Circulating CD4+ Treg, CD8+ Treg, and CD3+ γδ T Cell Subpopulations in Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Specimens
2.2. Blood Processing and Flow Cytometry
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Measurement of CA125, HE4 Concentrations
2.5. Statistical Analysis
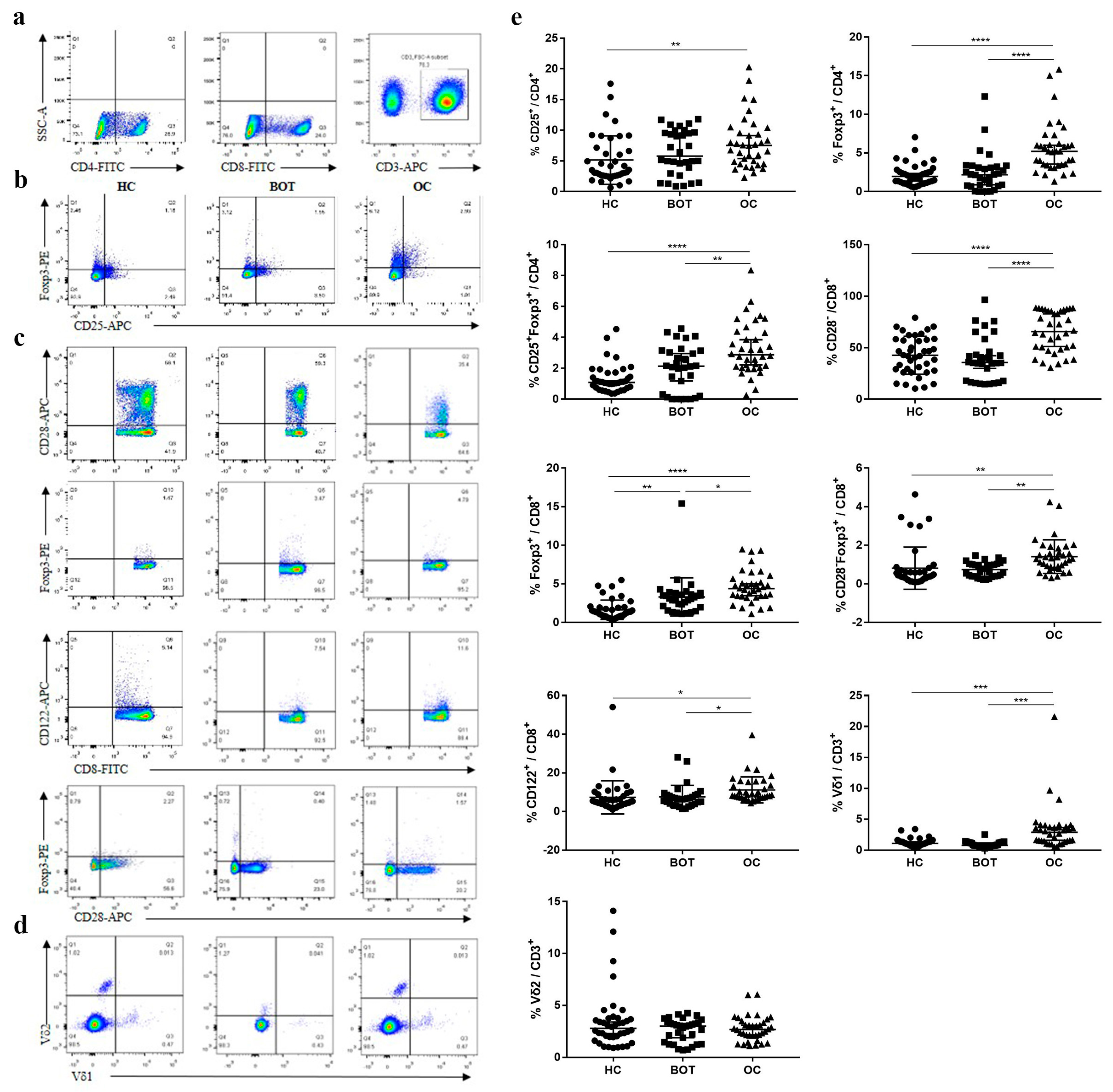
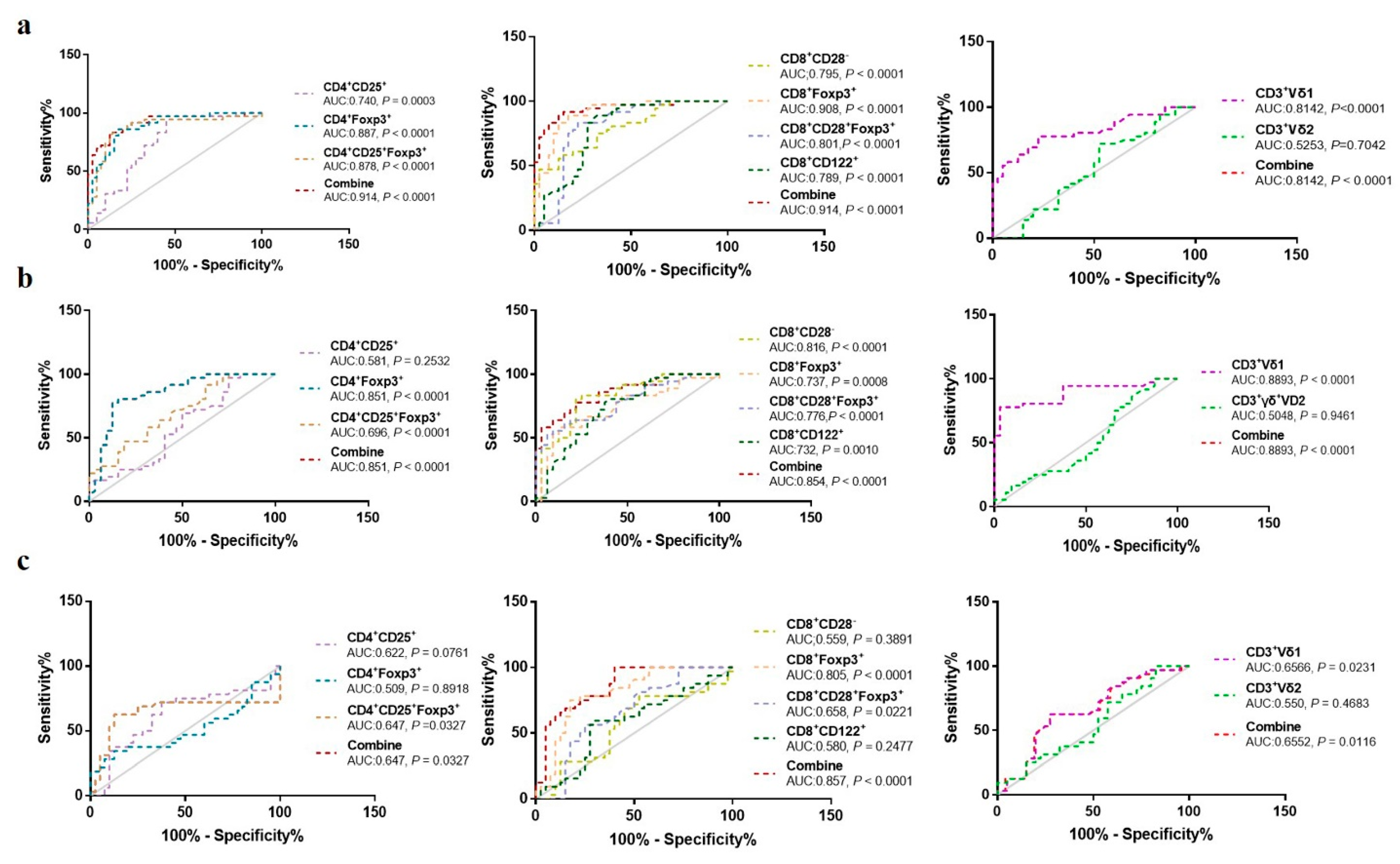
3. Results
3.1. Comparison of Proportion of Treg Subsets in Different Groups
3.2. The Relationship between Proportion of Different Treg Subsets and Clinical Characteristics of OC Patients
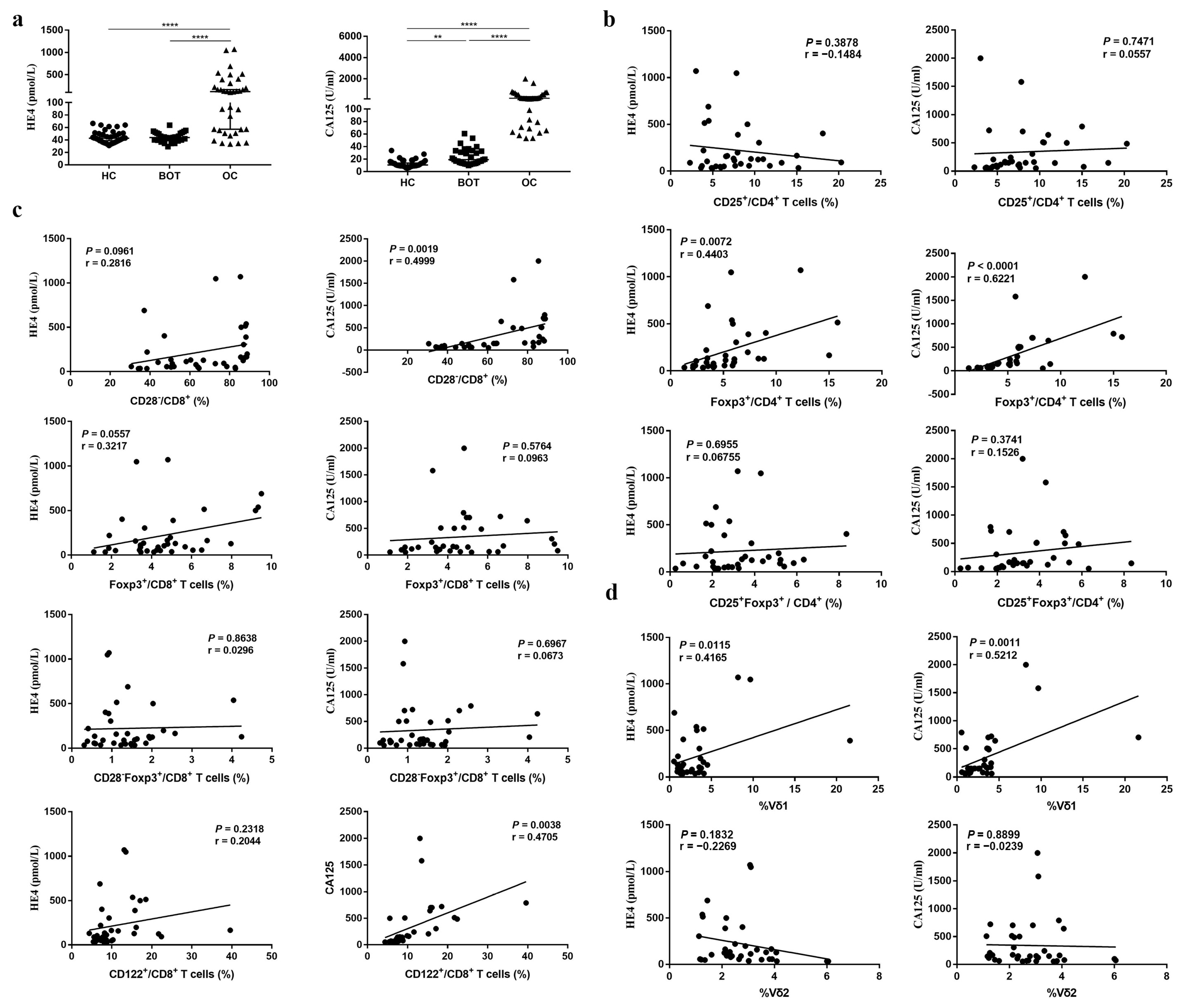
3.3. The Relationship between Proportion of Different Treg Subsets and CA125, HE4 of OC Patients
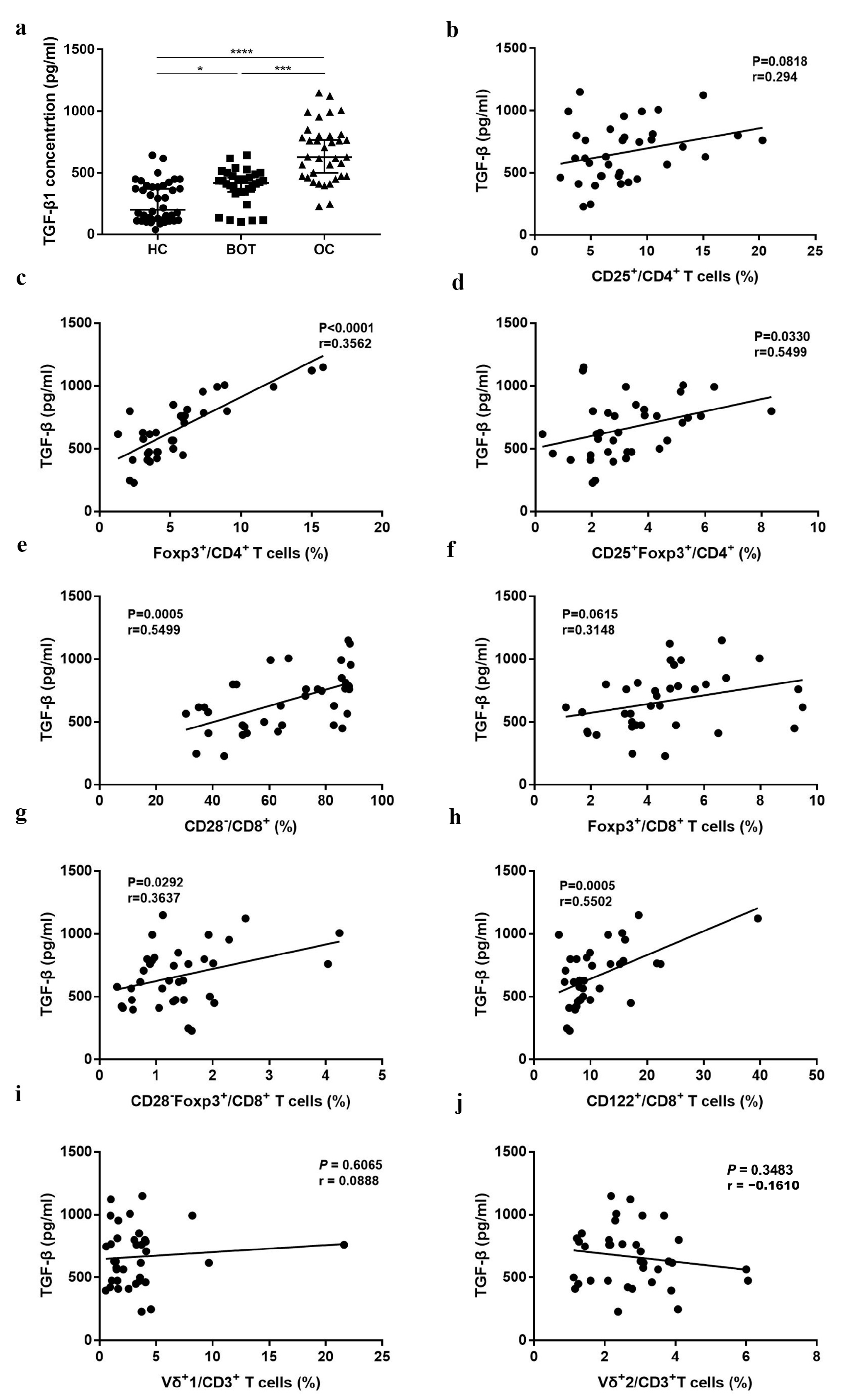
3.4. The Relationship between Proportion of Different Treg Subsets and TGF-β of OC Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zamwar, U.M.; Anjankar, A.P. Aetiology, Epidemiology, Histopathology, Classification, Detailed Evaluation, and Treatment of Ovarian Cancer. Cureus 2022, 14, e30561. [Google Scholar] [CrossRef] [PubMed]
- Cabasag, C.J.; Fagan, P.J.; Ferlay, J.; Vignat, J.; Laversanne, M.; Liu, L.; van der Aa, M.A.; Bray, F.; Soerjomataram, I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer 2022, 151, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Mazidimoradi, A.; Momenimovahed, Z.; Allahqoli, L.; Tiznobaik, A.; Hajinasab, N.; Salehiniya, H.; Alkatout, I. The global, regional and national epidemiology, incidence, mortality, and burden of ovarian cancer. Health Sci. Rep. 2022, 5, e936. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018: Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Trac, N.T.; Chung, E.J. Peptide-based targeting of immunosuppressive cells in cancer. Bioact. Mater. 2020, 5, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Workman, C.J.; Vignali, D.A.A. Targeting regulatory T cells in tumors. FEBS J. 2016, 283, 2731–2748. [Google Scholar] [CrossRef]
- De Simone, M.; Arrigoni, A.; Rossetti, G.; Gruarin, P.; Ranzani, V.; Politano, C.; Bonnal, R.J.; Provasi, E.; Sarnicola, M.L.; Panzeri, I.; et al. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity 2016, 45, 1135–1147. [Google Scholar] [CrossRef]
- Vanguri, R.; Benhamida, J.; Young, J.H.; Li, Y.; Zivanovic, O.; Chi, D.; Snyder, A.; Hollmann, T.J.; Mager, K.L. Understanding the impact of chemotherapy on the immune landscape of high-grade serous ovarian cancer. Gynecol. Oncol. Rep. 2022, 39, 100926. [Google Scholar] [CrossRef]
- Lo, C.S.; Sanii, S.; Kroeger, D.R.; Milne, K.; Talhouk, A.; Chiu, D.S.; Rahimi, K.; Shaw, P.A.; Clarke, B.A.; Nelson, B.H. Neoadjuvant Chemotherapy of Ovarian Cancer Results in Three Patterns of Tumor-Infiltrating Lymphocyte Response with Distinct Implications for Immunotherapy. Clin. Cancer Res. 2017, 23, 925–934. [Google Scholar] [CrossRef]
- Napoletano, C.; Bellati, F.; Landi, R.; Pauselli, S.; Marchetti, C.; Visconti, V.; Sale, P.; Liberati, M.; Rughetti, A.; Frati, L.; et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J. Cell. Mol. Med. 2010, 14, 2748–2759. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ke, X.; Zeng, S.; Wu, M.; Lou, J.; Wu, L.; Huang, P.; Huang, L.; Wang, F.; Pan, S. Analysis of CD8+ Treg cells in patients with ovarian cancer: A possible mechanism for immune impairment. Cell. Mol. Immunol. 2015, 12, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef]
- Asiry, S.; Kim, G.; Filippou, P.S.; Sanchez, L.R.; Entenberg, D.; Marks, D.K.; Oktay, M.H.; Karagiannis, G.S. The Cancer Cell Dissemination Machinery as an Immunosuppressive Niche: A New Obstacle Towards the Era of Cancer Immunotherapy. Front. Immunol. 2021, 12, 654877. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.; Zhou, X.; Wang, T.; Zhang, J. Changes in tumor markers, coagulation function and serum VEGF in patients with ovarian cancer and benign ovarian disease. J. BUON Off. J. Balk. Union Oncol. 2020, 25, 2287–2292. [Google Scholar]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188503. [Google Scholar] [CrossRef]
- Li, W.; Wang, D. The differential diagnostic value and clinical significance of serum HE4 in ovarian disease with elevated CA125. Arch. Gynecol. Obstet. 2020, 301, 1219–1225. [Google Scholar] [CrossRef]
- Plitas, G.; Rudensky, A.Y. Regulatory T Cells: Differentiation and Function. Cancer Immunol. Res. 2016, 4, 721–725. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wu, M.; Liu, S.; Shang, W.; Li, R.; Xu, J.; Huang, L.; Wang, F. Glucose metabolism characteristics and TLR8-mediated metabolic control of CD4+ Treg cells in ovarian cancer cells microenvironment. Cell Death Dis. 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Maurer, M.J.; Preston, C.C.; Moysich, K.B.; Goergen, K.; Hawthorne, K.M.; Cunningham, J.M.; Odunsi, K.; Hartmann, L.C.; Kalli, K.R.; et al. Regulatory T cells, inherited variation, and clinical outcome in epithelial ovarian cancer. Cancer Immunol. Immunother. 2015, 64, 1495–1504. [Google Scholar] [CrossRef]
- Rudensky, A.Y. Regulatory T cells and Foxp3: Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Vuddamalay, Y.; Attia, M.; Vicente, R.; Pomié, C.; Enault, G.; Leobon, B.; Joffre, O.; Romagnoli, P.; van Meerwijk, J.P. Mouse and human CD8(+) CD28(low) regulatory T lymphocytes differentiate in the thymus. Immunology 2016, 148, 187–196. [Google Scholar] [CrossRef]
- Vuddamalay, Y.; van Meerwijk, J.P.M. CD28- and CD28lowCD8+ Regulatory T Cells: Of Mice and Men. Front. Immunol. 2017, 8, 31. [Google Scholar] [CrossRef]
- Wang, L.-X.; Li, Y.; Yang, G.; Pang, P.Y.; Haley, D.; Walker, E.B.; Urba, W.J.; Hu, H.M. CD122+CD8+ Treg suppress vaccine-induced antitumor immune responses in lymphodepleted mice. Eur. J. Immunol. 2010, 40, 1375–1385. [Google Scholar] [CrossRef]
- Endharti, A.T.; Rifa’, M.; Shi, Z.; Fukuoka, Y.; Nakahara, Y.; Kawamoto, Y.; Takeda, K.; Isobe, K.I.; Suzuki, H. Cutting Edge: CD8 + CD122 + Regulatory T Cells Produce IL-10 to Suppress IFN-γ Production and Proliferation of CD8 + T Cells. J. Immunol. 2005, 175, 7093–7097. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, M.; Wang, F. Immune regulation by CD8+ Treg cells: Novel possibilities for anticancer immunotherapy. Cell. Mol. Immunol. 2018, 15, 805–807. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell 2019, 176, 998–1013.e16. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.; Ciampricotti, M.; Hawinkels, L.J.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wu, P.; Cheng, P.; Zhang, Z.; Wang, Z.; Yu, X.; Shao, X.; Wu, D.; Ye, J.; Zhang, T.; et al. Tumor-infiltrating CD39 + γ δ Tregs are novel immunosuppressive T cells in human colorectal cancer. OncoImmunology 2017, 6, e1277305. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shang, W.; Xu, R.; Wu, M.; Zhang, X.; Huang, P.; Wang, F.; Pan, S. Distribution and functions of γδ T cells infiltrated in the ovarian cancer microenvironment. J. Transl. Med. 2019, 17, 144. [Google Scholar] [CrossRef]
- Okła, K.; Czerwonka, A.; Wawruszak, A.; Bobiński, M.; Bilska, M.; Tarkowski, R.; Bednarek, W.; Wertel, I.; Kotarski, J. Clinical Relevance and Immunosuppressive Pattern of Circulating and Infiltrating Subsets of Myeloid-Derived Suppressor Cells (MDSCs) in Epithelial Ovarian Cancer. Front. Immunol. 2019, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Josefat, B.; Mulero-Navarro, S.; Dallas, S.L.; Fernandez-Salguero, P.M. Overexpression of latent transforming growth factor-beta binding protein 1 (LTBP-1) in dioxin receptor-null mouse embryo fibroblasts. J. Cell Sci. 2004, 117, 849–859. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Lee, H.-S.; Lee, J.-S.; Lee, Y.S.; Park, J.L.; Kim, S.Y.; Hwang, J.A.; Kunkeaw, N.; Jung, S.Y.; Kim, T.J.; et al. nc886 is induced by TGF-β and suppresses the microRNA pathway in ovarian cancer. Nat. Commun. 2018, 9, 1166. [Google Scholar] [CrossRef]
- De Streel, G.; Bertrand, C.; Chalon, N.; Liénart, S.; Bricard, O.; Lecomte, S.; Devreux, J.; Gaignage, M.; De Boeck, G.; Mariën, L.; et al. Selective inhibition of TGF-β1 produced by GARP-expressing Tregs overcomes resistance to PD-1/PD-L1 blockade in cancer. Nat. Commun. 2020, 11, 4545. [Google Scholar] [CrossRef]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Tone, Y.; Furuuchi, K.; Kojima, Y.; Tykocinski, M.L.; Greene, M.I.; Tone, M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 2008, 9, 194–202. [Google Scholar] [CrossRef]
- Marie, J.C.; Liggitt, D.; Rudensky, A.Y. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 2006, 25, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Quiros, J.; Mahuron, K.; Pai, C.C.; Ranzani, V.; Young, A.; Silveria, S.; Harwin, T.; Abnousian, A.; Pagani, M.; et al. Targeting EZH2 Reprograms Intratumoral Regulatory T Cells to Enhance Cancer Immunity. Cell Rep. 2018, 23, 3262–3274. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, X.; Lou, J.; Zhang, S.; Zhang, X.; Huang, L.; Sun, R.; Huang, P.; Wang, F. and Pan, S. TGF-β1 contributes to CD8+ Treg induction through p38 MAPK signaling in ovarian cancer microenvironment. Oncotarget 2016, 7, 44534–44544. [Google Scholar] [CrossRef] [PubMed]
| Variables | Patients, n | % |
|---|---|---|
| Age (year) | ||
| <50 | 14 | 38.9 |
| ≥50 | 22 | 61.1 |
| Tumor size (cm) | ||
| <5 | 4 | 11.1 |
| ≥5 | 32 | 88.9 |
| Histology | ||
| serous | 22 | 61.1 |
| mucinous | 1 | 2.8 |
| endometrioid | 4 | 11.1 |
| clear cell carcinoma | 8 | 22.2 |
| others | 1 | 2.8 |
| FIGO stage | ||
| I–II | 18 | 50.0 |
| III–IV | 18 | 50.0 |
| Lymph node metastasis | ||
| No | 21 | 58.3 |
| Yes | 15 | 41.7 |
| Distant metastasis | ||
| No | 19 | 52.8 |
| Yes | 17 | 47.2 |
| CA125 (U/mL) | ||
| ≤35 | 0 | 0.0 |
| >35 | 36 | 100.0 |
| HE4 (pmol/L) | ||
| ≤140 | 22 | 61.1 |
| >140 | 14 | 38.9 |
| Variables | OC | BOT | HC | P-Value |
|---|---|---|---|---|
| CD4+ Tregs | ||||
| CD4+CD25+, †† | 8.03 ± 4.24 | 6.39 ± 3.63 | 5.15 ± 3.95 | 0.008 |
| CD4+Foxp3+, ****, †††† | 5.62 ± 3.31 | 2.42 ± 2.53 | 2.15 ± 1.37 | <0.0001 |
| CD4+CD25+Foxp3+, **, †††† | 3.28 ± 1.70 | 2.00 ± 1.46 | 1.32 ± 0.91 | <0.0001 |
| CD8+ Tregs | ||||
| CD8+CD28−, ****, †††† | 65.61 ± 19.75 | 39.39 ± 21.25 | 42.73 ± 1.84 | <0.0001 |
| CD8+Foxp3+, *, ††† | 4.63 ± 2.09 | 3.31 ± 2.47 | 1.66 ± 1.25 | <0.0001 |
| CD8+CD28−Foxp3+, **, †† | 1.41 ± 0.87 | 0.74 ± 0.37 | 0.82 ± 1.09 | 0.002 |
| CD8+CD122+, *, † | 11.18 ± 6.73 | 7.56 ± 5.91 | 7.28 ± 8.59 | 0.043 |
| CD3+ γδ T cells | ||||
| CD3+Vδ1 T, ***, ††† | 3.36 ± 3.68 | 0.92 ± 0.44 | 1.22 ± 0.65 | <0.0001 |
| CD3+Vδ2 T | 2.76 ± 1.20 | 2.59 ± 2.57 | 3.43 ± 4.82 | 0.058 |
| Variables | CD4+CD25+ | P-Value | CD4+Foxp3+ | P-Value | CD4+CD25+Foxp3+ | P-Value |
|---|---|---|---|---|---|---|
| Age (year) | ||||||
| <50 | 7.33 ± 5.15 | 0.44 | 4.94 (2.90–9.70) | 0.365 | 2.43 ± 1.57 | 0.015 * |
| ≥50 | 8.47 ± 3.61 | 5.20 (3.54–6.08) | 3.81 ± 1.58 | |||
| Tumor size (cm) | ||||||
| <5 | 7.01 ± 1.40 | 0.618 | 4.74 ± 1.76 | 0.58 | 2.85 (2.62–3.14) | 0.195 |
| ≥5 | 8.15 ± 4.47 | 8.15 ± 4.47 | 3.00 (2.03–4.60) | |||
| Histology | ||||||
| serous | 8.64 ± 4.70 | 0.528 | 5.96 ± 3.26 | 0.205 | 3.55 ± 1.74 | 0.267 |
| endometrioid | 8.17 ± 5.22 | 7.33 ± 5.20 | 2.75 ± 1.90 | |||
| clear cell carcinoma | 7.20 ± 2.40 | 4.79 ± 1.96 | 3.30 ± 1.35 | |||
| others | 4.30 ± 0.97 | 1.72 ± 0.58 | 1.19 ± 1.31 | |||
| FIGO stage | ||||||
| I–II | 6.52 ± 3.08 | 0.031 * | 4.50 ± 3.01 | 0.040 * | 2.40 (2.01–3.01) | 0.003 * |
| III–IV | 9.53 ± 4.77 | 6.74 ± 3.29 | 3.86 (2.68–5.27) | |||
| Lymph node metastasis | ||||||
| No | 7.34 ± 3.68 | 0.256 | 4.48 ± 2.81 | 0.012 | 2.57 (2.03–3.23) | 0.011 * |
| Yes | 8.99 ± 4.89 | 7.21 ± 3.37 | 3.87 (2.81–5.40) | |||
| Distant metastasis | ||||||
| No | 5.38 (4.33–7.57) | 0.001 * | 3.99 (3.09–5.20) | 0.002 * | 2.57 (2.03–2.94) | 0.003 * |
| Yes | 9.55 (7.58–14.10) | 6.04 (5.47–8.93) | 3.87 (2.74–5.31) |
| Variables | CD8+CD28− | P-Value | CD8+Foxp3+ | P-Value | CD8+CD28−Foxp3+ | P-Value | CD8+CD122+ | P-Value |
|---|---|---|---|---|---|---|---|---|
| Age (year) | ||||||||
| <50 | 63.73 ± 21.87 | 0.655 | 4.80 (1.88–6.96) | 0.611 | 1.21 (0.64–2.17) | 0.417 | 10.55 (6.98–17.45) | 0.162 |
| ≥50 | 66.81 ± 18.72 | 4.30 (3.43–5.03) | 1.32 (0.88–1.68) | 8.66 (7.20–10.62) | ||||
| Tumor size (cm) | ||||||||
| <5 | 66.50 ± 15.72 | 0.926 | 3.4 ± 1.60 | 0.216 | 0.84 ± 0.48 | 0.171 | 9.65 ± 4.11 | 0.635 |
| ≥5 | 65.50 ± 20.41 | 4.78 ± 2.11 | 1.48 ± 0.89 | 11.37 ± 7.01 | ||||
| Histology | ||||||||
| serous | 70.43 ± 19.26 | 0.059 | 4.17 ± 1.73 | 0.259 | 4.71 ± 1.73 | 0.878 | 10.92 ± 5.23 | 0.481 |
| endometrioid | 67.72 ± 16.56 | 3.74 ± 0.71 | 3.74 ± 0.71 | 17.39 ± 15.02 | ||||
| clear cell carcinoma | 59.05 ± 17.89 | 5.43 ± 3.08 | 5.43 ± 3.08 | 10.19 ± 3.94 | ||||
| others | 34.60 ± 0.56 | 2.30 ± 1.66 | 2.30 ± 1.66 | 5.61 ± 0.32 | ||||
| FIGO stage | ||||||||
| I–II | 57.77 ± 20.72 | 0.015 * | 4.34 ± 2.19 | 0.41 | 1.30 ± 0.30 | 0.453 | 10.54 ± 7.90 | 0.573 |
| III–IV | 73.46 ± 15.60 | 4.92 ± 2.00 | 1.52 ± 1.08 | 11.83 ± 5.46 | ||||
| Lymph node metastasis | ||||||||
| No | 59.93 ± 20.11 | 0.039 * | 4.21 ± 2.09 | 0.157 | 1.23 ± 0.60 | 0.139 | 10.08 ± 7.40 | 0.249 |
| Yes | 73.56 ± 16.78 | 5.22 ± 2.01 | 1.67 ± 1.21 | 12.73 ± 5.53 | ||||
| Distant metastasis | ||||||||
| No | 57.40 ± 20.59 | 0.006 * | 4.36 ± 2.56 | 0.416 | 1.32 ± 0.86 | 0.518 | 8.01 (7.03–9.96) | 0.056 |
| Yes | 74.78 ± 14.39 | 4.93 ± 1.42 | 1.51 ± 0.90 | 10.30 (7.86–17.30) |
| Variables | CD3+Vδ1 | P-Value | CD3+Vδ2 | P-Value |
|---|---|---|---|---|
| Age (year) | ||||
| <50 | 2.92 ± 2.03 | 0.58 | 3.16 ± 1.48 | 0.105 |
| ≥50 | 3.63 ± 4.45 | 2.50 ± 0.92 | ||
| Tumor size (cm) | ||||
| <5 | 6.99 ± 9.75 | 0.464 | 2.29 ± 0.95 | 0.42 |
| ≥5 | 2.90 ± 2.04 | 2.82 ± 1.23 | ||
| Histology | ||||
| serous | 3.84 ± 4.28 | 0.782 | 2.42 ± 0.91 | 0.404 |
| endometrioid | 3.17 ± 4.35 | 3.20 ± 0.49 | ||
| clear cell carcinoma | 2.26 ± 1.38 | 3.01 ± 1.59 | ||
| others | 2.84 ± 1.80 | 4.53 ± 2.14 | ||
| FIGO stage | ||||
| I–II | 3.16 ± 4.75 | 0.752 | 2.82 ± 1.43 | 0.789 |
| III–IV | 3.56 ± 2.89 | 2.70 ± 0.96 | ||
| Lymph node metastasis | ||||
| No | 3.16 ± 4.38 | 0.707 | 2.83 ± 1.36 | 0.676 |
| Yes | 3.64 ± 2.51 | 2.66 ± 0.98 | ||
| Distant metastasis | ||||
| No | 3.24 ± 4.59 | 0.845 | 2.66 ± 1.41 | 0.6 |
| Yes | 3.49 ± 2.43 | 2.87 ± 0.95 |
| Variables | CA125 (U/mL) | P-Value | HE4 (pmol/L) | P-Value | ||
|---|---|---|---|---|---|---|
| <Median | >Median | ≤140 | >140 | |||
| CD4+CD25+ | 7.13 ± 4.10 | 9.02 ± 4.29 | 0.186 | 8.15 ± 4.28 | 7.84 ± 4.33 | 0.835 |
| CD4+Foxp3+ | 3.87 ± 1.95 | 7.57 ± 3.46 | 0.000 * | 4.02 (2.90–5.90) | 6.05 (5.21–9.84) | 0.008 |
| CD4+CD25+Foxp3+ | 2.86 ± 1.88 | 3.74 ± 1.37 | 0.122 | 3.18 ± 1.65 | 3.42 ± 1.81 | 0.683 |
| CD8+CD28− | 50.50 (38.40–60.40) | 85.80 (77.85–88.05) | 0.000 * | 58.78 ± 16.85 | 76.35 ± 19.71 | 0.007 * |
| CD8+Foxp3+ | 3.90 ± 2.01 | 5.44 ± 1.92 | 0.025 * | 4.19 (3.44–5.05) | 4.88 (3.24–7.38) | 0.114 |
| CD8+CD28−Foxp3+ | 1.12 ± 0.56 | 1.74 ± 1.05 | 0.032 * | 1.36 ± 0.83 | 1.49 ± 0.95 | 0.653 |
| CD8+CD122+ | 7.51 (6.31–8.05) | 15.20 (10.13–17.80) | 0.000 * | 9.14 ± 4.75 | 14.39 ± 8.20 | 0.020 |
| CD3+Vδ1 | 1.57 (1.01–2.56) | 3.71 (3.39–4.31) | 0.013 * | 1.88 (1.40–3.58) | 3.64 (1.48–5.11) | 0.103 |
| CD3+Vδ2 | 2.30 ± 1.36 | 2.49 ± 0.96 | 0.208 | 3.01 ± 1.33 | 2.36 ± 0.87 | 0.114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Xu, J.; Wu, M.; Liu, S.; Fu, X.; Shang, W.; Wang, T.; Jia, X.; Wang, F. Circulating CD4+ Treg, CD8+ Treg, and CD3+ γδ T Cell Subpopulations in Ovarian Cancer. Medicina 2023, 59, 205. https://doi.org/10.3390/medicina59020205
Li R, Xu J, Wu M, Liu S, Fu X, Shang W, Wang T, Jia X, Wang F. Circulating CD4+ Treg, CD8+ Treg, and CD3+ γδ T Cell Subpopulations in Ovarian Cancer. Medicina. 2023; 59(2):205. https://doi.org/10.3390/medicina59020205
Chicago/Turabian StyleLi, Rong, Juan Xu, Ming Wu, Shuna Liu, Xin Fu, Wenwen Shang, Ting Wang, Xuemei Jia, and Fang Wang. 2023. "Circulating CD4+ Treg, CD8+ Treg, and CD3+ γδ T Cell Subpopulations in Ovarian Cancer" Medicina 59, no. 2: 205. https://doi.org/10.3390/medicina59020205
APA StyleLi, R., Xu, J., Wu, M., Liu, S., Fu, X., Shang, W., Wang, T., Jia, X., & Wang, F. (2023). Circulating CD4+ Treg, CD8+ Treg, and CD3+ γδ T Cell Subpopulations in Ovarian Cancer. Medicina, 59(2), 205. https://doi.org/10.3390/medicina59020205









