pediatr. Hematol. Oncol. 2015, 32, 41–49. [Google Scholar] [CrossRef]
- Crocetti, L.; de Baére, T.; Pereira, P.L.; Tarantino, F.P. CIRSE Standards of Practice on Thermal Ablation of Liver Tumours. Cardiovasc. Interv. Radiol. 2020, 43, 951–962. [Google Scholar] [CrossRef]
- Marudanayagam, R.; Sandhu, B.; Perera, M.; Bramhall, S.; Mayer, D.; Buckels, J.; Mirza, D. Liver resection for metastatic soft tissue sarcoma: An analysis of prognostic factors. Eur. J. Surg. Oncol. 2011, 37, 87–92. [Google Scholar] [CrossRef]
- Okamoto, M.; Matsuoka, M.; Soma, T.; Arai, R.; Kato, H.; Harabayashi, T.; Adachi, H.; Shinohara, T.; Sagawa, T.; Nishiyama, N.; et al. Metastases of soft tissue sarcoma to the liver: A Historical Cohort Study from a Hospital-based Cancer Registry. Cancer Med. 2020, 9, 6159–6165. [Google Scholar] [CrossRef] [PubMed]
- Seesing, M.F.; Tielen, R.; van Hillegersberg, R.; van Coevorden, F.; de Jong, K.; Nagtegaal, I.; Verhoef, C.; de Wilt, J.; Hartgrink, H.; Sietses, C.; et al. Resection of liver metastases in patients with gastrointestinal stromal tumors in the imatinib era: A nationwide retrospective study. Eur. J. Surg. Oncol. 2016, 42, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Al-Mukthar, A.; Menon, K.V.; Toogood, G.J.; Lodge, J.P.; Prasad, K.R. Aggressive surgical resection for the management of hepatic metastases from gastrointestinal stromal tumours: A single centre experience. HPB 2007, 9, 64–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mussi, C.; Ronellenfitsch, U.; Jakob, J.; Tamborini, E.; Reichardt, P.; Casali, P.G.; Fiore, M.; Hohenberger, P.; Gronchi, A. Post-imatinib surgery in advanced/metastatic GIST: Is it worthwhile in all patients? Ann. Oncol. 2010, 21, 403–408. [Google Scholar] [CrossRef]
- Jung, J.H.; Won, H.J.; Shin, Y.M.; Kim, P.N. Safety and efficacy of radiofrequency ablation for hepatic metastases from gastrointestinal stromal tumor. J. Vasc. Interv. Radiol. 2015, 26, 1797–1802. [Google Scholar] [CrossRef]
- Jones, R.L.; McCall, J.; Adam, A.; O’Donnell, D.; Ashley, S.; Al-Muderis, O.; Thway, K.; Fisher, C.; Judson, I.R. Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma. Eur. J. Surg. Oncol. 2010, 36, 477–482. [Google Scholar] [CrossRef]
- Littrup, P.J.; Aoun, H.D.; Adam, B.; Krycia, M.; Prus, M.; Shields, A. Percutaneous cryoablation of hepatic tumors: Long-term experience of a large U.S. series. Abdom. Radiol. 2016, 41, 767–780. [Google Scholar] [CrossRef]
- Pretell-Mazzini, J.; Seldon, C.S.; D’Amato, G.; Subhawong, T.K. Musculoskeletal metastasis from soft-tissue sarcomas: A review of the literature. J. Am. Acad. Orthop. Surg. 2022, 30, 493–503. [Google Scholar] [CrossRef]
- Tomasian, A.; Filippiadis, D.K.; Tutton, S.; Deschamps, F.; Cazzato, R.L.; Prologo, J.D.; Kelekis, A.; Levy, J.; Gangi, A.; Garnon, J.; et al. Comprehensive palliative musculoskeletal interventional radiology care for patients with cancer. Radiographics 2022, 42, 1654–1669, Erratum in Radiographics 2023, 43, e229014. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Yevich, S.; Deschamps, F.; Jennings, J.W.; Tutton, S.; Kelekis, A. The role of ablation in cancer pain relief. Curr. Oncol. Rep. 2019, 21, 105. [Google Scholar] [CrossRef]
- Deschamps, F.; Farouil, G.; Ternes, N.; Gaudin, A.; Hakime, A.; Tselikas, L.; Teriitehau, C.; Baudin, E.; Auperin, A.; de Baere, T. Thermal ablation techniques: A curative treatment of bone metastases in selected patients? Eur. Radiol. 2014, 24, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- McMenomy, B.P.; Kurup, A.N.; Johnson, G.B.; Carter, R.E.; McWilliams, R.R.; Markovic, S.N.; Atwell, T.D.; Schmit, G.D.; Morris, J.M.; Woodrum, D.A.; et al. Percutaneous cryoablation of musculoskeletal oligometastatic disease for complete remission. J. Vasc. Interv. Radiol. 2013, 24, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.N.; Callstrom, M.R. Ablation of musculoskeletal metastases: Pain palliation, fracture risk reduction, and oligometastatic disease. Tech. Vasc. Interv. Radiol. 2013, 16, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, D.; Wallace, A.N.; Eiswirth, P.S.; Madaelil, T.P.; Chang, R.O.; Tomasian, A.; Jennings, J.W. Radiographic local tumor control and pain palliation of sarcoma metastases within the musculoskeletal system with percutaneous thermal ablation. Cardiovasc. Interv. Radiol. 2018, 41, 1223–1232. [Google Scholar] [CrossRef]
- Kurup, A.N.; Woodrum, D.A.; Morris, J.M.; Atwell, T.D.; Schmit, G.D.; Welch, T.J.; Yaszemski, M.J.; Callstrom, M.R. Cryoablation of recurrent sacrococcygeal tumors. J. Vasc. Interv. Radiol. 2012, 23, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.Z.; Niu, L.Z.; Wang, Y.; Yao, X.H.; Zhang, Y.Q.; Tan, G.S.; Yang, J.Y.; Li, J.P. Initial experience: Alleviation of pain with percutaneous ct-guided cryoablation for recurrent retroperitoneal soft-tissue sarcoma. J. Vasc. Interv. Radiol. 2016, 27, 1798–1805. [Google Scholar] [CrossRef]
- Littrup, P.J.; Bang, H.J.; Currier, B.P.; Goodrich, D.J.; Aoun, H.D.; Heilbrun, L.K.; Adam, B.A. Soft-tissue cryoablation in diffuse locations: Feasibility and intermediate term outcomes. J. Vasc. Interv. Radiol. 2013, 24, 1817–1825. [Google Scholar] [CrossRef]
- Yamakado, K.; Matsumine, A.; Nakamura, T.; Nakatsuka, A.; Takaki, H.; Matsubara, T.; Asanuma, K.; Sudo, A.; Sugimura, Y.; Sakuma, H. Radiofrequency ablation for the treatment of recurrent bone and soft-tissue sarcomas in non-surgical candidates. Int. J. Clin. Oncol. 2014, 19, 955–962. [Google Scholar] [CrossRef]
- Aubry, S.; Dubut, J.; Nueffer, J.P.; Chaigneau, L.; Vidal, C.; Kastler, B. Prospective 1-year follow-up pilot study of CT-guided microwave ablation in the treatment of bone and soft-tissue malignant tumours. Eur. Radiol. 2017, 27, 1477–1485. [Google Scholar] [CrossRef]
- Yu, W.; Tang, L.; Lin, F.; Yao, Y.; Shen, Z.; Zhou, X. High-intensity focused ultrasound: Noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg. Oncol. 2015, 24, 9–15. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Fan, W.; Huang, J.; Zhang, F.; Wu, P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer 2010, 116, 3934–3942. [Google Scholar] [CrossRef] [PubMed]

| Author | Type of Ablation | Number of Patients (N) | Numbers of Lesions | Mean Size (mm) | Mean Follow Up (Months) | Mean Overall Survival Rate | Median Progression-Free Survival Rate | Complications |
|---|---|---|---|---|---|---|---|---|
| Koelblinger et al. [26] | CT guided RFA | 22 | 55 | 9 | 20 | 94% and 85% in 2 & 3-years | 53% and 23% in 1 & 2 years Median time to local tumor progression was 12 months | 2 patients with grade III |
| Nakamura et al. [28] | CT guided RFA | 20 | 89 | 14 ± 9 | 18 | 58% and 29% in 1 & 3 years (medium 12.9 months) | Four of 20 patients (20%) experienced local tumor progression. Median time to local tumor progression was 7.5 months. | 38% needed pneumothorax tube |
| Palussière et al. [19] | CT guided RFA | 29 | 47 | 9 | 50 | 92.2% and 65.2% in 1 & 3 years | Local control rate was obtained in 42 of 47 ablated metastases (89%) Median time to local tumor progression was 7 months | 68.7% pneumothorax |
| Sato et al. [18] | CT guided RFA RFA | 46 | 144 | 13.5 ± 9.0 | 16.9 | 80.6%, 70.1% and 47.1% in 1, 2 & 3-years (medium 31.7 months) | Primary and secondary efficacy rates were 83.5% and 90.0% at 1 year and 76.3% and 81.4% at 2 years | 73% grade I 33% grade II |
| Bourgouin et al. [22] | 21 MWA sessions &18 CA | 27 | 65 | 11 | 23 | 100%, 89%, and 82% in 1, 2 & 3-years | For tumors < 1 cm local control rates were 97% and 95% after MWA & 99% and 98% after CA in 1 & 2 years For tumors > 1 cm, 74% and 62% after MWA & 86% and 79% after CA. | 44% < Grade III, chest tube placement in 23% |
| Yevich et al. [21] Pediatric population | CT guided RFA+ CRYO | 11 | 26 | 6.7 | 16.7 | Five patients remained in complete remission after 37.5 months & five patients developed new metastases | 3 pneumothoraxes | |
| Saumet et al. [29] Pediatric population | CT guided RFA | 10 | 22 | n/a | 24 | Median progression-free survival was 21.5 months | 3 hemoptysis and 3 pneumothorax |
| Authors | Type of Ablation | Type of Sarcoma | Number of Patients (N) | Mean Lesion Size | Mean Follow Up (Months) | Median Time to Progression | Overall Survival Rate/Time | Complications |
|---|---|---|---|---|---|---|---|---|
| Jones et al. [37] | CT guided RFA | GIST | 13 | - | 21 | 28 months | 2-year overall survival was 77% | 3 patients with sepsis |
| Jung et al. [36] | US guided RFA | GIST | 29 | 1.3 cm | 33.1 | 6% showed local recurrence at 3.2 and 10.5 months | 90.2 months | 1 patient with bleeding at the ablation site & 1 peritoneal seeding near the ablation tract |
| Littrup et al. [38] | CT guided CA | Various | 49 | - | 20 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efthymiou, E.; Charalampopoulos, G.; Velonakis, G.; Grigoriadis, S.; Kelekis, A.; Kelekis, N.; Filippiadis, D. Ablative Techniques for Sarcoma Metastatic Disease: Current Role and Clinical Applications. Medicina 2023, 59, 485. https://doi.org/10.3390/medicina59030485
Efthymiou E, Charalampopoulos G, Velonakis G, Grigoriadis S, Kelekis A, Kelekis N, Filippiadis D. Ablative Techniques for Sarcoma Metastatic Disease: Current Role and Clinical Applications. Medicina. 2023; 59(3):485. https://doi.org/10.3390/medicina59030485
Chicago/Turabian StyleEfthymiou, Evgenia, Georgios Charalampopoulos, Georgios Velonakis, Stauros Grigoriadis, Alexis Kelekis, Nikolaos Kelekis, and Dimitrios Filippiadis. 2023. "Ablative Techniques for Sarcoma Metastatic Disease: Current Role and Clinical Applications" Medicina 59, no. 3: 485. https://doi.org/10.3390/medicina59030485
APA StyleEfthymiou, E., Charalampopoulos, G., Velonakis, G., Grigoriadis, S., Kelekis, A., Kelekis, N., & Filippiadis, D. (2023). Ablative Techniques for Sarcoma Metastatic Disease: Current Role and Clinical Applications. Medicina, 59(3), 485. https://doi.org/10.3390/medicina59030485








