Abstract
Background and Objectives: Up to one-third of patients with acute biliary pancreatitis also present with choledocholithiasis. Guidelines from the European Society of Gastrointestinal Endoscopy (ESGE) and the American Society for Gastrointestinal Endoscopy (ASGE) for investigating suspected choledocholithiasis suggest endoscopic retrograde cholangiopancreatography in patients with high-likelihood (ESGE)/high-probability (ASGE) predictors and endoscopic ultrasound in those with intermediate-likelihood (ESGE)/intermediate-probability (ASGE) predictors. Although both guidelines are similar, they are not identical. Furthermore, these algorithms were mainly developed from cohorts of patients without pancreatitis and are therefore poorly validated in a subset of patients with acute pancreatitis. We aimed to assess the performance of the ESGE and ASGE algorithms for the prediction of choledocholithiasis in patients with acute biliary pancreatitis. Materials and Methods: This was a retrospective analysis of 86 consecutive patients admitted to a tertiary referral centre in the year 2020 due to acute biliary pancreatitis. Results: Choledocholithiasis was confirmed in 29/86 (33.7%) of patients (13 with endoscopic retrograde cholangiopancreatography and 16 with endoscopic ultrasound). All 10/10 (100%) ESGE high-likelihood and 14/19 (73.7%) ASGE high-probability patients had choledocholithiasis. Only 19/71 (26.8%) patients with ESGE intermediate likelihood and 15/67 (22.4%) with ASGE intermediate probability had choledocholithiasis. Only 8/13 (61.5%) patients with the ASGE high-probability predictor of dilated common bile duct plus bilirubin > 68.4 µmol/mL had choledocholithiasis. Since this predictor is not considered high likelihood by ESGE, this resulted in a superior specificity of the European compared to the American guideline (100% vs. 91.2%). Following the American instead of the European guidelines would have resulted in five unnecessary endoscopic retrograde cholangiopancreatographies and five unnecessary endoscopic ultrasound examinations. Conclusions: This retrospective analysis suggests that the European guidelines may perform better than the American guidelines at predicting choledocholithiasis in the setting of acute pancreatitis. This was because dilated common bile duct plus bilirubin > 68.4 µmol/mL was not a reliable predictor for persistent bile duct stones.
1. Introduction
The incidence of acute biliary pancreatitis (ABP) has been steadily increasing throughout most European countries in the past decades [1,2,3]. Although the pathogenesis of ABP is multifaceted, the key feature is ampullary obstruction by gallstones [4,5]. Fortunately, most offending stones spontaneously migrate into the duodenum [5,6,7]. However, in up to one-third of patients, the stones do not pass spontaneously from the common bile duct (CBD) to the duodenum [6,8]. These patients should be recognized and the CBD cleared by endoscopic retrograde cholangiopancreatography (ERCP) to relieve obstruction and prevent further complications. Clinicians often find it difficult to accurately identify this subset of patients with non-invasive modalities such as transabdominal ultrasound (US) or computerized tomography (CT), as these have somewhat low sensitivity (50–80% and 40%, respectively) [9]. The strategy of performing ERCP in all patients suspected to have CBD stones based on clinical, biochemical, and imaging techniques (transabdominal US or CT) would result in a high proportion of ERCP examinations with no stones found in the CBD. As ERCP has a significant risk of complications, such diagnostic ERCP should be avoided [10]. Magnetic resonance cholangiopancreatography (MRCP) provides high sensitivity and specificity for gallstones in ABP, but its availability is still very limited, especially in the emergency setting [9,11]. Due to these facts, either endoscopic ultrasound (EUS) or ERCP are performed to assess the CBD for pathology. Since EUS and ERCP are expensive and invasive procedures, with the latter also carrying a risk of serious complications, there is a need for a tool to help better stratify patients who require EUS or ERCP [9,12,13,14,15,16,17].
Up until 2019, clinicians relied on the American Society for Gastrointestinal Endoscopy (ASGE) 2010 guidelines on the role of endoscopy in the evaluation of suspected choledocholithiasis which classified clinical gallstone pancreatitis as a “moderate” predictor; thus, all patients with ABP were classified to have at least an intermediate probability for choledocholithiasis and were offered an EUS, provided the availability of local expertise and coverage by insurance [18]. However, in 2019, after reviewing new studies, the ASGE removed ABP from its guidelines as a predictor for choledocholithiasis [8,19,20,21]. This led to some uncertainty concerning the approach towards these patients. Despite ABP being removed from the 2019 ASGE guidelines, most clinicians still rely on these guidelines, as specific guidelines for patients with ABP are not available [19,22]. These guidelines suggest ERCP for patients with any high-probability predictor for choledocholithiasis (CBD stones on transabdominal US/cross-sectional imaging, signs of ascending cholangitis, or total bilirubin > 68.4 µmol/L plus dilated CBD on transabdominal US/cross-sectional imaging) or EUS for patients with any intermediate-probability predictor (dilated CBD on transabdominal US/cross-sectional imaging, pathologic liver tests or age over 55 years) [19]. Similar, but not identical, guidelines on this have also been published by the European Society of Gastrointestinal Endoscopy (ESGE) in 2019 [22]. An important difference between the two guidelines is that the ESGE guidelines suggest immediate ERCP only in patients with features of cholangitis or CBD stones identified on a transabdominal US, but not in patients with abnormal liver function tests plus dilated CBD on a transabdominal US as is the case with the ASGE 2019 guidelines [19,22].
However, there is one caveat—it is difficult to apply the ASGE 2019 or ESGE guidelines to patients with ABP as these have not been validated in patients with ABP, but are generally intended for patients with suspected choledocholithiasis without pancreatitis [19,22]. Pathogenesis in ABP may directly interfere with the predictors used by both guidelines and influence their reliability [4,19,22]. For example, spontaneous passage of a gallstone or sludge can cause oedema and spasms of the ampulla of Vater, resulting in biliary obstruction, which can present as a moderate elevation of bilirubin and dilation of the CBD despite the absence of choledocholithiasis [13,23]. Moreover, pancreatitis can cause high fever due to systemic inflammatory response in the absence of bacterial infection [24]. High fever in such patients can be misinterpreted as a sign of cholangitis, leading to unnecessary ERCP [13,15,19,22,25]. The sonographic visibility of the upper abdomen is typically reduced in patients with ABP due to bowel paresis, further decreasing the reliability of transabdominal US for assessing distal CBD [26]. Consequently, the utility of transabdominal US to assess two high-probability ASGE predictors (the presence of a CBD stone and CBD dilation plus elevated levels of bilirubin) and one high-likelihood ESGE predictor (the presence of a CBD stone) is decreased in the settings of pancreatitis [4,19,22,26].
Due to these limitations, our institution has a low threshold for EUS before ERCP in patients with ABP, as similarly suggested by the ESGE guidelines [19,22]. The global aim of this report was to evaluate if such a strategy is justified. Our specific aim was to assess the performance of both the ASGE and ESGE guidelines for the prediction of choledocholithiasis in patients with ABP.
2. Materials and Methods
In this retrospective cohort study, we included all patients admitted to the tertiary referral gastroenterology centre due to ABP in the year 2020. Using an electronic database, we identified 92 patients hospitalized for ABP in this period. Only 6 out of 92 (6.5%) were not assessed for the presence of CBD stones by EUS or ERCP, or had insufficient laboratory data and were therefore excluded from further analysis. Patient flow is depicted in Figure 1.
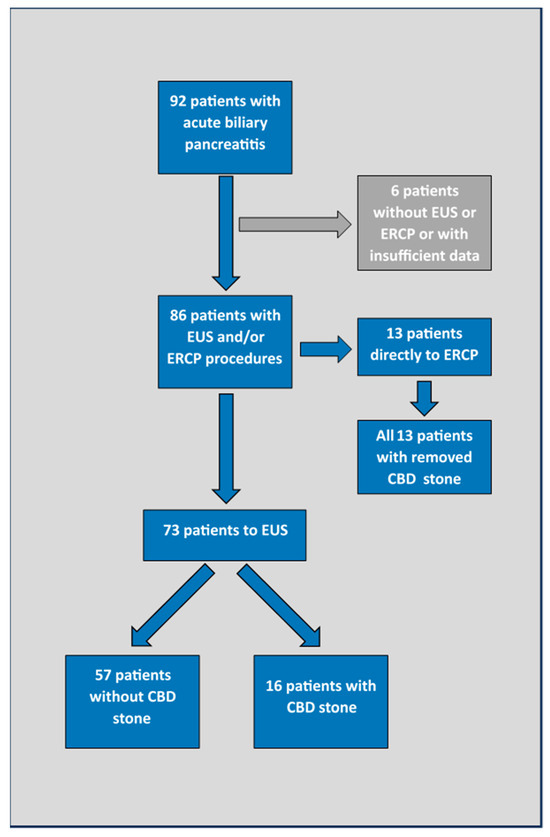
Figure 1.
Patient flow. EUS—endoscopic ultrasound; ERCP—endoscopic retrograde cholangiopancreatography; CBD—common bile duct.
The diagnosis of ABP was made in accordance with the revised Atlanta classification system [27].
The diagnosis of ABP was based on the finding of stones in the gallbladder or in the CBD, in combination with typical biochemical results after excluding other aetiologies [4].
The diagnosis of concomitant acute cholangitis was made at the discretion of the treating physician (an experienced gastroenterologist), in line with the Tokyo 2018 criteria [28], but also relying on indicators of bacterial infection such as elevated serum procalcitonin levels and positive blood cultures. As per local clinical practices, an EUS was performed on all patients with ABP apart from patients with clinical and laboratory signs of cholangitis or choledocholithiasis clearly visualized on the transabdominal US or CT. Patients with the latter two criteria were referred directly to ERCP. Exceptionally, patients proceeded directly to ERCP based on the decision made by the treating gastroenterologist.
Thus, EUS was performed on all patients with the intermediate-probability ASGE and intermediate-likelihood ESGE predictors for choledocholithiasis [19,22]. Importantly, all patients with the ASGE high-probability predictor of a total bilirubin > 68.4 µmol/L plus dilated CBD on their transabdominal US were also referred to EUS before potential ERCP (contrary to the ASGE 2019 guideline) [19].
The transabdominal US was performed at the emergency department by a radiologist. The common bile duct was considered dilated when wider than 6 mm, or in the case of prior cholecystectomy, wider than 8 mm, in line with previous definitions [8,20,21,29,30]. A common bile duct stone was defined as a hyperechogenic finding with the diameter ≥ 3mm, with dorsal acoustic shadow (transabdominal US and EUS), or stone visualization during ERCP [19]. Endoscopic ultrasound procedures were performed by 5 different gastroenterologists with a total annual volume of around 1300 procedures, using a Fujifilm EG-580 UR endoscope, and 5 different gastroenterologists with a total annual volume of around 1400 procedures performed ERCP.
All patients had standard biochemical tests performed at the emergency department. A subset of patients had repeated biochemical tests just before EUS/ERCP, typically the morning after their admission to the ward. Liver biochemical tests were considered abnormal if at least one of the parameters (total or direct bilirubin, transaminases, or alkaline phosphatase) was >2× above the upper normal level [19,22].
The reliability of the ASGE and ESGE predictors (Table 1) for CBD stones was assessed by calculating the sensitivity and specificity of each predictor. In a subset of patients with available paired biochemical measurements, we also evaluated whether the dynamics of liver biochemistry could predict spontaneous passage of CBD stones.

Table 1.
American society for Gastrointestinal Endoscopy 2019 and European society of Gastrointestinal Endoscopy algorithms for suspected common bile duct stones.
Statistical Analysis
For the statistical analysis we used Mann–Whitney U, Chi square/Fisher’s exact tests and receiver operating characteristic (ROC) curve analysis with Youden’s J statistics [31]. A p-value < 0.05 was considered statistically significant. Reported p values are nominal unless otherwise stated. Analyses were performed using IBM SPSS (Version 22) and GraphPad Prism (Version 8). Ethical approval was obtained from the Slovenian Commission for Medical Ethics (application number 0120-486/2022/3).
3. Results
A total of 86 out of 92 (93.5%) patients who were admitted in 2020 due to ABP were included in this analysis (Figure 1).
Patients’ characteristics, duration of symptoms relative to examinations performed, and laboratory values are summarized in Table 2.

Table 2.
Patients’ evaluation details before endoscopic ultrasound examination.
3.1. Endoscopic Ultrasound and Endoscopic Retrograde Cholangiopancreatography Findings
Common bile duct stones were confirmed in all 13 patients who were directly referred to ERCP. Ten of these had cholangitis, a CBD stone visualized on the transabdominal US, or both criteria. Three patients were referred to ERCP based on clinical decisions (one patient with a rapid increase in serum bilirubin and two older, fragile patients not suitable for cholecystectomy where endoscopic biliary sphincterotomy was considered to be the definite treatment).
All other patients (73/86 (84.9%)) underwent EUS after a median time of 2 days (interquartile range 1–3 days) from their first presentation at the emergency department. EUS eventually confirmed CBD stones in 16/73 (21.9%) of these patients. In one patient, the EUS detected a very small fragment with a diameter of only 3 mm located in the distal part of the CBD. In this patient, ERCP was not performed (clinical decision) and a second EUS a few days later confirmed spontaneous passage.
3.2. Performance of American Society for Gastrointestinal Endoscopy 2019 Guidelines and European Society of Gastrointestinal Endoscopy Guidelines for Prediction of Choledocholithiasis in Patients with Acute Biliary Pancreatitis
Predictors used in the ASGE and ESGE guidelines are summarised in Table 1. Table 3 shows the proportion of patients with an eventually confirmed CBD stone according to the presence of different ASGE and ESGE predictors. In Table 4, the high-, intermediate-, and low-probability predictors of ASGE, and the high-, intermediate-, and low-likelihood ESGE predictors are compared.

Table 3.
Incidence of American Society for Gastrointestinal Endoscopy 2019 and European Society of Gastrointestinal Endoscopy predictors for detection of common bile duct stones in acute biliary pancreatitis.

Table 4.
Comparison of American Society for Gastrointestinal Endoscopy 2019 probability and European Society of Gastrointestinal Endoscopy likelihood groups for common bile duct stones in acute biliary pancreatitis.
The sensitivity and specificity of each predictor and risk group of the ASGE and ESGE guidelines are depicted in Table 5.

Table 5.
Sensitivity and specificity of American Society for Gastrointestinal Endoscopy 2019 and European Society of Gastrointestinal Endoscopy algorithms and single predictors for prediction of choledocholithiasis in acute biliary pancreatitis.
3.3. Predictive Values of Liver Biochemistry for the Presence of Common Bile Duct Stones in Patients with Acute Biliary Pancreatitis
Liver biochemistry at admission (Table 6) was not associated with the presence of CBD stones as patients with and without choledocholithiasis had similar values. Only the alkaline phosphatase values were slightly higher in patients who later had confirmed CBD stones (area under the ROC curve of 69.8% (95% confidence interval: 57.3–82.3%, p = 0.004)) (Figure 2) [31].

Table 6.
Liver biochemistry in patients with and without choledocholithiasis.
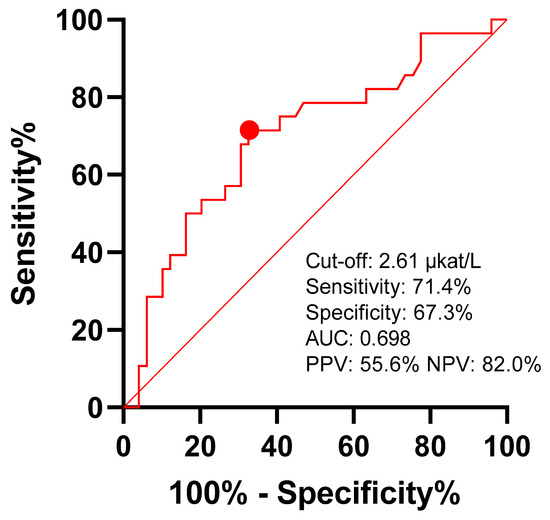
Figure 2.
Predictive value of alkaline phosphatase for choledocholithiasis. Youden’s J statistics cut-off point is marked with red dot. AUC—area under curve; PPV—positive predictive value; NPV—negative predictive value.
Similarly, by analysing the data of 70/87 (80.5%) patients who had available paired measurements of biochemistry (baseline at the emergency department and follow-up value after admission to the ward; the time span between both measurements was median 1 day (interquartile range: 1–3 days)), we did not find any predictive value of follow-up biochemistry for choledocholithiasis. Furthermore, the dynamics of biochemistry were similar in patients with or without CBD stones, apart from one patient, whose total bilirubin levels rose from 33 to 115 µmol/L and was consequently referred directly to ERCP that confirmed choledocholithiasis. The dynamics of total bilirubin are depicted in Figure 3.
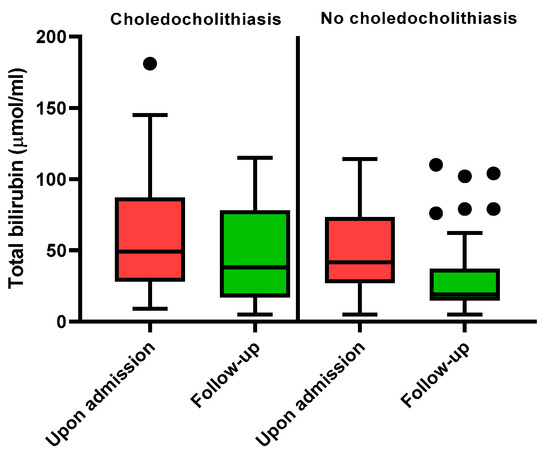
Figure 3.
Bilirubin levels in patients with and without choledocholithiasis.
4. Discussion
The removal of persistent CBD stones is important in the management of ABP [22,32,33,34,35,36]. Since ERCP can have serious, potentially life-threatening complications, every effort is made to confirm the presence of stones in the CBD before ERCP [13,19,22]. The ASGE and ESGE guidelines suggest using algorithms that stratify patients with suspected CBD stones in three groups: low, intermediate, and high probability/likelihood. However, these guidelines were mainly developed from cohorts of patients without pancreatitis [19,22]. Furthermore, the presence of ABP was historically used as a variable in patients with suspected choledocholithiasis, mainly decreasing the chance of CBD stone persistence [8,19,20,21]. However, the performance of predictors was scarcely tested within the subgroup of patients with ABP [29].
Because of specific pathological changes in ABP that may impact the sensitivity of transabdominal US and influence liver biochemistry, which are the two input variables central to the ASGE and ESGE algorithms for predicting CBD stones, it is important to validate the guidelines in this subset of patients [4,19,22]. We did this in our retrospective cohort study. Our findings confirmed that the high-probability/likelihood predictors (visualized stones in the CBD by transabdominal US and features of cholangitis) correctly identified patients with persistent CBD stones who can bypass EUS and proceed directly to ERCP. Conversely, the third high-probability ASGE predictor for choledocholithiasis, total bilirubin > 68.4 µmol/L plus dilated CBD, was only correct in predicting CBD stones in half of the patients with ABP. The value of this predictor was numerically similar to the intermediate-probability ASGE predictors (age > 55 years, dilated CBD, and abnormal liver function tests) and intermediate-likelihood ESGE predictors (abnormal liver function tests and dilated CBD). We speculate that one of the reasons for this deviation might be oedema and spasms of the ampulla of Vater after the spontaneous passage of CBD stones resulting in the transient elevation of bilirubin and CBD dilation [4,19,23]. Strictly following the ASGE algorithm in our cohort would have resulted in five patients undergoing unnecessary ERCP. We therefore suggest, that in the setting of ABP, patients with elevated bilirubin and dilated CBD are first referred to EUS rather than directly to ERCP. However, it should be noted that we did not address the timing of ERCP in our cohort. This is relevant since early ERCP with sphincterotomy did not change the outcome of patients with predicted severe pancreatitis and nor did EUS-guided ERCP [37,38].
Applying the ESGE algorithm to our cohort resulted in five patients being classified as low likelihood. These five patients were classified as intermediate probability by the ASGE 2019 guidelines as they were older than 55 years. None of them had choledocholithiasis. If the ESGE guidelines were followed, these patients could have been spared an EUS. Additionally, since all patients with choledocholithiasis showed dilated CBDs on the transabdominal US or abnormal liver biochemical tests, there was no additional benefit in using the third ASGE intermediate-probability predictor (age over 55) in our cohort.
Normal liver biochemistry has a high negative predictive value for choledocholithiasis [18,19,22]. However, since most patients in our cohort had increased liver function tests this was not useful for excluding CBD stones. Our analysis also did not reveal useful differences in the levels of liver function tests (total and direct bilirubin, alkaline phosphatase, and aspartate and alanine aminotransferase) between patients with or without CBD stones. Alkaline phosphatase had the highest predictive value among biochemical tests, but the area under ROC was still below 70%, thus precluding its clinical utility. The predictive value of liver function tests also did not improve after incorporating the secondary laboratory set after 1–3 days from presentation. The same conclusions have been previously published by other authors. Repeating these tests before deciding to proceed with either EUS or ERCP appears not to be clinically useful [8,39]. The only test that may be worth repeating is total bilirubin, since its doubling to >68.4 µmol/L could be a sign of persistent CBD obstruction by a gallstone [30]. However, this occurred in only one of our patients and the reported positive predictive values from He and colleagues (62%) do not justify the immediate use of ERCP in these patients [30].
A dilated CBD was the most reliable intermediate-probability/likelihood predictor of choledocholithiasis in our cohort, which is in line with previous studies that were not exclusively performed in patients with ABP [20,21,30]. However, the sensitivity of CBD dilatation for choledocholithiasis was lower in our patients with ABP (55.2%) compared to the sensitivity reported in studies that predominantly included patients without pancreatitis (from 58% to 83.8%) [20,21,30]. This could be due to the aforementioned decreased performance of transabdominal US caused by bowel paresis in the setting of acute pancreatitis (26). Alternatively, patients with ABP may be more likely to have small and distally located stones, which are more difficult to find with transabdominal US [6,30].
Our study was an open-label cohort study with all the potential intrinsic limitations. Despite its retrospective design, we still believe we largely avoided selection bias since we analysed all patients admitted in the year 2020 to a large tertiary referral centre covering a predefined urban area. Also, the majority of patients were systematically approached for CBD stones in a similar way, with a low threshold for EUS examination (following ESGE guidelines) [22]. The literature has shown that EUS is more sensitive compared to MRCP in the detection of very small stones and sludge [9,11]. Therefore, we might have more accurately assessed choledocholithiasis compared to some other studies that used the latter two modalities as the golden standard [29,30]. However, very small stones or sludge, that could potentially be missed with MRCP, might not be a clinically important finding in ABP. Nevertheless, performing EUS instead of MRCP might be more practical in some institutions as in the case of CBD stones where the patient can have ERCP performed during the same session.
In the setting of acute pancreatitis, it is challenging to reliably establish the diagnosis of concomitant acute cholangitis. Using the Tokyo 2018 guidelines for acute cholangitis [28], virtually all the criteria can also be observed in acute pancreatitis due to pathophysiological, non-infectious processes ongoing during pancreatitis. Nevertheless, the diagnosis of concomitant acute cholangitis in our study was not only made at the discretion of the treating physician based on the Tokyo guidelines, but also on the indicators of bacterial infection. Despite this somewhat arbitrary approach, the strategy proved to be a highly accurate predictor of persistent CBD stones in our cohort. Further studies are needed to validate a tool that would promptly discern cholangitis in patients with ABP.
The APEC trials failed to demonstrate a difference for the primary outcome, which was a composite of mortality or major complication occurring within 6 months of randomization. The only component of the primary outcome with a difference between groups was cholangitis, which was less common in the urgent ERCP group than in the conservative treatment group (2% versus 10%) [37,38]. The findings of the APEC studies, in conjunction with prior work, emphasize that expecting ERCP to stop or reverse the pathophysiological mechanisms resulting in systemic and local complications in acute pancreatitis is overly optimistic. Biliary obstruction is removed with ERCP, which is reflected by the lower incidence of cholangitis in the urgent ERCP group but does not impact the development of organ failure or pancreatic necrosis, the driving mechanisms of mortality in acute pancreatitis. Hypothetically, an even earlier intervention could conceivably alter the disease course, but the intervention (either urgent ERCP or EUS to assess the need for ERCP) was performed within 24 h of presentation, with a quarter of ERCP performed within 12 h in APEC-1 [38], which is probably already at the limit of feasibility in most, if not all, healthcare settings.
In this study, the data on the complications following EUS or ERCP were not collected. However, we believe that our approach with early EUS was safe as the safety of EUS is well documented [40,41]. To the contrary, ERCP may be associated with significant procedure-related complications [42]. Here it should also be acknowledged that our approach with early EUS might not have always been justified since the EUS-guided ERCP approach, even in predicted severe pancreatitis, did not reduce major complications or mortality compared to the conservative strategy in APEC trials [37,38]. This might be even more relevant in predicted mild pancreatitis. Unfortunately, we were unable to perform more focused analyses (stratified by predicted severity), since retrospectively assessing predicted disease severity would be unreliable from clinical charts.
5. Conclusions
In conclusion, our retrospective analysis of patients with ABP suggests that the ESGE 2019 high-likelihood predictors for CBD stone prediction perform better than the ASGE 2019 high-probability predictors in the setting of ABP. The difference can be attributed to the fact that half of the patients with the ASGE 2019 predictor of dilated CBD plus bilirubin above > 68.4 µmol/mL did not have CBD stones on their EUS. Our observation might be specific to a subgroup of patients with ABP and needs further exploration in larger cohorts to help prevent unnecessary ERCP procedures. Future guidelines should separately address the assessment of the CBD for persistent stones in a subgroup of patients with ABP.
Author Contributions
Conceptualization, Ž.P.Č. and D.D.; methodology, Ž.P.Č., L.S., S.P., J.D., D.S. and D.D.; software, Ž.P.Č. and D.D.; validation, Ž.P.Č. and D.D.; formal analysis, Ž.P.Č. and D.D.; investigation, Ž.P.Č. and D.D.; resources, Ž.P.Č., L.S., S.P., J.D., D.S. and D.D.; data curation, Ž.P.Č. and D.D.; writing—original draft preparation, Ž.P.Č. and D.D.; writing—review and editing, N.S., J.H. and D.D.; visualization, Ž.P.Č. and D.D.; supervision, B.Š., J.H. and D.D.; project administration, Ž.P.Č.; funding acquisition, D.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Slovenian Commission for Medical Ethics (application number 0120-486/2022/3) on 2 February 2023.
Informed Consent Statement
Patient consents were waived due to the retrospective design of the study. The study did not include any intervention.
Data Availability Statement
Data used for this report are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Knudsen, J.S.; Heide-Jørgensen, U.; Mortensen, F.V.; Sørensen, H.T.; Ehrenstein, V. Acute pancreatitis: 31-Year trends in incidence and mortality—A Danish population-based cohort study. Pancreatology 2020, 20, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Lindkvist, B.; Appelros, S.; Manjer, J.; Borgström, A. Trends in incidence of acute pancreatitis in a Swedish population: Is there really an increase? Clin. Gastroenterol. Hepatol. 2004, 2, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.E.; Morrison-Rees, S.; John, A.; Williams, J.G.; Brown, T.H.; Samuel, D.G. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology 2017, 17, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Van Geenen, E.J.M.; van der Peet, D.L.; Bhagirath, P.; Mulder, C.J.J.; Bruno, M.J. Etiology and diagnosis of acute biliary pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.M.; Ledesma, C.L. Gallstone Migration as a Cause of Acute Pancreatitis. N. Engl. J. Med. 1974, 290, 484–487. [Google Scholar] [CrossRef]
- Tranter, S.; Thompson, M. Spontaneous passage of bile duct stones: Frequency of occurrence and relation to clinical presentation. Ann. R. Coll. Surg. Engl. 2003, 85, 174–177. [Google Scholar] [CrossRef]
- Acosta, J.M.; Rossi, R.; Ledesma, C.L. The usefulness of stool screening for diagnosing cholelithiasis in acute pancreatitis: A description of the technique. Dig. Dis. Sci. 1977, 22, 168–172. [Google Scholar] [CrossRef]
- Adams, M.A.; Hosmer, A.E.; Wamsteker, E.J.; Anderson, M.A.; Elta, G.H.; Kubiliun, N.M.; Kwon, R.S.; Piraka, C.R.; Scheiman, J.M.; Waljee, A.K.; et al. Predicting the likelihood of a persistent bile duct stone in patients with suspected choledocholithiasis: Accuracy of existing guidelines and the impact of laboratory trends. Gastrointest. Endosc. 2015, 82, 88–93. [Google Scholar] [CrossRef][Green Version]
- Șurlin, V. Imaging tests for accurate diagnosis of acute biliary pancreatitis. World J. Gastroenterol. 2014, 20, 16544. [Google Scholar] [CrossRef]
- Buscarini, E.; Tansini, P.; Vallisa, D.; Zambelli, A.; Buscarini, L. EUS for suspected choledocholithiasis: Do benefits outweigh costs? A prospective, controlled study. Gastrointest. Endosc. 2003, 57, 510–518. [Google Scholar] [CrossRef]
- Afzalpurkar, S.; Giri, S.; Kasturi, S.; Ingawale, S.; Sundaram, S. Magnetic resonance cholangiopancreatography versus endoscopic ultrasound for diagnosis of choledocholithiasis: An updated systematic review and meta-analysis. Surg. Endosc. 2023, 37, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Palazzo, L. Choledocholithiasis: Role of US and endoscopic ultrasound. Abdom. Imaging 2001, 26, 7–14. [Google Scholar] [CrossRef] [PubMed]
- De Lisi, S.; Leandro, G.; Buscarini, E. Endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography in acute biliary pancreatitis: A systematic review. Eur. J. Gastroenterol. Hepatol. 2011, 23, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.S.; DaVee, T.; Bernstam, E.V.; Kao, L.S.; Wandling, M.; Hussain, M.R.; Rashtak, S.; Ramireddy, S.; Guha, S.; Thosani, N. Cost-effectiveness analysis of optimal diagnostic strategy for patients with symptomatic cholelithiasis with intermediate probability for choledocholithiasis. Gastrointest. Endosc. 2022, 95, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Enochsson, L.; Swahn, F.; Arnelo, U.; Nilsson, M.; Löhr, M.; Persson, G. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest. Endosc. 2010, 72, 1175–1184.e3. [Google Scholar] [CrossRef]
- Tse, F.; Yuan, Y. Early routine endoscopic retrograde cholangiopancreatography strategy versus early conservative management strategy in acute gallstone pancreatitis. Cochrane Database Syst. Rev. 2012, 5, CD009779. [Google Scholar]
- Chan, K.S.; Shelat, V.G. Diagnosis, severity stratification and management of adult acute pancreatitis-current evidence and controversies. World J. Gastrointest. Surg. 2022, 14, 1179–1197. [Google Scholar] [CrossRef]
- Maple, J.T.; Ben-Menachem, T.; Anderson, M.A.; Appalaneni, V.; Banerjee, S.; Cash, B.D.; Fisher, L.; Harrison, M.E.; Fanelli, R.D.; Fukami, N.; et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest. Endosc. 2010, 71, 1–9. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Abbas Fehmi, S.M.; Sultan, S.; Fishman, D.S.; Qumseya, B.J.; Cortessis, V.K.; Schilperoort, H.; Kysh, L.; Matsuoka, L.; Yachimski, P.; et al. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest. Endosc. 2019, 89, 1075–1105.e15. [Google Scholar] [CrossRef]
- Rubin, M.I.N.; Thosani, N.C.; Tanikella, R.; Wolf, D.S.; Fallon, M.B.; Lukens, F.J. Endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis: Testing the current guidelines. Dig. Liver Dis. 2013, 45, 744–749. [Google Scholar] [CrossRef]
- Magalhães, J. Endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis: From guidelines to clinical practice. World J. Gastrointest. Endosc. 2015, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Manes, G.; Paspatis, G.; Aabakken, L.; Anderloni, A.; Arvanitakis, M.; Ah-Soune, P.; Barthet, M.; Domagk, D.; Dumonceau, J.-M.; Gigot, J.-F.; et al. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2019, 51, 472–491. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Underhill, L.H.; Steer, M.L.; Meldolesi, J. The Cell Biology of Experimental Pancreatitis. N. Engl. J. Med. 1987, 316, 144–150. [Google Scholar] [CrossRef]
- Bohidar, N.P.; Garg, P.K.; Khanna, S.; Tandon, R.K. Incidence, etiology, and impact of fever in patients with acute pancreatitis. Pancreatology 2003, 3, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Charcot, J.M. Leçons sur les maladies du foie, des voies biliaires et des reins faites à la Faculté de Médecine de Paris. In Recueillies et Publliées par Bourneville et Sevestre; Bureaux du Progrés Médical & Adrien Delahaye: Paris, France, 1877; 480p. [Google Scholar]
- Neoptolemos, J.P.; Hall, A.W.; Finlay, D.F.; Berry, J.M.; Carr-Locke, D.L.; Fossard, D.P. The urgent diagnosis of gallstones in acute pancreatitis: A prospective study of three methods. Br. J. Surg. 2005, 71, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.-H.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepato-Biliary Pancreat. 2018, 25, 17–30. [Google Scholar] [CrossRef]
- Tintara, S.; Shah, I.; Yakah, W.; Ahmed, A.; Sorrento, C.S.; Kandasamy, C.; Freedman, S.D.; Kothari, D.J.; Sheth, S.G. Evaluating the accuracy of American Society for Gastrointestinal Endoscopy guidelines in patients with acute gallstone pancreatitis with choledocholithiasis. World J. Gastroenterol. 2022, 28, 1692–1704. [Google Scholar] [CrossRef]
- He, H.; Tan, C.; Wu, J.; Dai, N.; Hu, W.; Zhang, Y.; Laine, L.; Scheiman, J.; Kim, J.J. Accuracy of ASGE high-risk criteria in evaluation of patients with suspected common bile duct stones. Gastrointest. Endosc. 2017, 86, 525–532. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Crockett, S.D.; Wani, S.; Gardner, T.B.; Falck-Ytter, Y.; Barkun, A.N.; Crockett, S.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology 2018, 154, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology Guideline: Management of Acute Pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.M.; Katkhouda, N.; Debian, K.A.; Groshen, S.G.; Tsao-Wei, D.D.; Berne, T.V. Early Ductal Decompression Versus Conservative Management for Gallstone Pancreatitis With Ampullary Obstruction: A Prospective Randomized Clinical Trial. Ann. Surg. 2006, 243, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.M. Repetitive Short-Term Obstructions of the Common Bile–Pancreatic Duct Induce Severe Acute Pancreatitis in the Opossum. Dig. Dis. Sci. 1999, 44, 1653–1661. [Google Scholar] [CrossRef]
- Neoptolemos, J.P. The theory of ‘persisting’ common bile duct stones in severe gallstone pancreatitis. Ann. R. Coll. Surg. Engl. 1989, 71, 326–331. [Google Scholar]
- Hallensleben, N.D.; Stassen, P.M.C.; Schepers, N.J.; Besselink, M.G.; Anten, M.P.G.F.; Bakker, O.J.; Vollen, T.L.; da Costa, D.W.; van Dijk, S.M.; van Dullemen, H.M.; et al. Patient selection for urgent endoscopic retrograde cholangio-pancreatography by endoscopic ultrasound in predicted severe acute biliary pancreatitis (APEC-2): A multicentre prospective study. Gut 2023, 72, 1534–1542. [Google Scholar] [CrossRef]
- Schepers, N.J.; Hallensleben, N.D.L.; Besselink, M.G.; Anten, M.P.G.F.; Bollen, T.L.; da Costa, D.W.; van Delft, F.; van Dijk, S.M.; van Dullemen, H.M.; Dijkgraaf, M.G.W.; et al. Urgent endoscopic retrograde cholangiopancreatography with sphincterotomy versus conservative treatment in predicted severe acute gallstone pancreatitis (APEC): A multicentre randomised controlled trial. Lancet 2020, 396, 167–176. [Google Scholar] [CrossRef]
- Suarez, A.L.; LaBarre, N.T.; Cotton, P.B.; Payne, K.M.; Coté, G.A.; Elmunzer, B.J. An assessment of existing risk stratification guidelines for the evaluation of patients with suspected choledocholithiasis. Surg. Endosc. 2016, 30, 4613–4618. [Google Scholar] [CrossRef]
- Van Der Merwe, S.W.; Van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef]
- Iglesias-Garcia, J.; Sample Organization. Endoscopic ultrasound elastography. Endosc. Ultrasound 2012, 1, 8. [Google Scholar] [CrossRef]
- Dumonceau, J.M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).