Clinical and Ultrasonographic Characteristics of the Achilles Tendon in Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Clinical Evaluation of the Achilles Tendon
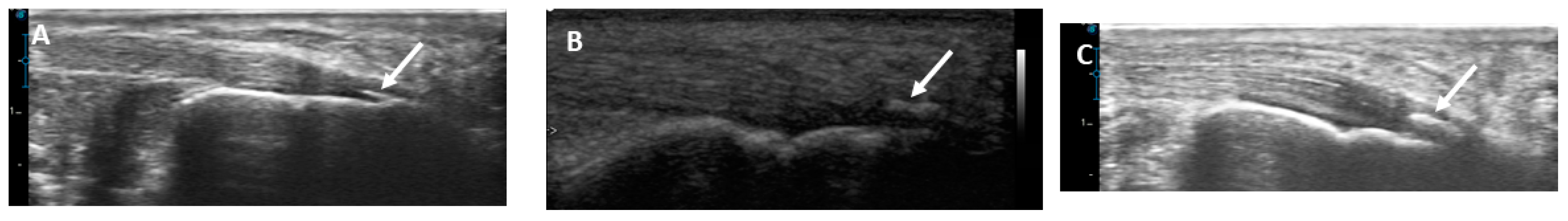
2.4. Musculoskeletal Ultrasound of the Achilles Tendon
2.5. Blood Sampling and Laboratory Tests
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ca | calcium |
| CBC | complete blood count |
| CRF | chronic renal failure |
| ESRD | end-stage renal disease |
| GSUS | grayscale ultrasound |
| GUESS | Glasgow Ultrasound Enthesitis Scoring System |
| HD | hemodialysis |
| HRQoL | health-related quality of life |
| iPTH | intact parathyroid hormone |
| MSUS | musculoskeletal ultrasound |
| PO4 | phosphorus |
References
- Liew, A. Perspectives in renal replacement therapy: Haemodialysis. Nephrol. Carlton Vic. 2018, 23 (Suppl. S4), 95–99. [Google Scholar] [CrossRef] [PubMed]
- SSaran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W.; et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2018, 71, A7. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.; Tharwat, S.; Abdelsalam, S.; Eltoraby, E.E. Musculoskeletal Symptoms in Hemodialysis Patients and their Effect on Health-Related Quality of Life. Blood Purif. 2020, 49, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- Ilan, D.I.; Tejwani, N.; Keschner, M.; Leibman, M. Quadriceps tendon rupture. J. Am. Acad. Orthop. Surg. 2003, 11, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Carrero, J.J.; von Walden, F.; Ikizler, T.A.; Nader, G.A. Muscle wasting in end-stage renal disease promulgates premature death: Established, emerging and potential novel treatment strategies. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2016, 31, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Taşoğlu, Ö.; Ekiz, T.; Yenigün, D.; Akyüz, M.; Özgirgin, N. Bilateral quadriceps and triceps tendon rupture in a hemodialysis patient. Hemodial. Int. Int. Symp. Home Hemodial. 2016, 20, E19–E21. [Google Scholar] [CrossRef]
- Wu, W.; Wang, C.; Ruan, J.; Wang, H.; Huang, Y.; Zheng, W.; Chen, F. Simultaneous spontaneous bilateral quadriceps tendon rupture with secondary hyperparathyroidism in a patient receiving hemodialysis: A case report. Medicine 2019, 98, e14809. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Krieger, N.S. Effects of acid on bone. Kidney Int. 2022, 101, 1160–1170. [Google Scholar] [CrossRef]
- Tuite, M.J. Musculoskeletal ultrasound: Its impact on your MR practice. AJR Am. J. Roentgenol. 2009, 193, 605–606. [Google Scholar] [CrossRef]
- Bureau, N.J.; Ziegler, D. Economics of Musculoskeletal Ultrasound. Curr. Radiol. Rep. 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.A.; Lancaster, S.; Prasad, A.; van Holsbeeck, M.T.; Craig, J.G.; Kolowich, P. Full-thickness and partial-thickness supraspinatus tendon tears: Value of US signs in diagnosis. Radiology 2004, 230, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.C.; Schneider, M.E.; Malliaras, P.; Chadwick, M.; Connell, D.A. Diagnostic performance of axial-strain sonoelastography in confirming clinically diagnosed Achilles tendinopathy: Comparison with B-mode ultrasound and color Doppler imaging. Ultrasound Med. Biol. 2015, 41, 15–25. [Google Scholar] [CrossRef]
- Chandler, J.; Shapiro, D. Conducting Clinical Research Using Crowdsourced Convenience Samples. Annu. Rev. Clin. Psychol. 2016, 12, 53–81. [Google Scholar] [CrossRef]
- Hutchison, A.-M.; Evans, R.; Bodger, O.; Pallister, I.; Topliss, C.; Williams, P.; Vannet, N.; Morris, V.; Beard, D. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. Off. J. Eur. Soc. Foot Ankle Surg. 2013, 19, 112–117. [Google Scholar] [CrossRef]
- Maffulli, N.; Kenward, M.G.; Testa, V.; Capasso, G.; Regine, R.; King, J.B. Clinical diagnosis of Achilles tendinopathy with tendinosis. Clin. J. Sport. Med. Off. J. Can. Acad. Sport. Med. 2003, 13, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sabry, R.; Tharwat, S.; Nassar, M.K.; Eltoraby, E.E. Ultrasonographic assessment of entheseal sites of upper and lower extremities in hemodialysis patients using Madrid Sonography Enthesitis Index. BMC Musculoskelet. Disord. 2022, 23, 606. [Google Scholar] [CrossRef]
- Sigaux, J.; Abdelkefi, I.; Bardin, T.; Laredo, J.-D.; Ea, H.-K.; Ureñatorres, P.; Cohen-Solal, M. Tendon thickening in dialysis-related joint arthritis is due to amyloid deposits at the surface of the tendon. Jt. Bone Spine 2019, 86, 233–238. [Google Scholar] [CrossRef]
- Basic-Jukic, N.; Juric, I.; Racki, S.; Kes, P. Spontaneous tendon ruptures in patients with end-stage renal disease. Kidney Blood Press. Res. 2009, 32, 32–36. [Google Scholar] [CrossRef]
- Jones, N.; Kjellstrand, C.M. Spontaneous tendon ruptures in patients on chronic dialysis. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1996, 28, 861–866. [Google Scholar] [CrossRef]
- Spencer, J.D. Spontaneous rupture of tendons in dialysis and renal transplant patients. Injury 1988, 19, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Hussein, D.A.; El-Azizi, N.O.; Abdel Meged, A.H.; Al-Hoseiny, S.A.; Hamada, A.M.; Sabry, M.H. Ultrasonographic tendon alteration in relation to parathyroid dysfunction in chronic hemodialysis patients. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Rahatli, F.K.; Turnaoglu, H.; Haberal, M.; Kirnap, M.; Sayin, B.; Fidan, C.; Uslu, N.; Haberal, M. Shear Wave Elastography Findings of Achilles Tendons in Patients on Chronic Hemodialysis and Patients with renal Transplantation. Transplantation 2018, 102, S523. [Google Scholar] [CrossRef]
- Gutierrez, M.; Zeiler, M.; Filippucci, E.; Salaffi, F.; Becciolini, A.; Bertolazzi, C.; Chavez, M.; Monteburini, T.; Agostinelli, R.M.; Marinelli, R.; et al. Sonographic subclinical entheseal involvement in dialysis patients. Clin. Rheumatol. 2011, 30, 907–913. [Google Scholar] [CrossRef]
- Brountzos, E.; Syrgiannis, K.; Panagiotou, I.; Nikolaos, K.; Kalogeropoulou, S.; Balanika, A.; Tzavara, C.; Vlahakos, D. Ultrasonographic alterations in Achilles tendon in relation to parathormone in chronic hemodialysis patients. J. Nephrol. 2009, 22, 476–483. [Google Scholar] [PubMed]
- Fredberg, U.; Stengaard-Pedersen, K. Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand. J. Med. Sci. Sports 2008, 18, 3–15. [Google Scholar] [CrossRef]
- Yuan, J.; Murrell, G.A.C.; Trickett, A.; Wang, M.-X. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim. Biophys. Acta 2003, 1641, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Almekinders, L.C.; Weinhold, P.S.; Maffulli, N. Compression etiology in tendinopathy. Clin. Sports Med. 2003, 22, 703–710. [Google Scholar] [CrossRef]
- Rees, J.D.; Stride, M.; Scott, A. Tendons—time to revisit inflammation. Br. J. Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef]
- Cook, J.L.; Rio, E.; Purdam, C.R.; Docking, S.I. Revisiting the continuum model of tendon pathology: What is its merit in clinical practice and research? Br. J. Sports Med. 2016, 50, 1187–1191. [Google Scholar] [CrossRef]
- Fu, S.-C.; Rolf, C.; Cheuk, Y.-C.; Lui, P.P.; Chan, K.-M. Deciphering the pathogenesis of tendinopathy: A three-stages process. Sports Med. Arthrosc. Rehabil. Ther. Technol. SMARTT 2010, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.; Teber, M.A.; Yakut, Z.I.; Unlu, H.A.; Hekimoglu, B. Sonoelastographıc assessment of the age-related changes of the Achilles tendon. Med. Ultrason. 2015, 17, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Sargon, M.F.; Ozlu, K.; Oken, F. Age-related changes in human tendo calcaneus collagen fibrils. Saudi Med. J. 2005, 26, 425–428. [Google Scholar] [PubMed]
- Tuite, D.J.; Renström, P.A.; O’Brien, M. The aging tendon. Scand. J. Med. Sci. Sports 1997, 7, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Carcia, C.R.; Martin, R.L.; Houck, J.; Wukich, D.K.; Orthopaedic Section of the American Physical Therapy Association. Achilles pain, stiffness, and muscle power deficits: Achilles tendinitis. J. Orthop. Sports Phys. Ther. 2010, 40, A1–A26. [Google Scholar] [CrossRef] [PubMed]
- Wakker, J.; PhD for the Elasto Study Group; Kratzer, W.; Graeter, T.; Schmidberger, J. Elasticity standard values of the Achilles tendon assessed with acoustic radiation force impulse elastography on healthy volunteers: A cross section study. BMC Musculoskelet. Disord. 2018, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Delabastita, T.; Hollville, E.; Catteau, A.; Cortvriendt, P.; De Groote, F.; Vanwanseele, B. Distal-to-proximal joint mechanics redistribution is a main contributor to reduced walking economy in older adults. Scand. J. Med. Sci. Sports 2021, 31, 1036–1047. [Google Scholar] [CrossRef]
- Shiota, E.; Tsuchiya, K.; Yamaoka, K.; Kawano, O. Spontaneous major tendon ruptures in patients receiving long-term hemodialysis. Clin. Orthop. 2002, 394, 236–242. [Google Scholar] [CrossRef]
- Achilles Tendon Tear|Radiology Reference Article|Radiopaedia.org n.d. Available online: https://radiopaedia.org/articles/achilles-tendon-tear (accessed on 1 December 2023).
- Terai, K.; Nara, H.; Takakura, K.; Mizukami, K.; Sanagi, M.; Fukushima, S.; Fujimori, A.; Itoh, H.; Okada, M. Vascular calcification and secondary hyperparathyroidism of severe chronic kidney disease and its relation to serum phosphate and calcium levels. Br. J. Pharmacol. 2009, 156, 1267–1278. [Google Scholar] [CrossRef]
- Taş, S.; Yılmaz, S.; Onur, M.R.; Soylu, A.R.; Altuntaş, O.; Korkusuz, F. Patellar tendon mechanical properties change with gender, body mass index and quadriceps femoris muscle strength. Acta Orthop. Traumatol. Turc. 2017, 51, 54–59. [Google Scholar] [CrossRef]
- Burgess, K.E.; Graham-Smith, P.; Pearson, S.J. Effect of acute tensile loading on gender-specific tendon structural and mechanical properties. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009, 27, 510–516. [Google Scholar] [CrossRef]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Stokes, M. Measurement of ageing effects on muscle tone and mechanical properties of rectus femoris and biceps brachii in healthy males and females using a novel hand-held myometric device. Arch. Gerontol. Geriatr. 2016, 62, 59–67. [Google Scholar] [CrossRef]
- Morse, C.I. Gender differences in the passive stiffness of the human gastrocnemius muscle during stretch. Eur. J. Appl. Physiol. 2011, 111, 2149–2154. [Google Scholar] [CrossRef]
- Morrison, S.M.; Dick, T.J.M.; Wakeling, J.M. Structural and mechanical properties of the human Achilles tendon: Sex and strength effects. J. Biomech. 2015, 48, 3530–3533. [Google Scholar] [CrossRef]
- Fu, S.; Cui, L.; He, X.; Sun, Y. Elastic Characteristics of the Normal Achilles Tendon Assessed by Virtual Touch Imaging Quantification Shear Wave Elastography. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2016, 35, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Burgess, K.E.; Pearson, S.J.; Breen, L.; Onambélé, G.N.L. Tendon structural and mechanical properties do not differ between genders in a healthy community-dwelling elderly population. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009, 27, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Chino, K.; Takahashi, H. Association of Gastrocnemius Muscle Stiffness With Passive Ankle Joint Stiffness and Sex-Related Difference in the Joint Stiffness. J. Appl. Biomech. 2018, 34, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Saeki, J.; Ikezoe, T.; Yoshimi, S.; Nakamura, M.; Ichihashi, N. Menstrual cycle variation and gender difference in muscle stiffness of triceps surae. Clin. Biomech. 2019, 61, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; LeBrasseur, N.K.; An, K.-N. Shear wave elastography of passive skeletal muscle stiffness: Influences of sex and age throughout adulthood. Clin. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kerimoglu, U.; Hayran, M.; Ergen, F.B.; Kirkpantur, A.; Turgan, C. Sonographic evaluation of entheseal sites of the lower extremity in patients undergoing hemodialysis. J. Clin. Ultrasound JCU 2007, 35, 417–423. [Google Scholar] [CrossRef]







| Variable n (%), Mean ± SD, Median (Min–Max) | HD Patients (n = 46) |
|---|---|
| Sociodemographic data | |
| Age, years | 52.34 ± 14.99 |
| Gender | |
| Male | 25 (54.3) |
| Female | 21 (45.7) |
| Occupation | |
| Not employed | 24 (52.2) |
| Employed | 9 (19.6) |
| Retired | 6 (13) |
| Not able to work due to disability | 7 (15.2) |
| Active lifestyle | 10 (21.7) |
| Socioeconomic level | |
| Low | 21 (45.7) |
| Average | 21 (45.7) |
| High | 4 (8.7) |
| Smoking | |
| Never | 33 (71.7) |
| Former smoker | 12 (26.1) |
| Current Smoker | 1 (2.2) |
| Anthropometric measures | |
| Weight, kg | 84.34 ± 17.72 |
| Height, m | 168.11 ± 6.94 |
| Body mass index, kg/m2 | 29.93 ± 6.42 |
| Clinical data | |
| Cause of chronic renal failure | |
| ADPKD | 1 (2.2) |
| Drug induced | 2 (4.3) |
| Glomerulonephritis | 3 (6.5) |
| Hypertension | 8 (17.4) |
| Diabetes Miletus | 3 (6.5) |
| Neurogenic bladder | 1 (2.2) |
| Obstructive uropathy | 1 (2.2) |
| Unknown cause | 14 (30.4) |
| Duration of HD, years | 3 (0.6–13) |
| Associated comorbidities | |
| Diabetes Miletus | 5 (10.9) |
| Hypertension | 25 (54.3) |
| Ischemic heart disease | 4 (8.6) |
| Therapeutic data | |
| Erythropoietin | 32 (69.6) |
| Calcium supplementation | 27 (58.7) |
| Iron supplementation | 22 (47.8) |
| Antihypertensives | 20 (43.5) |
| Antidiabetics | 5 (10.9) |
| Laboratory data | |
| Calcium, mg/dL | 8.5 (5.20–9.7) |
| Phosphorus, mg/dL | 5.4 (1.9–17.5) |
| Intact PTH, pg/mL | 531 (4.6–1978) |
| Hemoglobin, g/dL | 11.2 (8–14.3) |
| Serum ferritin, ng/mL | 263.4 (12.6–1730) |
| Transferrin saturation, % | 21 (5–43) |
| Variable n (%), Mean ±SD, Median (Min–Max) | Healthy Subjects (n = 24) (Achilles Tendons = 48) | Hemodialysis Patients (n = 46) (Achilles Tendons = 92) | p |
|---|---|---|---|
| Clinical findings | |||
| Pain | 4 (8.3) | 3 (3.3) | 0.231 |
| Swelling | 0 | 2 (2.2) | 0.546 |
| Tenderness | 0 | 2 (2.2) | 0.546 |
| Ultrasonographic features | |||
| Thickness, mm | |||
| Proximal | 3.84 ± 0.86 | 5.19 ± 1.05 | <0.001 * |
| Middle | 3.86 ± 0.86 | 5.06 ± 0.93 | <0.001 * |
| Distal | 3.94 ± 0.87 | 5 ± 0.87 | <0.001 * |
| Tendinosis | 0 | 12 (13) | 0.008 * |
| Achilles tendon entheses | |||
| Structural abnormalities | 0 | 13 (14.1) | |
| Retrocalcaneal bursitis | 2 (4.2) | 7 (7.6) | 0.005 * |
| Bone erosions | 0 | 11 (12) | 0.716 |
| Calcifications | 0.016 * | ||
| Mild | 1 (2.1) | 6 (6.5) | |
| Moderate | 1 (2.1) | 14 (15.2) | 0.015 * |
| Severe | 0 | 4 (4.3) |
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.062 | 1.022–1.104 | 0.002 * |
| Gender | |||
| Female | Ref | Ref | Ref |
| Male | 2.717 | 1.131–6.531 | 0.025 * |
| Weight | 1.000 | 0.972–1.029 | 0.979 |
| Height | 0.981 | 0.911–1.056 | 0.604 |
| Body mass index | 1.007 | 0.932–1.088 | 0.861 |
| Parathyroid hormone | 0.999 | 0.998–1.000 | 0.259 |
| Serum calcium | 0.729 | 0.427–1.243 | 0.246 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharwat, S.; Saleh, M.; Elrefaey, R.; Nassar, M.K.; Nassar, M.K. Clinical and Ultrasonographic Characteristics of the Achilles Tendon in Hemodialysis Patients. Medicina 2023, 59, 2181. https://doi.org/10.3390/medicina59122181
Tharwat S, Saleh M, Elrefaey R, Nassar MK, Nassar MK. Clinical and Ultrasonographic Characteristics of the Achilles Tendon in Hemodialysis Patients. Medicina. 2023; 59(12):2181. https://doi.org/10.3390/medicina59122181
Chicago/Turabian StyleTharwat, Samar, Marwa Saleh, Rabab Elrefaey, Mona Kamal Nassar, and Mohammed Kamal Nassar. 2023. "Clinical and Ultrasonographic Characteristics of the Achilles Tendon in Hemodialysis Patients" Medicina 59, no. 12: 2181. https://doi.org/10.3390/medicina59122181
APA StyleTharwat, S., Saleh, M., Elrefaey, R., Nassar, M. K., & Nassar, M. K. (2023). Clinical and Ultrasonographic Characteristics of the Achilles Tendon in Hemodialysis Patients. Medicina, 59(12), 2181. https://doi.org/10.3390/medicina59122181






