Abstract
Background and Objectives: Periodontitis is a chronic multifactorial inflammatory infectious disease marked by continuous degradation of teeth and surrounding parts. One of the most important periodontal pathogens is P. intermedia, and with its interpain A proteinase, it leads to an increase in lethal infection. Materials and Methods: The current study was designed to create a multi-epitope vaccine using an immunoinformatics method that targets the interpain A of P. intermedia. For the development of vaccines, P. intermedia peptides InpA were found appropriate. To create a multi-epitope vaccination design, interpain A, B, and T-cell epitopes were found and assessed depending on the essential variables. The vaccine construct was evaluated based on its stability, antigenicity, and allergenicity. Results: The vaccine construct reached a more significant population and was able to bind to both the binding epitopes of major histocompatibility complex (MHC)-I and MHC-II. Through the C3 receptor complex route, P. intermedia InpA promotes an immunological subunit. Utilizing InpA-C3 and vaccination epitopes as the receptor and ligand, the molecular docking and dynamics were performed using the ClusPro 2.0 server. Conclusion: The developed vaccine had shown good antigenicity, solubility, and stability. Molecular docking indicated the vaccine’s 3D structure interacts strongly with the complement C3. The current study describes the design for vaccine, and steady interaction with the C3 immunological receptor to induce a good memory and an adaptive immune response against Interpain A of P. intermedia.
1. Introduction
With the advances in technology and science, significant progress has been made in understanding microorganisms and disease processes. The knowledge of the disease process has undergone a paradigm shift from early models that assumed the quantity of plaque to current concepts of host-microbial interactions [1]. Periodontitis is a microbial-induced, host-mediated, chronic inflammatory disease characterized by dysbiotic plaque biofilms that cause progressive attachment loss [2,3]. Some bacteria, even in small quantities, can interact with the host’s immune system and other bacteria, increasing the pathogenicity of the microbiome. The dysbiotic microbiota includes a variety of microorganisms, of which Prevotella intermedia, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans have significant effects on the microbiota, disrupting tissue homeostasis [4,5].
A localized chronic inflammation and subsequent loss of the tooth’s supporting structures result from the intricate interplay of the bacterial virulence factors and defense mechanisms of the host [6,7]. Many periodontal pathogens create proteinases, which are key virulence factors that can lead to the host’s proteins being broken down for vital nutrients. Many strains of P. intermedia have evolved defenses against complement killing to become successful pathogens [8]. The function of the complement system is to promote the uptake and destruction of pathogens by phagocytic cells. Complement receptors (CRs) on phagocytes detect bound complement components. These complement receptors bind pathogens that have been opsonized by complement proteins, which is one of the main roles of C3b and its proteolytic derivatives. Since C3b is produced in greater quantities than C4b, C4b also functions as an opsonin but plays a considerably smaller role. Hence, microbes have found ways to evade this system for better survival. Similar to how gingipains have favoured the chain of C3 and C4, Interpain A (InpA) also demonstrated this preference. Gingipains primarily bind the chains of these proteins, allowing them to release the complements C3a and C5a, which serve as anaphylatoxins, and their induced versions C3b, C4b, and C5b at low doses [9].
Prevotella intermedia, an oral Gram-negative anaerobe, helps in converting hemoglobin to an iron (III) protoporphyrin IX pigment. The bacterium produces InpA (interpain A), a 90-kDa cysteine protease, a homolog of streptopain from Streptococcus pyogenes (SpeB). Under situations of low redox potential and higher pH in the infected gingival crevice and diseased periodontal pocket, where the host closely regulates the availability of haeme, InpA greatly contributes to the acquisition of haeme [10]. Haem albumin is more sensitive to InpA than apo albumin. In order to extract haemoglobin’s hemosiderin from the cell, Prevotella intermedia’s cysteine protease Interpain A (InpA) collaborates with P. gingivalis’ HmuY hameophore, demonstrating further post-translational interaction. Notably, gingipains work in concert with karilysin or Interpain A to suppress complement, indicating that these complementary proteases may still prevent complement activation after being released and diffused across the biofilm that is defending the entire microbial community [11].
Computational immunology, often known as immunoinformatics, is a subfield of bioinformatics that uses bioinformatics methods to comprehend and analyze immunological data [12]. Utilizing databases and other technologies to predict B- and T-cell epitopes is one of the most researched aspects of applied immunology. Researchers can now utilize an organism’s genome information to identify vaccine candidates computationally, moving beyond the old vaccinology method thanks to advancements in sequencing tools [13,14]. Major colonization factors, adhesion proteins, and other well-characterized virulence components essential for infection initiation and additional host damage have been the main targets of vaccine development. The pathogen genome does, however, encode several uncharacterized proteins that have yet to be investigated for their potential to encode antigenic regions. Immunoinformatics techniques can be beneficial, especially for diseases with minimal information on pathogenesis mechanisms or antigenic determinants [15,16].
Since P. intermedia (Interpain A) has a significant role in host immune modulation and providing the required nutrition to the microbiome, the current study aimed to design a vaccine that targets Interpain A using an immunoinformatics approach.
2. Materials and Methods
2.1. Sequence Analysis
Immune epitope database analysis was used to identify the protein structure of the epitope of P. intermedia with the help of positive assays for linear epitopes [17]. The network assembled was examined for hubs, shortest path, and clustering coefficient. The Protein Data Bank (PDB) database was used to retrieve the amino acid (FASTA) reference identity (ID) of 3BBA, which belongs to P. intermedia. The antigenic peptides prediction tool (http://imed.med.ucm.es/Tools/antigenic.pl) (accessed on 12 March 2022) and the AllerTop v2.0 servers (http://ddg-pharmfac.net/AllergenFP/) (accessed on 12 March 2022) were used to screen 3BBA for average antigenic propensity and allergenicity [12].
2.2. Prediction of Epitope
Using NetCTL1.2 (DTU Health Tech, Lyngby, Denmark) [18], lymphocyte (CTL-cytotoxic-T cells) epitopes for 3BBA were predicted for serotypes that had a threshold value of 0.75, 0.97 (specificity), or 0.80 (sensitivity). Default levels of C-terminal cleavage and transporter associated with the antigen were used. Both immune and antigen reactivity were ascertained using Class-I immunogenicity of the IEDB server and VaxiJen v2.0. Using a traditional method and a percentile rank of 2, the MHC-I specific gene sequence of a subset of CTL epitopes (17 epitopes/ligands) was found with MHC-I related predictions in the Immune Epitope Database (http://tools.iedb.org/mhci/), (accessed on 14 March 2022). The percentile rank and IC50 value of peptide-MHC-II interactions were determined using the NN Align technique and the IEDB MHC-II epitope prediction tool. The origin species was a person. Further analysis was conducted on the Human Leukocyte Antigen–DR isotype (HLA-DR), HLA-DP, and HLA-DQ loci. Since these results represent a greater affinity, IC50 values of 10 nM and a percentile rank of 1.5 were utilized for prediction. We assessed the antigenic properties of anticipated HTL epitopes. Finally, the allergenicity, toxicity, and antigenicity of the 3BBA epitopes from Cytotoxic T lymphocytes (CTL), Helper T cell (HTL), and B cell lymphoma (BCL) were considered. For the creation of multi-epitope vaccines, the predicted 3BBA epitopes were utilized [13].
2.3. Population Coverage Analysis
Expression and distribution of HLA alleles expressed diverse presentations between regions and ethnicity, which may impact the creation of multi-epitope-based vaccines [19]. The population coverage tool of IEDB was used to assess the CTL and HTL.
2.4. Construction of Multi Epitope Vaccine
Adjuvant, CTL, HTL, and BCL epitopes were combined to form appropriate links to allow the epitopes, in vivo, adequate room to function. Human-defensin-2 (PDB ID: 1FD 3) served as an adjuvant along with a B-cell epitope utilizing an EAAAK linker to boost the immunogenicity of the vaccine candidate [14,20]. The same GSGSGS, GSGSGS, and AAY linkers were used to bind BCL to HTL, HTL to CTL, and intra-CTL epitopes, respectively.
2.5. Structure Prediction and Validation
Through the use of the Swiss model, the iterative threading modeling method was used to predict and validate the three-dimensional structure of the vaccine construct.
2.6. Molecular Docking Analysis
Utilizing 2a73 complement C3 and the vaccine receptor and ligand, molecular docking with ClusPro 2.0 program was used in order to assess the co-action of the vaccine and with the host immune receptor. So, using three sequential steps—rigid body docking, clustering of lowest energy structures, and structural refinement—complexes were created. The docked structure was examined using PyMol (http://www.pymol.org) (accessed on 15 March 2022), and the ideal complex was selected to assess which complex had a lesser energy score [12,13].
2.7. Molecular Dynamics Simulation
The dynamics and structural stability of protein complexes are effectively investigated by molecular dynamics (MD) simulation. Desmond software (D. E. Shaw Research, New York, NY, United States) used MD to imitate vaccine-C3. Desmond (Schrödinger LLC, New York, NY, USA) ran a 100-nanosecond simulation of molecular dynamics. The receptor-ligand complex was reduced and optimized by Maestro’s Protein Preparation Wizard. Using System Builder, all systems were created. An orthorhombic solvent model is TIP3P.
(Points from TIIP3) The simulation made use of OPLS 2005. NaCl at 0.15 M simulated physiological conditions. The ensemble of 300 K and 1 atm NPT was used throughout the simulation. Simulation models were lax. The simulation’s stability was evaluated by comparing the root-mean-square deviation (RMSD) over time for the protein and ligand.
3. Results
3.1. Analysis of P. intermedia Peptide Sequences
The molecular weight of the peptide 3BBA was approximately 251 amino acids bases. In vaccine development, epitope identification assesses proteins whose antigen prediction was higher than 0.8 (Supplementary Figure S1). The antigenic susceptibility of 3BBA was found to be 1.0122 on average, and they were not allergic (Supplementary Figure S2). For the creation of a multi-epitope vaccine against P. intermedia, 3BBA was chosen based on its antigenic propensity (Figure 1 and Supplementary Figure S3).
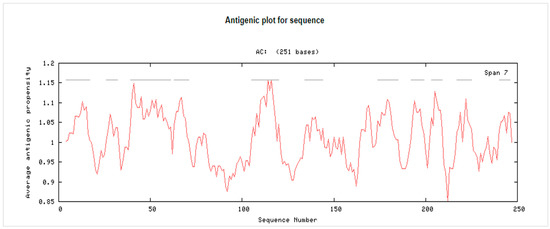
Figure 1.
The antigenic propensity of reference ID 3BBA. AC: Amino acids.
3.2. Prediction and Assessment of T-Lymphocyte Epitope
Cytotoxic T-lymphocyte epitopes are essential for eliciting robust immune reactions involving the histocompatibility complex. The NetCTL1.2 service was used to find the epitopes of 3BBA. Fifteen epitopes from 3BBA with cumulative scores greater than 0.75 were discovered from all MHC-I serotypes (Table 1 and Supplementary Table S1). Helper T-lymphocytes activate cytotoxic T-cells to produce antibodies and kill infected target cells. For the HLA-DR, HLA-DQ, and HLA-DP loci, HTL epitopes for 3BBA were predicted using IC50 values (10 nM) and percentile rank (1.5). The HTL epitopes at the HLA-DR gene met several requirements. The HTL epitope (obtained from MHC-II) was discovered to be similar. The C-terminal dimerization domain employed in the development of vaccines was chosen to contain the HTL epitope (LAEVKALTTELTAEN) (Table 2).

Table 1.
MHC-I binding predictions of IEDB server.

Table 2.
MHC-II binding peptides (Consensus (comb.lib./smm/nn)) accessed on 17 March 2022.
3.3. Prediction and Assessment of B-Lymphocytes
B-cell epitopes are crucial in the production of antibodies. The BepiPred server confirmed the B-cell epitopes identified by ABCPred (Table 3) with a 16-mer length score of 0.5 or above (Figure 2). The BCL epitopes for 3BBA were discovered to satisfy server requirements by demonstrating antigenic, non-allergic, and non-toxic characteristics (Table 4 and Table 5). Ultimately, 3BBA was chosen for vaccine production based on the study and prediction of CTL, HTL, and BCL.

Table 3.
Identification of B-cell epitopes (ABCPred).
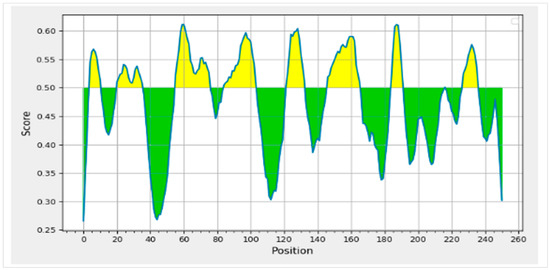
Figure 2.
Graphical representation of the BCL epitopes’ confirmation with the BepiPred server identified by ABCPred with a 16-mer length score of 0.5 or above.

Table 4.
Predicted peptides.

Table 5.
Toxicity prediction of epitopes.
3.4. Analysis of Population Coverage
The population coverage of the peptide epitope 3BBA from P. intermedia was examined (Table 6). The combined MHC-I and MHC-II epitopes revealed 89.44% and 29.81% of population coverage worldwide, respectively, using the chosen T-cell epitopes with cognate HLA alleles (Figure 3). Notably, our vaccine candidate has demonstrated wider population coverage to combat P. intermedia globally.

Table 6.
India Asian-class II coverage.
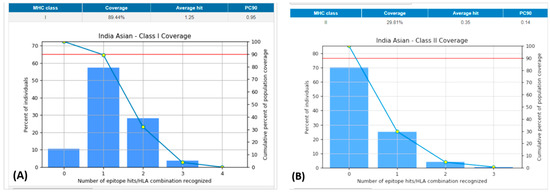
Figure 3.
Population coverage of all the selected epitopes for both MHC-I and II classes. (A): Indian Asian class-I coverage. (B): Indian Asian class-II coverage. MHC: major histocompatibility complex.
3.5. Construction of Multi-Epitope Vaccine
The epitopes chosen from the top-scoring MHC classes 1 and 2 were stitched together by appropriate linkers to create a multi-epitope vaccine. An EAAAK linker was used to bind the adjuvant to the BCL epitope to prevent adjuvant interaction with the vaccine design. An AAY linker was used to unite CTL epitopes, maintaining the structural configuration of the epitopes while increasing the likelihood of antigenic reactivity. Additionally, BCL to HTL and HTL to CTL epitopes were linked together using the linker GSGSGS, which gives proteins structural flexibility without affecting the function of vaccine candidates.
3.6. Molecular Docking
ClusPro 2.0-based molecular docking was conducted to evaluate the vaccine components’ interactions with Human Complement Component C3 PDB id-2a73. The best complex of vaccine-C3 was preferred as it presented the lowest energy score (596.9 kJ mol−1) and center energy (the energy between receptor and ligand) of −661.2 kJ mol−1 (Supplementary Figure S4). The vaccine candidate’s residues displayed polar contact with the C3 receptor residues (Figure 4B). The Molecular Dynamic Simulation Analysis has been shown in Supplementary Figure S5.
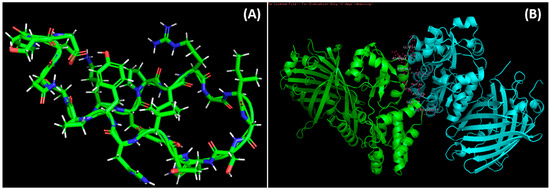
Figure 4.
Docked complexes of the vaccine and the epitopes. (A): Predicted structure of modeled vaccine construct using a Swiss model. (B): Molecular docking of epitopes with 2a37 complement c3 receptor.
The stability and compactness of the docked vaccine-C3 complex were investigated using the Desmond tool and a 100 ns molecular dynamics simulation. The complicated RMSD (1) plot demonstrates that stability was obtained at 40 ns. Following that, fluctuations in protein RMSD values remained within 1.5 throughout the simulation time. The RMSD values of ligands range from 2.0 angstrom to 100 ns.
4. Discussion
The primary goal of the periodontal vaccine is to depreciate disease progression and eventually eradicate periodontal disease [21]. P. intermedia is a bacterium that is closely associated with various periodontal diseases and other infections [22,23]. P. intermedia has frequently been found in subgingival plaque in human patients with necrotizing gingivitis, pregnancy gingivitis, and adult periodontitis [24]. Additionally, P. intermedia is challenging to eliminate because it quickly develops antibiotic resistance [25]. An in-depth molecular understanding of infection and resistance is essential for developing alternative treatments [26]. Numerous proteases, including trypsin-like serine proteases, dipeptidyl peptidase IV, and cysteine proteases, have been identified in P. intermedia [27]. However, the development of structural studies enabled us to comprehend their unique mode of action and aid in designing vaccines. Epitope-based vaccines have frequently been created using immunoinformatics, an innovative and practical approach [28].
Interpain A, a cysteine protease from the cysteine-histidine-dyad class, was investigated in its zymogenic and mature self-processed forms. The latter is made up of a bivalved portion with two subdomains. Complement is an important component of the innate immune defense system, with the primary purpose of recognizing and killing bacteria [28,29].
Heat inactivation of the complement system greatly reduces the opsonic activity in vitro, indicating that complement is required for host defense against P. intermedia [30,31]. Moreover, in the absence of the classical system, the alternative pathway opsonized P. intermedia, most likely due to a reaction to endotoxin. Yet, kinetic tests demonstrated that opsonization occurred substantially faster when the classical pathway was intact [32]. Surprisingly, the alternative pathway contributed to the death of serum-sensitive strains, whereas the traditional pathway was predominantly responsible for the death of intermediate-sensitivity strains [33]. As a result, the complement appears to recognize P. intermedia through many sensory chemicals. However, it appears that P. intermedia can bind to C3 and, to some extent, overwhelm complement defenses, allowing chronic infections to develop in the oral cavity [34]. Furthermore, Interpain A acts synergistically with P. gingivalis gingipains [35]. When applied in equal quantities, C3b deposition was reduced by 85%, compared to 55% when added individually. Moreover, combining three gingipains with InpA reduced the C3b deposition by 93%. Hence, the C3 target used in the current study is relevant and practical since the multiple vaccine epitope inhibits the binding of Interpain A to C3, besides eliminating the interaction or association with other periodontopathogens [27].
Epitopes induce cytotoxic T- and B-cell lymphocytes to destroy pathogenic microbes through cytokine action [36]. Cytokines utilize helper T-lymphocytes to trigger the immune system. The P. intermedia 3BBA epitope peptide depicted higher antigenicity, immunogenicity, non-allergenic and non-toxic nature, and increased MHC-I and II binding than other epitopes. The present study results show that the current vaccine design is non-allergic, non-antigenic, non-toxic, and does exhibit good immunogenicity. Moreover, molecular docking and dynamics results exhibit excellent and stable binding throughout the stimulation period. This is due to the increased affinity of the vaccine for the C3 receptors. Further studies with DNA cloning are required to make this vaccine a reality.
5. Conclusions
The current study provides the first vaccine design for Interpain A using an Immunoinformatic approach. P. intermedia is a common bacterium that is associated with periodontal infections. It also contributes to the sustainability of the microbiome by providing essential substrates such as albumin and haem. Future well-designed studies are required to evaluate the efficacy of this vaccine design. Furthermore, it will be interesting to observe the overall effect of the elimination of P. intermedia on the microbiome.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina59020302/s1, Figure S1: Antigenic Peptides determinants; Figure S2: Prediction of Allergenicity; Figure S3: Antigenicity Prediction; Figure S4: Binding energy of the Predicted vaccine structure with C3; Figure S5: Molecular dynamic simulation analysis; Table S1: CTL epitopes for N protein (NetCTL1.2).
Author Contributions
Conceptualization, P.K.Y., R.V.A., R.R. and A.A.R.; methodology, S.M., P.K.Y., R.V.A., R.R., S.R. (Santhiya Rengaraj) and S.R. (Sindhu Ramesh).; software, M.A., B.R.A.S., A.A.A., A.S.S.A., S.A.T., A.A. (Abdulsalam Alawfi), P.K.Y., R.V.A., R.R. and A.A.R.; validation, A.A. (Amer Alshengeti), M.G., A.A.S., M.A.A. and F.S.A.; formal analysis, M.A., B.R.A.S., A.A.A., A.S.S.A., S.A.T., A.A. (Abdulsalam Alawfi), P.K.Y., R.V.A., R.R. and A.A.R.; validation, A.A. (Amer Alshengeti); investigation, S.M., P.K.Y., R.V.A., R.R., S.R. (Santhiya Rengaraj) and S.R. (Sindhu Ramesh).; resources, R.R., M.A., B.R.A.S. and M.G.; data curation, P.K.Y., R.V.A., R.R. and A.A.R.; writing—original draft preparation, A.A. (Abdulsalam Alawfi), P.K.Y., R.V.A., R.R. and A.A.R.; validation, A.A. (Amer Alshengeti); writing—review and editing, P.K.Y., R.V.A., R.R. and A.A.R.; visualization, P.K.Y. and R.V.A.; supervision, R.V.A. and A.A.R.; project administration, P.K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lamont, R.J.; Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2015, 21, 172–183. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Arshad, S.; Basheer, S.N.; Karobari, M.I.; Marya, A.; Marya, C.M.; Taneja, P.; Messina, P.; Yean, C.Y.; Scardina, G.A. Smoking a Dangerous Addiction: A Systematic Review on an Underrated Risk Factor for Oral Diseases. Int. J. Environ. Res. Public Health 2021, 18, 11003. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The nexus between periodontal inflammation and dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Myneni, S.R.; Brocavich, K.; Wang, H.H. Biological strategies for the prevention of periodontal disease: Probiotics and vaccines. Periodontology 2000 2020, 84, 161–175. [Google Scholar] [CrossRef]
- Karobari, M.I.; Arshad, S.; Noorani, T.Y.; Ahmed, N.; Basheer, S.N.; Peeran, S.W.; Marya, A.; Marya, C.M.; Messina, P.; Scardina, G.A. Root and Root Canal Configuration Characterization Using Microcomputed Tomography: A Systematic Review. J. Clin. Med. 2022, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Moen, K.; Brun, J.G.; Madland, T.M.; Tynning, T.; Jonsson, R. Immunoglobulin G and A antibody responses to Bacteroides forsythus and Prevotella intermedia in sera and synovial fluids of arthritis patients. Clin. Vaccine Immunol. 2003, 10, 1043–1050. [Google Scholar] [CrossRef]
- Amano, A. Bacterial adhesins to host components in periodontitis. Periodontology 2000 2010, 52, 12–37. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Byrne, D.P.; Wawrzonek, K.; Jaworska, A.; Birss, A.J.; Potempa, J.; Smalley, J.W. Role of the cysteine protease interpain A of Prevotella intermedia in breakdown and release of haem from haemoglobin. Biochem. J. 2010, 425, 257–264. [Google Scholar] [CrossRef]
- Byrne, D.P.; Manandhar, S.P.; Potempa, J.; Smalley, J.W. Breakdown of albumin and haemalbumin by the cysteine protease interpain A, an albuminase of Prevotella intermedia. BMC Microbiol. 2015, 15, 185. [Google Scholar] [CrossRef]
- Naveed, M.; Ali, U.; Karobari, M.I.; Ahmed, N.; Mohamed, R.N.; Abullais, S.S.; Kader, M.A.; Marya, A.; Messina, P.; Scardina, G.A. A Vaccine Construction against COVID-19-Associated Mucormycosis Contrived with Immunoinformatics-Based Scavenging of Potential Mucoralean Epitopes. Vaccines 2022, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hassan, J.-u.; Ahmad, M.; Naeem, N.; Mughal, M.S.; Rabaan, A.A.; Aljeldah, M.; Shammari, B.R.A.; Alissa, M.; Sabour, A.A. Designing mRNA-and Peptide-Based Vaccine Construct against Emerging Multidrug-Resistant Citrobacter freundii: A Computational-Based Subtractive Proteomics Approach. Medicina 2022, 58, 1356. [Google Scholar] [CrossRef]
- Naveed, M.; Yaseen, A.R.; Khalid, H.; Ali, U.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Al Mutair, A.; Alhumaid, S.; Al Alawi, Z. Execution and Design of an Anti HPIV-1 Vaccine with Multiple Epitopes Triggering Innate and Adaptive Immune Responses: An Immunoinformatic Approach. Vaccines 2022, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Tomar, N. Immunoinformatics; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Naveed, M.; Bukhari, B.; Afzal, N.; Sadia, H.; Meer, B.; Riaz, T.; Ali, U.; Ahmed, N. Geographical, Molecular, and Computational Analysis of Migraine-Causing Genes. J. Comput. Biophys. Chem. 2021, 20, 391–403. [Google Scholar] [CrossRef]
- Rappuoli, R. Reverse vaccinology. Curr. Opin. Microbiol. 2000, 3, 445–450. [Google Scholar] [CrossRef]
- Ashfaq, U.A.; Ahmed, B. De novo structural modeling and conserved epitopes prediction of Zika virus envelop protein for vaccine development. Viral Immunol. 2016, 29, 436–443. [Google Scholar] [CrossRef]
- Naveed, M.; Jabeen, K.; Naz, R.; Mughal, M.S.; Rabaan, A.A.; Bakhrebah, M.A.; Alhoshani, F.M.; Aljeldah, M.; Shammari, B.R.A.; Alissa, M. Regulation of Host Immune Response against Enterobacter cloacae Proteins via Computational mRNA Vaccine Design through Transcriptional Modification. Microorganisms 2022, 10, 1621. [Google Scholar] [CrossRef]
- Ahmad, B.; Ashfaq, U.A.; Rahman, M.-u.; Masoud, M.S.; Yousaf, M.Z. Conserved B and T cell epitopes prediction of ebola virus glycoprotein for vaccine development: An immuno-informatics approach. Microb. Pathog. 2019, 132, 243–253. [Google Scholar] [CrossRef]
- Meenakshi, S.; Varghese, S. Periodontal Vaccines-A systematic Review. Braz. Dent. Sci. 2020, 23, 17-p. [Google Scholar] [CrossRef]
- Dixitraj, P.; Nayak, A.; Bansal, S.; Bhat, K. Detection of antibodies against Prevotella Intermedia in patients with chronic periodontitis and periodontally healthy individuals. Dent. Med. Res. 2021, 9, 45. [Google Scholar] [CrossRef]
- Persson, G.R. Immune responses and vaccination against periodontal infections. J. Clin. Periodontol. 2005, 32, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Borgo, P.V.; Rodrigues, V.A.A.; Feitosa, A.C.R.; Xavier, K.C.B.; Avila-Campos, M.J. Association between periodontal condition and subgingival microbiota in women during pregnancy: A longitudinal study. J. Appl. Oral Sci. 2014, 22, 528–533. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Alhumaid, S.; Mutair, A.A.; Garout, M.; Abulhamayel, Y.; Halwani, M.A.; Alestad, J.H.; Bshabshe, A.A.; Sulaiman, T.; AlFonaisan, M.K. Application of Artificial Intelligence in Combating High Antimicrobial Resistance Rates. Antibiotics 2022, 11, 784. [Google Scholar] [CrossRef]
- Zeb, S.; Mushtaq, M.; Ahmad, M.; Saleem, W.; Rabaan, A.A.; Naqvi, B.S.Z.; Garout, M.; Aljeldah, M.; Al Shammari, B.R.; Al Faraj, N.J. Self-Medication as an Important Risk Factor for Antibiotic Resistance: A Multi-Institutional Survey among Students. Antibiotics 2022, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Potempa, M.; Potempa, J.; Kantyka, T.; Nguyen, K.-A.; Wawrzonek, K.; Manandhar, S.P.; Popadiak, K.; Riesbeck, K.; Eick, S.; Blom, A.M. Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3. PLoS Pathog. 2009, 5, e1000316. [Google Scholar] [CrossRef]
- Rakib, A.; Sami, S.A.; Islam, M.A.; Ahmed, S.; Faiz, F.B.; Khanam, B.H.; Marma, K.K.S.; Rahman, M.; Uddin, M.M.N.; Nainu, F. Epitope-based immunoinformatics approach on nucleocapsid protein of severe acute respiratory syndrome-coronavirus-2. Molecules 2020, 25, 5088. [Google Scholar] [CrossRef] [PubMed]
- Mallorquí-Fernández, N.; Manandhar, S.P.; Mallorqui-Fernandez, G.; Uson, I.; Wawrzonek, K.; Kantyka, T.; Sola, M.; Thøgersen, I.B.; Enghild, J.J.; Potempa, J. A new autocatalytic activation mechanism for cysteine proteases revealed by Prevotella intermedia interpain A. J. Biol. Chem. 2008, 283, 2871–2882. [Google Scholar] [CrossRef]
- Potempa, M.; Potempa, J.; Okroj, M.; Popadiak, K.; Eick, S.; Nguyen, K.-A.; Riesbeck, K.; Blom, A.M. Binding of complement inhibitor C4b-binding protein contributes to serum resistance of Porphyromonas gingivalis. J. Immunol. 2008, 181, 5537–5544. [Google Scholar] [CrossRef]
- Dhingra, K.; Vandana, K. Prophylactic vaccination against periodontal disease: A systematic review of preclinical studies. J. Periodontol. 2010, 81, 1529–1546. [Google Scholar] [CrossRef]
- Krauss, J.L.; Potempa, J.; Lambris, J.D.; Hajishengallis, G. Complementary Tolls in the periodontium: How periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontology 2000 2010, 52, 141. [Google Scholar] [CrossRef] [PubMed]
- Potempa, J.; Pike, R.N. Corruption of innate immunity by bacterial proteases. J. Innate Immun. 2009, 1, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.P.; Potempa, J.; Olczak, T.; Smalley, J.W. Evidence of mutualism between two periodontal pathogens: Co-operative haem acquisition by the H mu Y haemophore of P orphyromonas gingivalis and the cysteine protease interpain A (I np A) of P revotella intermedia. Mol. Oral Microbiol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yi, N.N.; Kim, U.S.; Choi, J.S.; Kim, S.J.; Choi, J.I. Porphyromonas gingivalis heat shock protein vaccine reduces the alveolar bone loss induced by multiple periodontopathogenic bacteria. J. Periodontal Res. 2006, 41, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Qureshi, R.; Sagurthi, S.R.; Qureshi, I.A. Designing of nucleocapsid protein based novel multi-epitope vaccine against SARS-COV-2 using immunoinformatics approach. Int. J. Pept. Res. Ther. 2021, 27, 941–956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).