Histopathologic and Preneoplastic Changes in Tubal Ligation Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, S.; Abramovitz, A. Tubal Ligation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Şentürk, M.B.; Budak, M.S.; Toğrul, C.; Tahaoğlu, A.E.; Balsak, D.; Akgöl, S. The Pregnancy Rates After Tubal Reanastomosis. Okmeydanı Tıp Derg. 2016, 32, 79–82. [Google Scholar] [CrossRef]
- Özkan, H.; Özer, B.U.; Arı, Ö. Sezaryen İle Doğuma Güncel Bir Bakış: Modern Sezaryen Teorisi. Arşiv Kaynak Tarama Derg. 2021, 30, 226–235. [Google Scholar]
- Uaamnuichai, S.; Phutrakool, P.; Thammasitchai, N.; Sathitloetsakun, S.; Santibenchakul, S.; Jaisamrarn, U. Does socioeconomic factors and healthcare coverage affect postpartum sterilization uptake in an urban, tertiary hospital? Reprod. Health 2023, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Ely, L.K.; Truong, M. The role of opportunistic bilateral salpingectomy vs tubal occlusion or ligation for ovarian cancer prophylaxis. J. Minim. Invasive Gynecol. 2017, 24, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yanai-Inbar, I.; Silverberg, S.G. Mucosal epithelial proliferation of the fallopian tube: Prevalence, clinical associations, and optimal strategy for histopathologic assessment. Int. J. Gynecol. Pathol. 2000, 19, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Vang, R.; Junge, J.; Hannibal, C.G.; Kjaer, S.K.; Shih, I.M. Papillary tubal hyperplasia. The putative precursor of ovarian atypical proliferative (borderline) serous tumors, noninvasive implants, and endosalpingiosis. Am. J. Surg. Pathol. 2011, 35, 1605. [Google Scholar] [CrossRef]
- Lehn, K.; Gu, L.; Creinin, M.D.; Chen, M.J. Successful completion of total and partial salpingectomy at the time of cesarean delivery. Contraception 2018, 98, 232–236. [Google Scholar] [CrossRef]
- Koç, N.; Ayas, S.; Uygur, L. The association of serous tubal intraepithelial carcinoma with gynecologic pathologies and its role in pelvic serous cancer. Gynecol. Oncol. 2014, 134, 486–491. [Google Scholar] [CrossRef]
- Aslani, F.S.; Maleknasab, M.; Akbarzadeh-Jahromi, M. Fallopian tube epithelial changes in ovarian serous tumors compared with control group: A single-center study. Niger. Med. J. 2019, 60, 47. [Google Scholar]
- Luke, S.; Addae-Konadu, K.; Davidson, B.; Kuller, J.; Dotters-Katz, S. Benefits and Risks of Bilateral Salpingectomy Compared with Standard Tubal Ligation During Cesarean Delivery for Permanent Postpartum Contraception. Obstet. Gynecol. Surv. 2022, 77, 167–173. [Google Scholar] [CrossRef]
- Ida, T.; Fujiwara, H.; Kiriu, T.; Taniguchi, Y.; Kohyama, A. Relationship between the precursors of high grade serous ovarian cancer and patient characteristics: Decreased incidence of the p53 signature in pregnant women. J. Gynecol. Oncol. 2019, 30, e96. [Google Scholar] [CrossRef] [PubMed]
- Gaitskell, K.; Green, J.; Pirie, K.; Reeves, G.; Beral, V.; on behalf of the Million Women Study Collaborators. Tubal ligation and ovarian cancer risk in a large cohort: Substantial variation by histological type. Int. J. Cancer 2016, 138, 1076–1084. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.H.; Kim, Y.B.; Kim, J.; Kim, J.W.; Park, M.H.; Park, J.H.; Rhee, J.H.; Lim, M.C.; Hong, J.S. Bilateral salpingectomy to reduce the risk of ovarian/fallopian/peritoneal cancer in women at average risk: A position statement of the Korean Society of Obstetrics and Gynecology (KSOG). Obstet. Gynecol. Sci. 2018, 61, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Sungu, N.; Kiran, N.U.; Tatli Dogan, H.; Kilicarslan, A.; Karakok, E.; Akyol, M.E. Evaluation of p53 and Ki67 expression profiles in basal cell carcinomas in a usual and an unusual location. Turk Patoloji Derg. 2018, 34, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Gül, A.; Şimşek, Y.; Şahin, G. The Review of the Cases of Tubal Sterilization Which Were Performed in the Department of Obstetrics and Gynecology, Yüzüncü Yıl University. Van Med. J. 2000, 7, 57–59. [Google Scholar]
- Christopher, G.L. Lalana Newborn Resuscitation. J. Clin. Med. Res. 2021, 2, 1–55. [Google Scholar] [CrossRef]
- Moreno, J.M.; Bartual, E.; Carmona, M.; Araico, F.; Miranda, J.A.; Herruzo, A.J. Changes in the rate of tubal ligation done after cesarean section. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 97, 147–151. [Google Scholar] [CrossRef]
- Mandelbaum, R.S.; Matsuzaki, S.; Sangara, R.N.; Klar, M.; Matsushima, K.; Roman, L.D.; Paulson, R.J.; Wright, J.D.; Matsuo, K. Paradigm shift from tubal ligation to opportunistic salpingectomy at cesarean delivery in the United States. Am. J. Obstet. Gynecol. 2021, 225, 399.e1–399.e32. [Google Scholar] [CrossRef]
- Werawatakul, Y.; Prasit, M.; Leelapongwattana, K.; Kleebkaow, P. Evaluation of female sterilization and tubal tissue confirmation at Srinagarind Hospital. J. Med. Assoc. Thail. 2012, 95, 1252. [Google Scholar]
- Yener, A.; Giray, B. Pregnancy Outcomes After Tubal Reanastomosis: A Novel Technique. Dicle Tıp Derg. 2021, 48, 839–843. [Google Scholar] [CrossRef]
- Akalın, A.; Bostancı, Ş. Aile planlaması yöntemi kullanan üreme çağındaki kadınlarda cinsel fonksiyonlar ve cinsel yaşam kalitesi. Androloji Bülteni (Androl. Bullettin) 2022, 24, 110–117. [Google Scholar] [CrossRef]
- Piek, J.M. Hereditary Serous Ovarian Carcinogenesis, a Hypothesis. Ph.D. Thesis, Medicine, Vrije Universiteit, Amsterdam, The Netherlands, 2004. [Google Scholar]
- Elnory, M.A.; Elmantwe, A. Impact of bilateral total salpingectomy versus standard tubal ligation at time of cesarean section on ovarian reserve: A randomized controlled trial. Evid. Based Women’s Health J. 2019, 9, 458–467. [Google Scholar] [CrossRef][Green Version]
- Powell, C.B.; Alabaster, A.; Simmons, S.; Garcia, C.; Martin, M.; McBride-Allen, S.; Littell, R.D. Salpingectomy for sterilization: Change in practice in a large integrated health care system, 2011–2016. Obstet. Gynecol. 2017, 130, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Rigby, C.H.; Aljassim, F.; Powell, S.G.; Wyatt, J.N.; Hill, C.J.; Hapangama, D.K. The immune cell profile of human fallopian tubes in health and benign pathology: A systematic review. J. Reprod. Immunol. 2022, 152, 103646. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.L.; Lynn, A.A. Histologic features of surgically removed fallopian tubes. Arch. Pathol. Lab. Med. 2002, 126, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, N.; Perween, S.; Gupta, K. Histopathological study of surgically resected specimens of fallopian tube. Int. J. Clin. Diagn. Pathol. 2020, 3, 37–43. [Google Scholar] [CrossRef]
- Kuhn, E.; Kurman, R.J.; Sehdev, A.S.; Shih, I.M. Ki-67 labeling index as an adjunct in the diagnosis of serous tubal intraepithelial carcinoma. Int. J. Gynecol. Pathol. 2012, 31, 416. [Google Scholar] [CrossRef]
- Sowamber, R.; Nelson, O.; Dodds, L.; DeCastro, V.; Paudel, I.; Milea, A.; Considine, M.; Cope, L.; Pinto, A.; Schlumbrecht, M.; et al. Integrative transcriptome analyses of the human fallopian tube: Fimbria and ampulla—Site of origin of serous carcinoma of the ovary. Cancers 2020, 12, 1090. [Google Scholar] [CrossRef]
- Chen, E.Y.; Mehra, K.; Mehrad, M.; Ning, G.; Miron, A.; Mutter, G.L.; Monte, N.; Quade, B.J.; McKeon, F.D.; Yassin, Y.; et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J. Pathol. 2010, 222, 110–116. [Google Scholar] [CrossRef]
- Zheng, W.; Sung, C.J.; Cao, P.; Zhang, Z.F.; Cai, R.; Godwin, T.A.; Kramer, E.E.; Lauchlan, S.C. Early occurrence and prognostic significance of p53 alteration in primary carcinoma of the fallopian tube. Gynecol. Oncol. 1997, 64, 38–48. [Google Scholar] [CrossRef]
- Mittal, N.; Srinivasan, R.; Gupta, N.; Rajwanshi, A.; Nijhawan, R.; Gautam, U.; Sood, S.; Dhaliwal, L. Secretory cell outgrowths, p53 signatures, and serous tubal intraepithelial carcinoma in the fallopian tubes of patients with sporadic pelvic serous carcinoma. Indian J. Pathol. Microbiol. 2016, 59, 481. [Google Scholar]
- Briseño Campos, A.G.; Cruz Rodríguez, A.; García Perales, M.O.; Serna Vela, F.J.; Camarillo Elizalde, D.G.; Robles Martínez, M.D. Incidence of intraepithelial fallopian tube neoplasias in Mexican women over 40 years of age that underwent elective hysterectomy. J. Ovarian Res. 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, K.; Shaw, P.; May, B.J.; Bahadirli-Talbott, A.; Kaushiva, A.; Risch, H.; Narod, S.; Wang, T.L.; Parkash, V.; Vang, R.; et al. Fallopian tube lesions in women at high risk for ovarian cancer: A multicenter study. Cancer Prev. Res. 2018, 11, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Tissot, M.; Lecointre, L.; Faller, E.; Afors, K.; Akladios, C.; Audebert, A. Clinical presentation of endometriosis identified at interval laparoscopic tubal sterilization: Prospective series of 465 cases. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 647–650. [Google Scholar] [CrossRef]
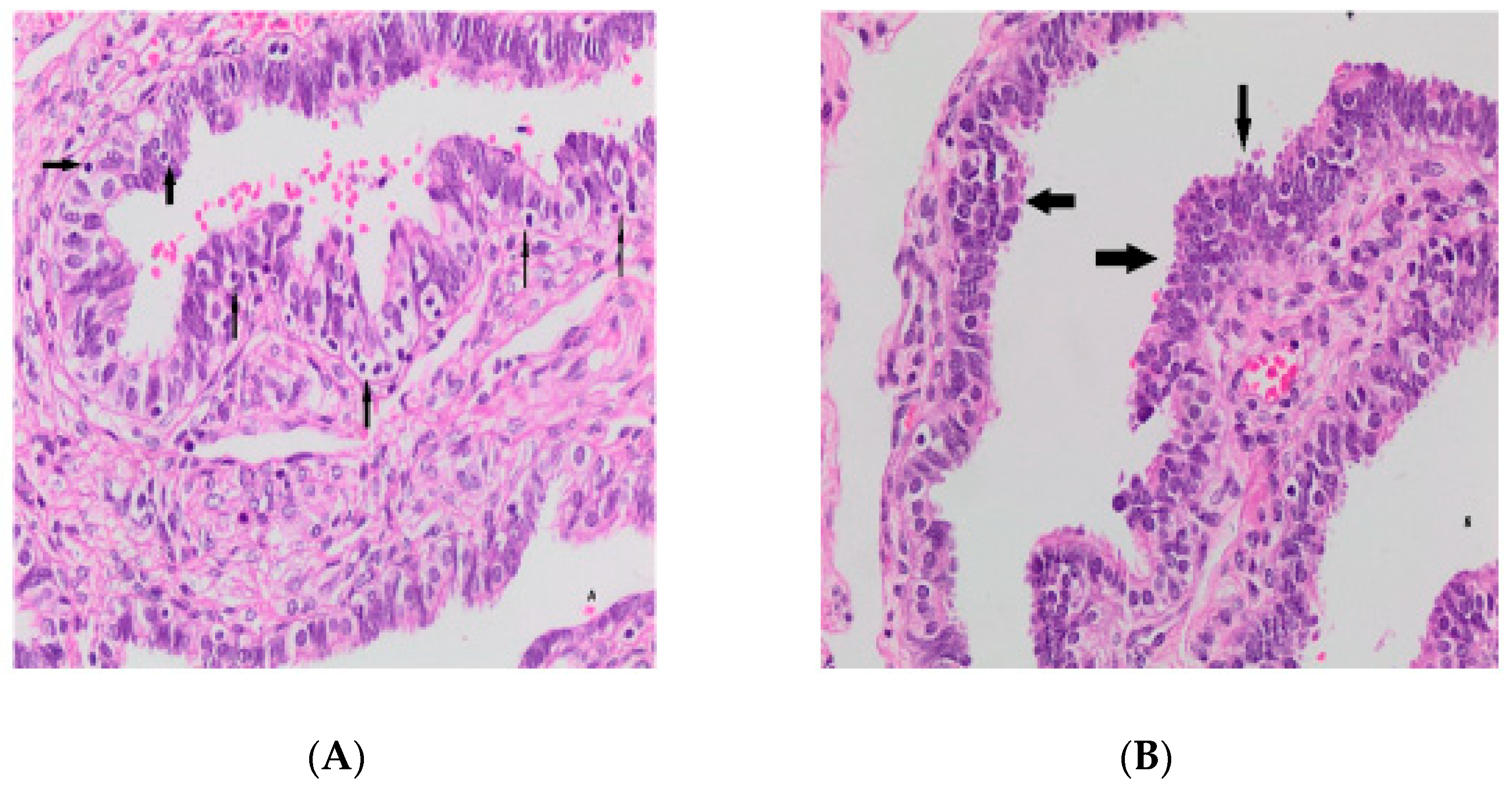


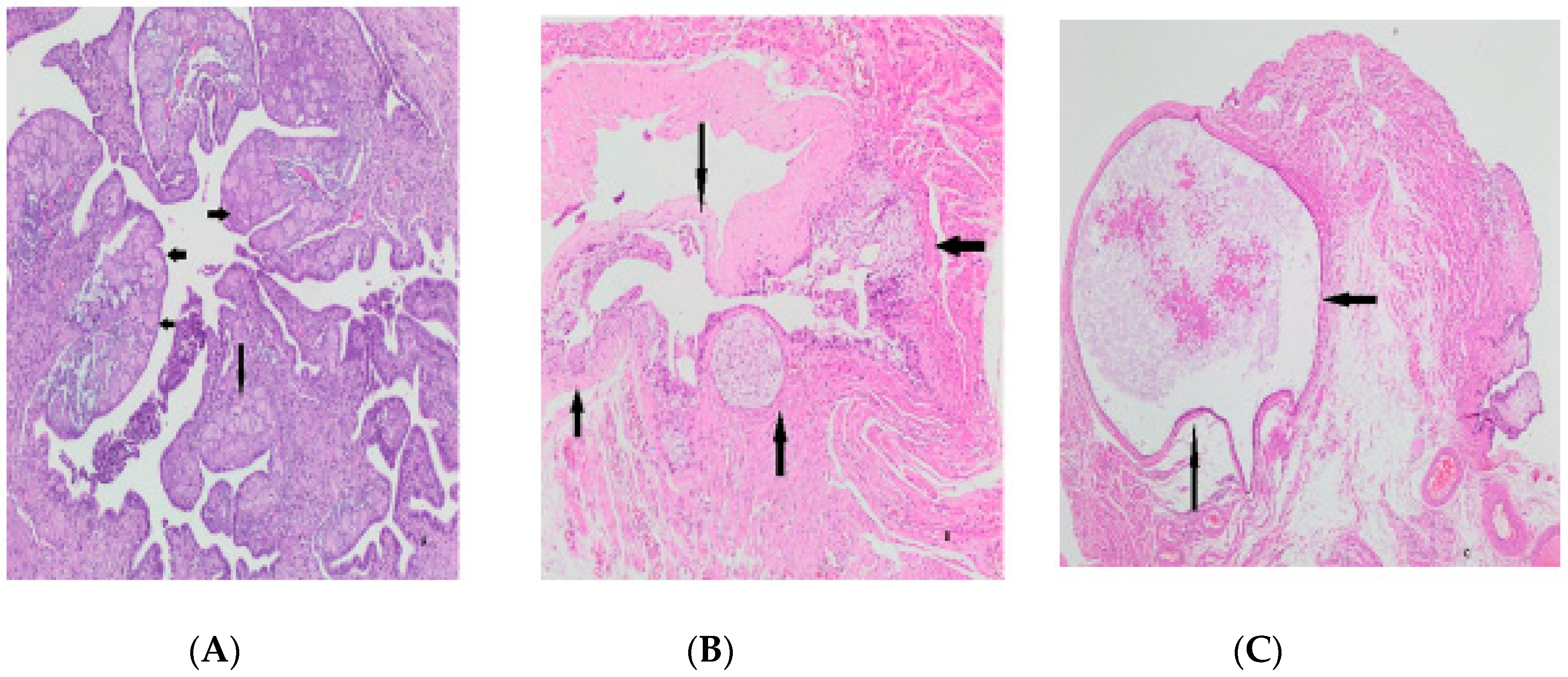
| NCL ≤ 2 | NCL ≥ 3 | p-Value | ||
|---|---|---|---|---|
| Age | <25 years | 2 (1.45%) | 1 (0.72%) | 0.024 |
| 26–30 years | 19 (13.77%) | 7 (5.07%) | ||
| 31–35 years | 44 (31.89%) | 4 (2.89%) | ||
| >40 years | 16 (11.59%) | 1 (0.72%) | ||
| Gravity Number | ≤3 | 18 (13.04%) | 5 (3.63%) | 0.90 |
| 4–6 | 83 (60.14%) | 11 (7.98%) | ||
| 6–9 | 16 (11.60%) | 1 (0.72%) | ||
| ≥10 | 3 (2.17%) | 1 (0.72%) | ||
| Parity Number | ≤3 | 86 (62.32%) | 2 (1.44%) | 0.71 |
| 4–6 | 28 (20.29%) | 3 (2.18%) | ||
| 6–9 | 5 (3.63%) | 1 (0.72%) | ||
| ≥10 | 1 (0.72%) | 1 (0.72%) | ||
| Papillary Hyperplasia | Absent (n) | 115 (83.34%) | 5 (3.63%) | 0.92 |
| Present (n) | 16 (11.59%) | 2 (1.44%) | ||
| Tufting | Absent (n) | 104 (88.41%) | 16 (11.59%) | 0.003 |
| Present (n) | 10 (7.2%) | 8 (92.8%) | ||
| Inflammatory cells | Absent (n) | 84 (60.86) | 8 (92.8%) | 0.029 |
| Present (n) | 36 (39.14%) | 10 (7.2%) | ||
| Parameters | Scores | N/% |
|---|---|---|
| Ki-67 | 1 | 127 (92.02%) |
| 2 | 8 (5.79%) | |
| 3 | 3 (2.17%) | |
| p53 | 0 | 50 (36.23%) |
| 1 | 38 (27.53%) | |
| 2 | 27(19.56%) | |
| 3 | 18 (13.04%) | |
| 4 | 5 (3.62%) | |
| SCOUT | 0 | 130 (94.21%) |
| 1 | 8 (5.79%) | |
| STIL | 0 | 137 (99.28%) |
| 1 | 1 (0.72%) | |
| p53 signature | 0 | 125 (90.58%) |
| 1 | 13 (9.42%) |
| SCOUT | p53 Score | p53 Signature | Kİ-67 Score | |
|---|---|---|---|---|
| Age | 0.4 | 0.036 | 0.2 | 0.2 |
| Gravity | 0.2 | 0.8 | 0.7 | 0.016 |
| Parity | 0.6 | 0.7 | >0.9 | 0.049 |
| Atypia | 0.5 | 0.2 | 0.6 | 0.3 |
| NCL | 0.3 | 0.7 | >0.9 | 0.7 |
| Papillary Hyperplasia | <0.9 | 0.6 | 0.5 | >0.9 |
| Tufting | 0.14 | 0.030 | >0.9 | 0.6 |
| Inflammatory cells | 0.4 | 0.3 | <0.9 | 0.7 |
| SCOUT | - | 0.3 | 0.028 | >0.9 |
| p53 | 0.3 | - | <0.001 | 0.053 |
| p53 Signature | 0.028 | <0.001 | - | 0.020 |
| Ki-67 | >0.9 | 0.053 | 0.02 | - |
| Variant | N | OR 1 | 95% CI 1 | p |
|---|---|---|---|---|
| Age | 138 | 0.88 | 0.79, 0.98 | 0.024 |
| Gravity | 138 | 0.98 | 0.74, 1.23 | 0.90 |
| Parity | 138 | 0.94 | 0.63, 1.27 | 0.71 |
| p53 | 138 | 0.17 | ||
| 0 | — | — | ||
| 1 | 0.30 | 0.06, 1.07 | ||
| 2 | 0.28 | 0.04, 1.17 | ||
| 3 | 0.44 | 0.06, 1.90 | ||
| 4 | 0.00 | |||
| Ki-67 | 138 | 0.65 | ||
| 0 | — | — | ||
| 1 | 0.92 | 0.05, 5.67 | ||
| 2 | 0.00 | |||
| Tufting | 138 | 0.003 | ||
| 0 | — | — | ||
| 1 | 5.20 | 1.76, 15.3 | ||
| p53 signature | 138 | 0.52 | ||
| 0 | — | — | ||
| 1 | 0.53 | 0.03, 2.96 | ||
| SCOUT | 138 | 0.35 | ||
| 0 | — | — | ||
| 1 | 2.37 | 0.33, 11.4 | ||
| Papillary Hyperplasia | 138 | 0.92 | ||
| 0 | — | — | ||
| 1 | 1.12 | 0.06, 7.12 | ||
| Atypia | 138 | 0.088 | ||
| 0 | — | — | ||
| 1 | 0.00 | |||
| Inflammation | 138 | 0.29 | ||
| 0 | — | — | ||
| 1 | 0.58 | 0.21, 1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ömeroğlu, E.; Ünlü, Y.; Uğur Kılınç, A.N.; Günler, T.; Günenc, O. Histopathologic and Preneoplastic Changes in Tubal Ligation Materials. Medicina 2023, 59, 2117. https://doi.org/10.3390/medicina59122117
Ömeroğlu E, Ünlü Y, Uğur Kılınç AN, Günler T, Günenc O. Histopathologic and Preneoplastic Changes in Tubal Ligation Materials. Medicina. 2023; 59(12):2117. https://doi.org/10.3390/medicina59122117
Chicago/Turabian StyleÖmeroğlu, Ethem, Yaşar Ünlü, Ayşe Nur Uğur Kılınç, Tuğba Günler, and Oğuzhan Günenc. 2023. "Histopathologic and Preneoplastic Changes in Tubal Ligation Materials" Medicina 59, no. 12: 2117. https://doi.org/10.3390/medicina59122117
APA StyleÖmeroğlu, E., Ünlü, Y., Uğur Kılınç, A. N., Günler, T., & Günenc, O. (2023). Histopathologic and Preneoplastic Changes in Tubal Ligation Materials. Medicina, 59(12), 2117. https://doi.org/10.3390/medicina59122117





