Our C-Arm-Free Minimally Invasive Technique for Spinal Surgery: The Thoracolumbar and Lumbar Spine—Based on Our Experiences
Abstract
:1. Introduction
2. Thoracolumbar Spine
2.1. Thoracolumbar Anterior Application
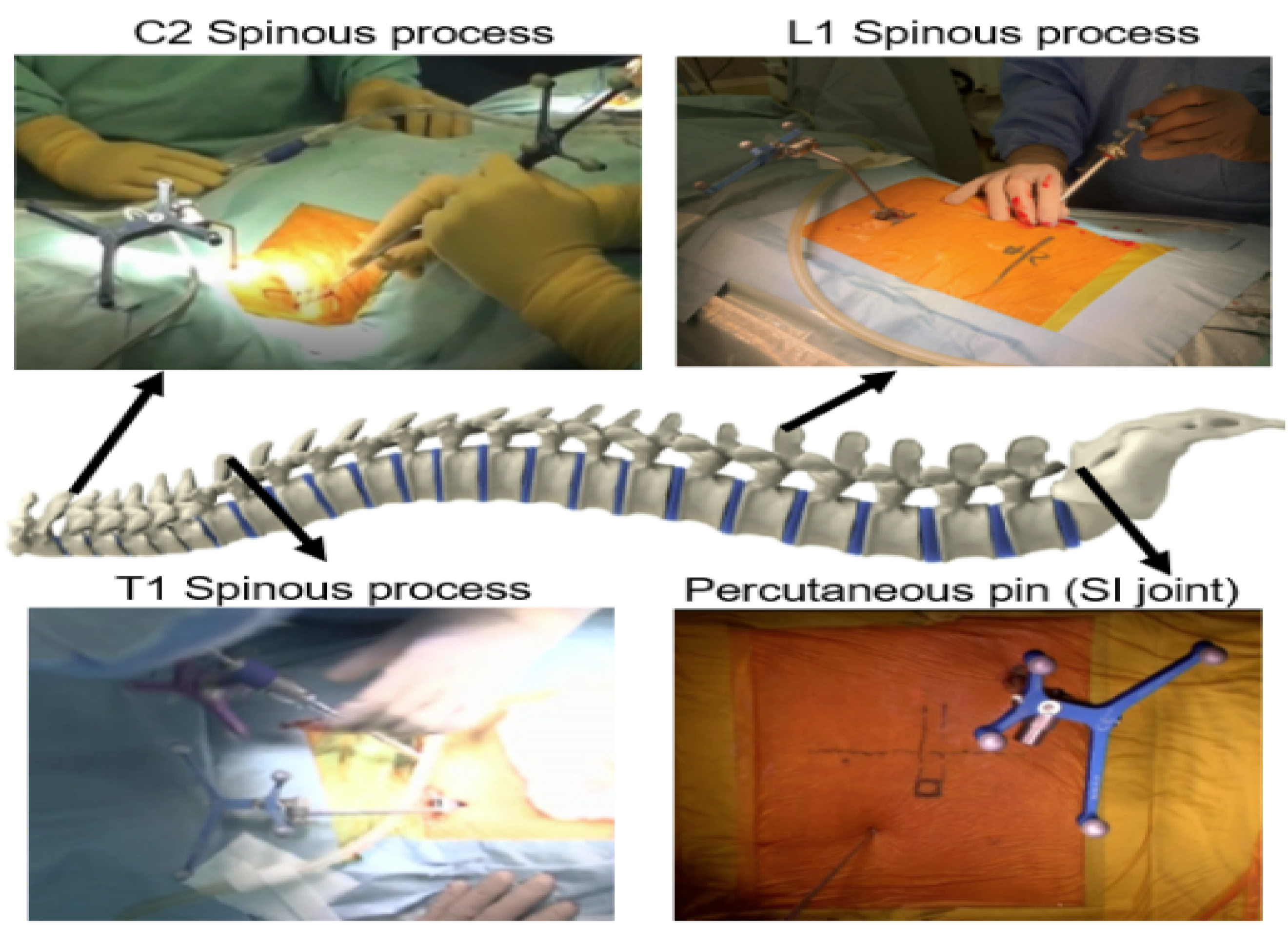
2.2. Thoracolumbar Posterior Application
2.2.1. Lateral Bendini
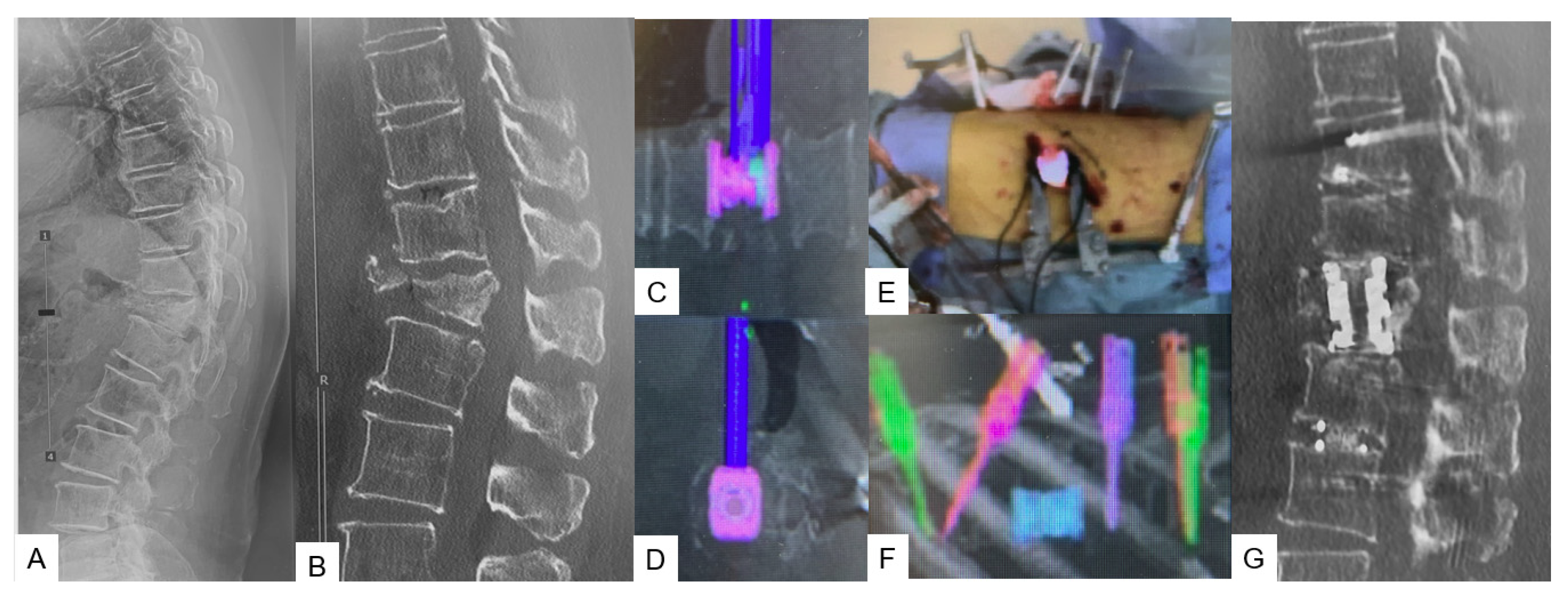
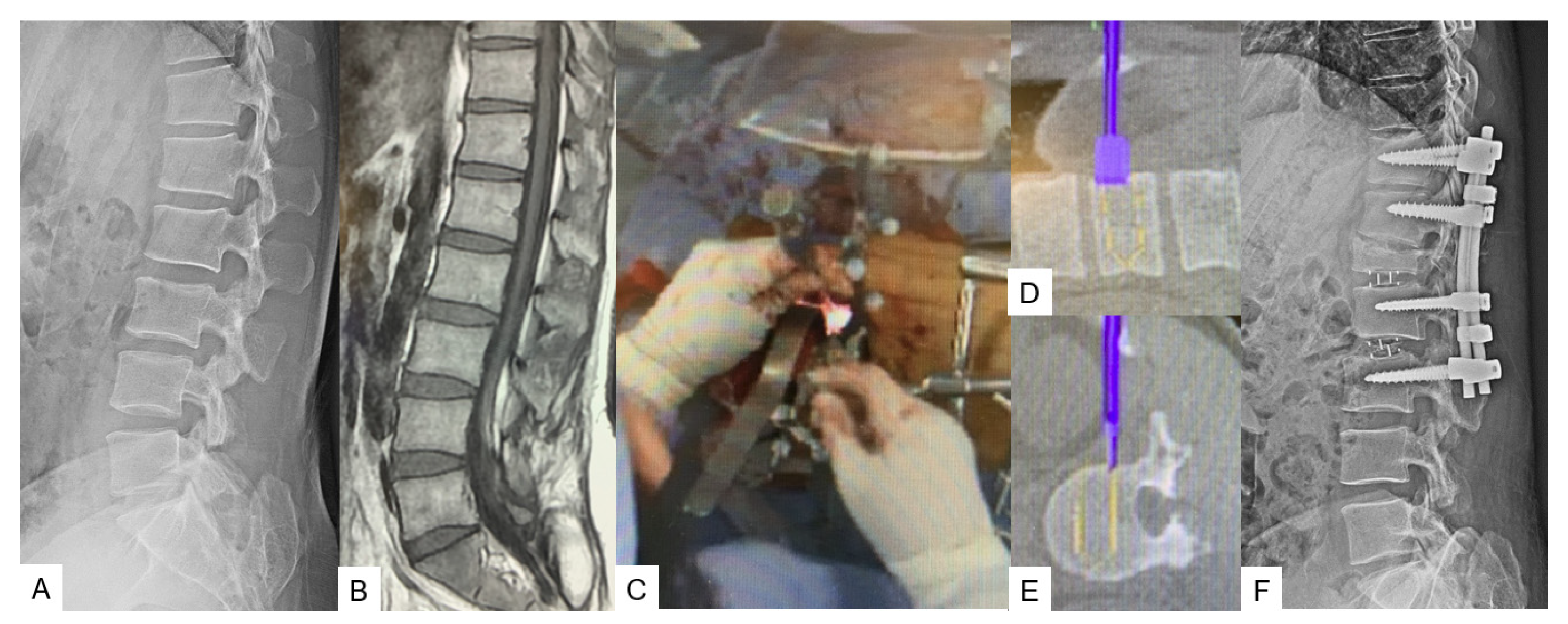
2.2.2. C-Arm-Free Trauma
3. Lumbar Spine
3.1. Lumbar Anterior Application
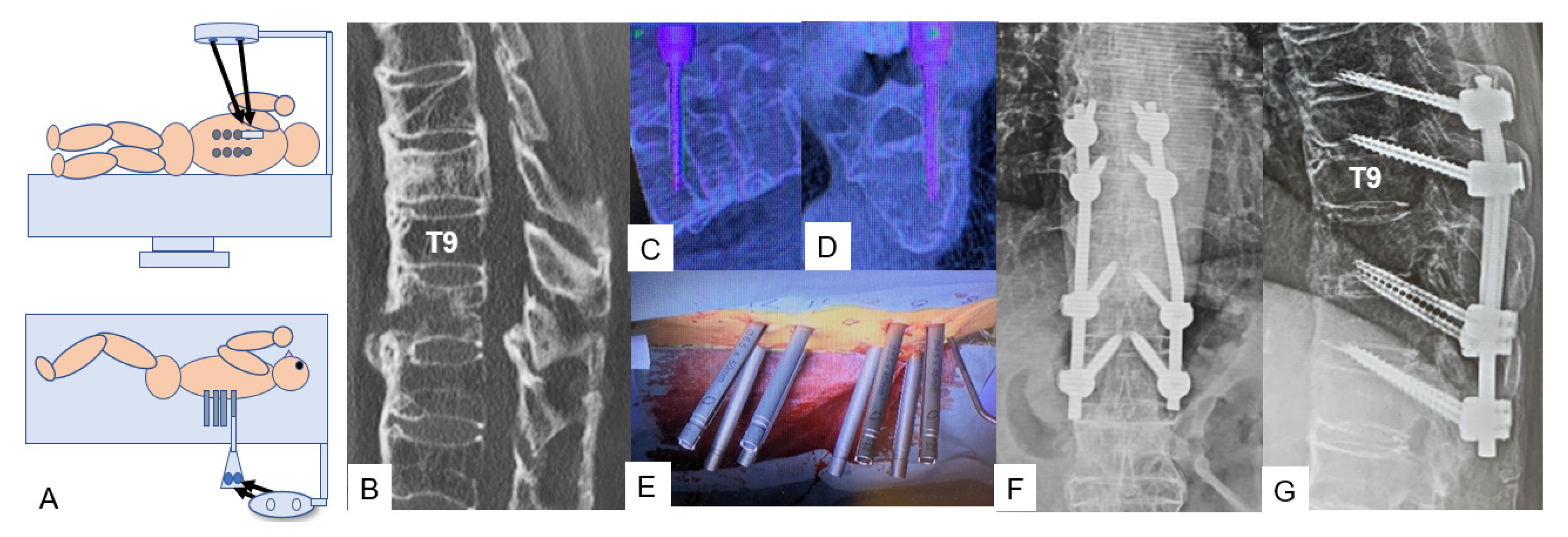
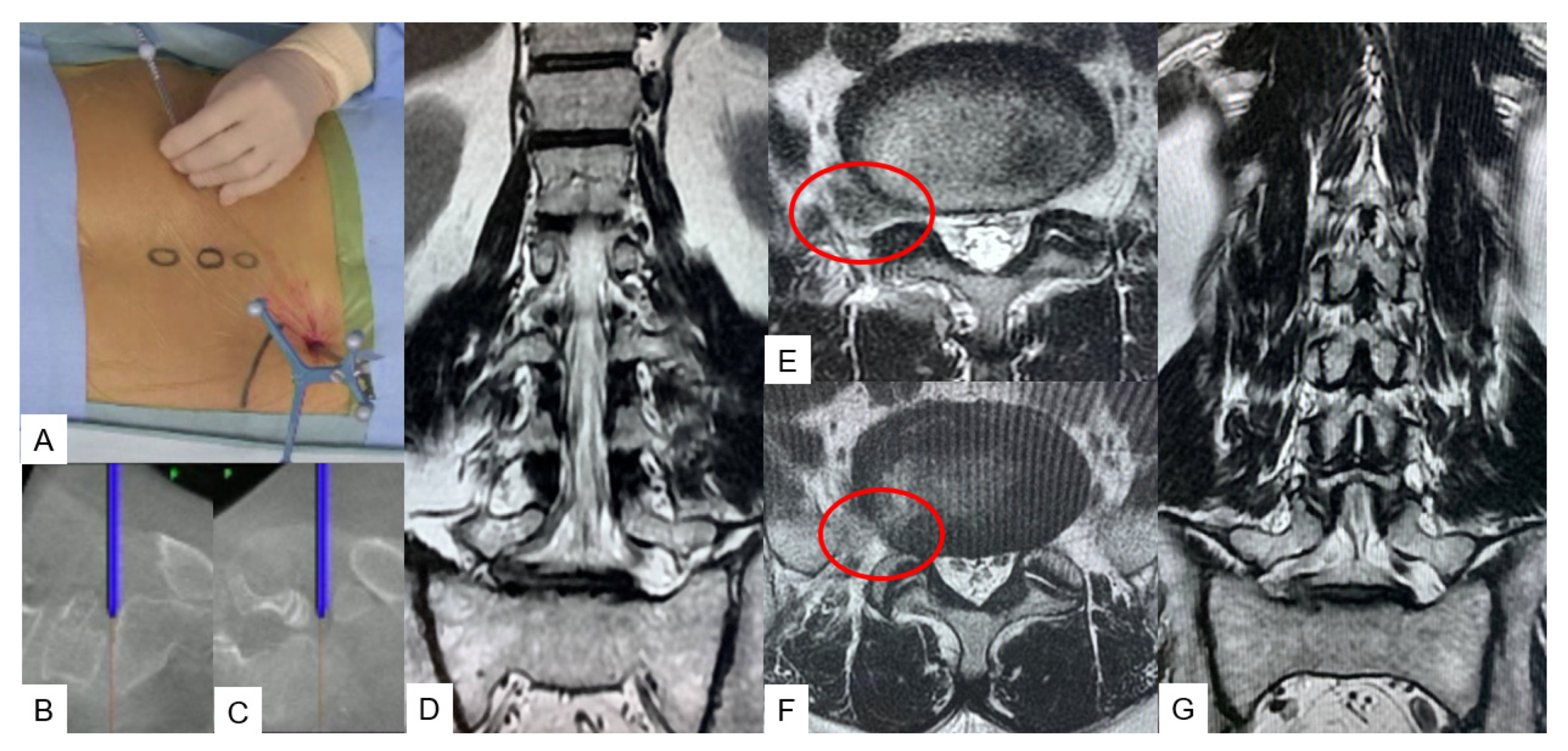
3.1.1. OLIF51 [10]
3.1.2. Lateral Osteotomy [11]
3.2. Lumbar Posterior Application
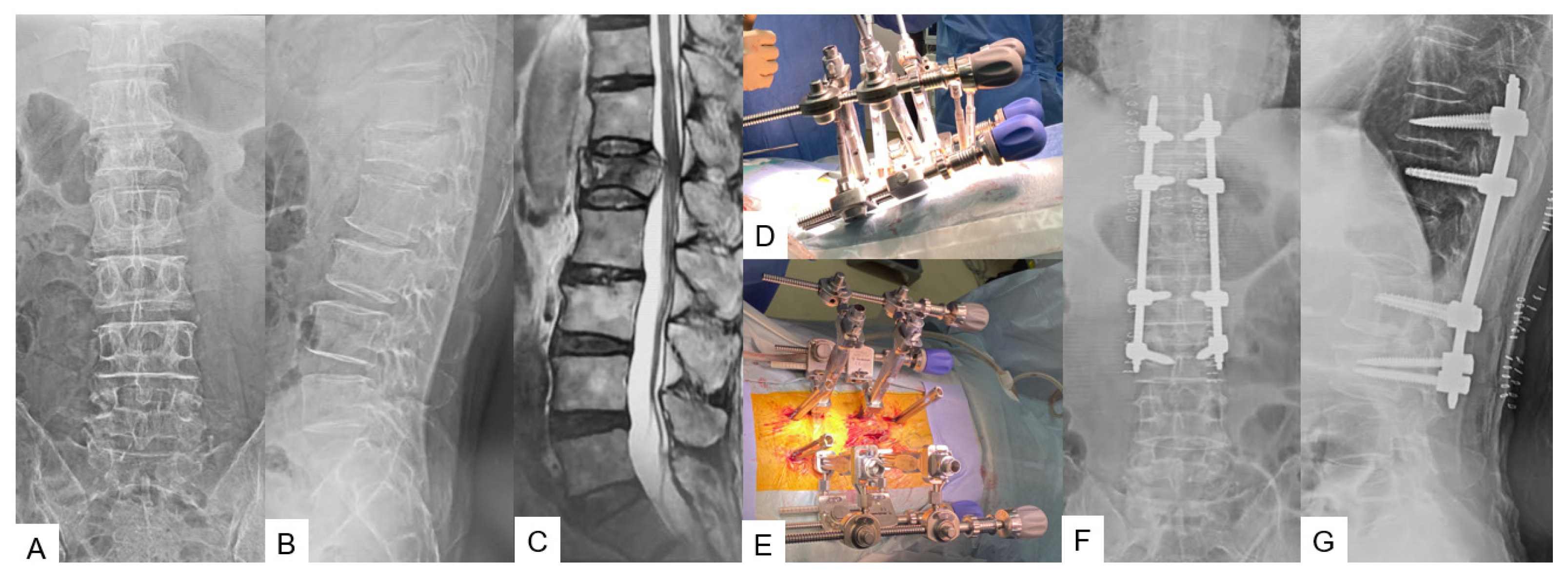
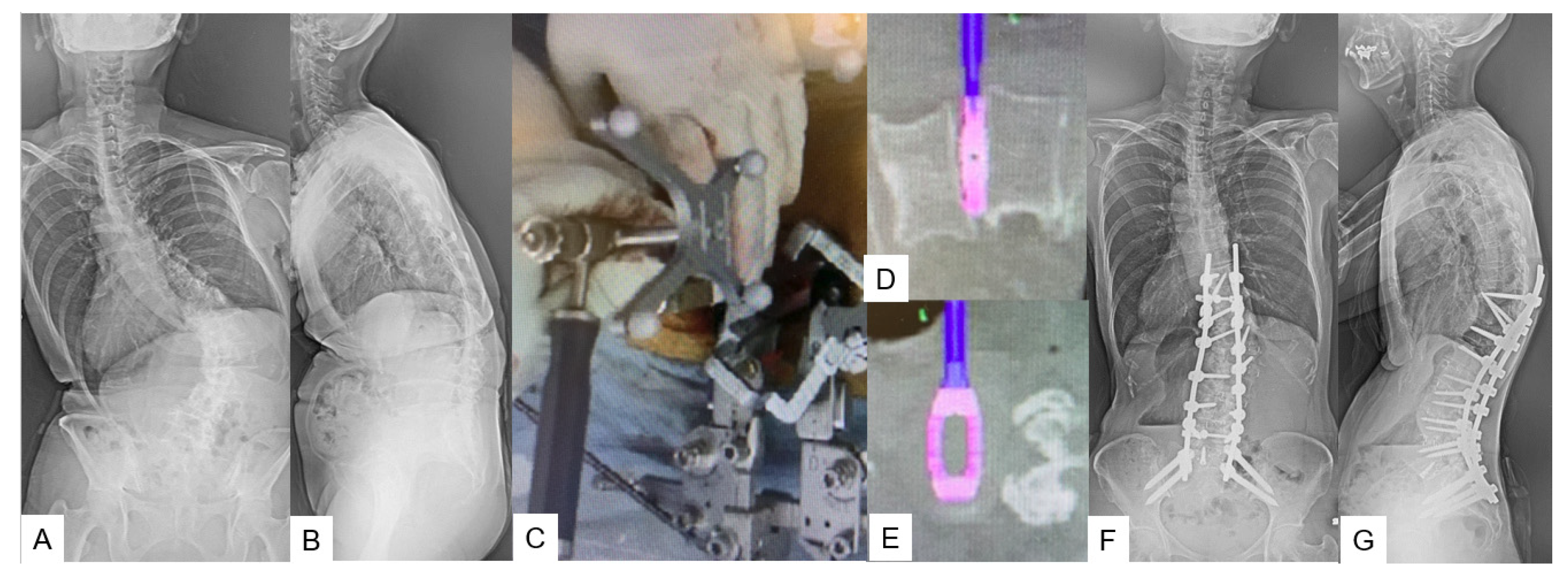
3.2.1. Adult Spinal Deformity [12]
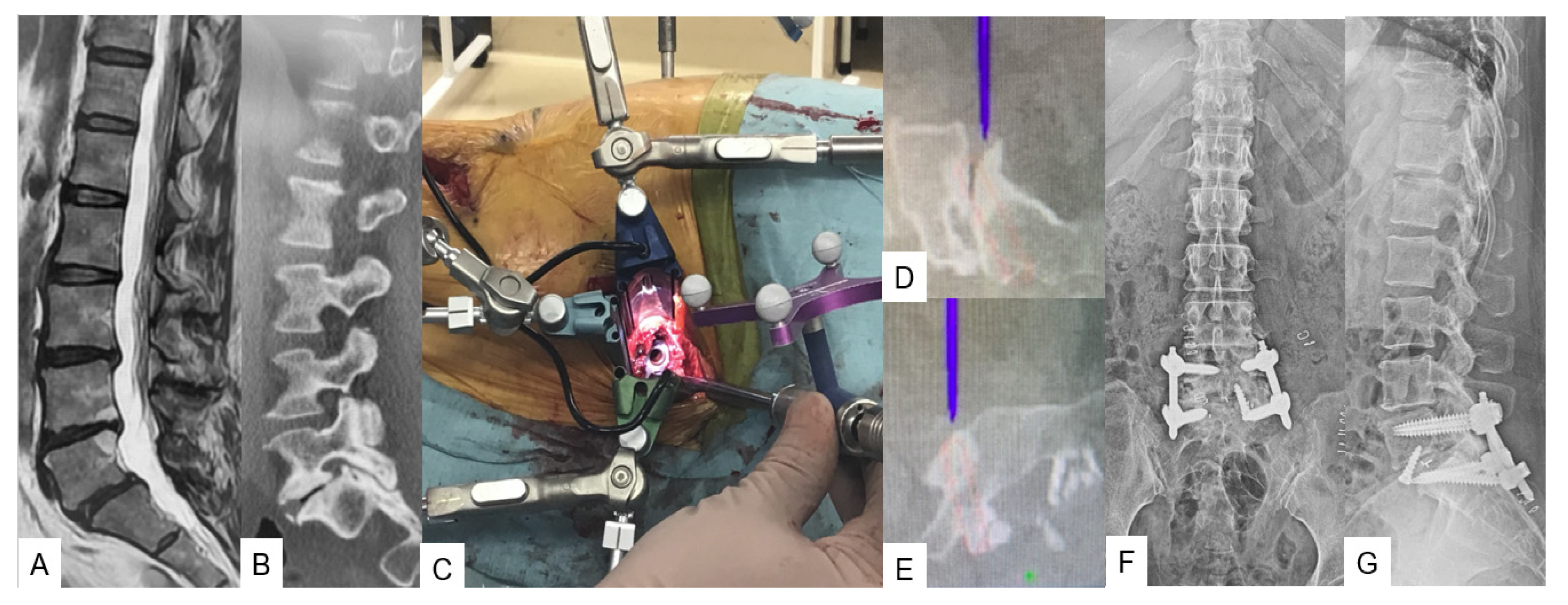
3.2.2. C-Arm-Free Biopsy [13]
3.2.3. C-Arm-Free Endoscopic Technique [14]
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helm, P.A.; Teichman, R.; Hartmann, S.L.; Simon, D. Spinal Navigation and Imaging: History, Trends, and Future. IEEE Trans. Med. Imaging 2015, 34, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Rawicki, N.; Dowdell, J.E.; Sandhu, H.S. Current state of navigation in spine surgery. Ann. Transl. Med. 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Mirza, S.K.; Li, C.; Evans, L.T.; Ji, S.; Paulsen, K.D. Accuracy of Stereovision-Updated Versus Preoperative CT-Based Image Guidance in Multilevel Lumbar Pedicle Screw Placement: A Cadaveric Swine Study. JB JS Open Access 2022, 7, e21.00129. [Google Scholar] [CrossRef] [PubMed]
- Rampersaud, Y.R.; Foley, K.T.; Shen, A.C.; Williams, S.; Solomito, M. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine 2000, 25, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Mroz, T.E.; Abdullah, K.G.; Steinmetz, M.P.; Klineberg, E.O.; Lieberman, I.H. Radiation exposure to the surgeon during percutaneous pedicle screw placement. J. Spinal Disord. Tech. 2011, 24, 264–267. [Google Scholar] [CrossRef]
- Shuman, W.H.; Valliani, A.A.; Chapman, E.K.; Martini, M.L.; Neifert, S.N.; Baron, R.B.; Schupper, A.J.; Steinberger, J.M.; Caridi, J.M. Intraoperative Navigation in Spine Surgery: Effects on Complications and Reoperations. World Neurosurg. 2022, 160, e404–e411. [Google Scholar] [CrossRef]
- Bratschitsch, G.; Leitner, L.; Stücklschweiger, G.; Guss, H.; Sadoghi, P.; Puchwein, P.; Leithner, A.; Radl, R. Radiation Exposure of Patient and Operating Room Personnel by Fluoroscopy and Navigation during Spinal Surgery. Sci. Rep. 2019, 9, 17652. [Google Scholar] [CrossRef]
- Van de Kelft, E.; Costa, F.; Van der Planken, D.; Schils, F. A prospective multicenter registry on the accuracy of pedicle screw placement in the thoracic, lumbar, and sacral levels with the use of the O-arm imaging system and stealthstation navigation. Spine 2012, 37, E1580–E1587. [Google Scholar] [CrossRef]
- Yamauchi, T.; Jaiswal, A.; Tanaka, M.; Fujiwara, Y.; Oda, Y.; Arataki, S.; Misawa, H. Minimally Invasive L5 Corpectomy with Navigated Expandable Vertebral Cage: A Technical Note. Brain Sci. 2021, 11, 1241. [Google Scholar] [CrossRef]
- Tanaka, M.; Singh, M.; Fujiwara, Y.; Uotani, K.; Oda, Y.; Arataki, S.; Yamauchi, T.; Takigawa, T.; Ito, Y. Comparison of Navigated Expandable Vertebral Cage with Conventional Expandable Vertebral Cage for Minimally Invasive Lumbar/Thoracolumbar Corpectomy. Medicina 2022, 58, 364. [Google Scholar] [CrossRef]
- Tanaka, M.; Uotani, K.; Fujiwara, Y.; Yamane, K.; Sonawane, S.; Arataki, S.; Yamauchi, T. Navigated Lateral Osteotomy for Adult Spinal Deformity: A Technical Note. World Neurosurg. 2021, 150, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.S.; Tanaka, M.; Arataki, S.; Fujiwara, Y.; Mushtaq, M.; Taoka, T.; Zygogiannnis, K.; Ruparel, S. Lateral access minimally invasive spine surgery in adult spinal deformity. J. Orthop. 2023, 45, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Sonawane, S.; Uotani, K.; Fujiwara, Y.; Sessumpun, K.; Yamauchi, T.; Sugihara, S. Percutaneous C-Arm Free O-Arm Navigated Biopsy for Spinal Pathologies: A Technical Note. Diagnostics 2021, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Arataki, S.; Mehta, R.; Tsai, T.T.; Fujiwara, Y.; Uotani, K.; Yamauchi, T. Transtubular Endoscopic Posterolateral Decompression for L5-S1 Lumbar Lateral Disc Herniation. J. Vis. Exp. 2022, 14, e63603. [Google Scholar]
- Xu, D.S.; Walker, C.T.; Farber, S.H.; Godzik, J.; Gandhi, S.V.; Koffie, R.M.; Turner, J.D.; Uribe, J.S. Surgical anatomy of minimally invasive lateral approaches to the thoracolumbar junction. J. Neurosurg. Spine 2022, 36, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, N.A.; Konig, M.; Booker, S.J.; Shafafy, M.; Boszczyk, B.M.; Grevitt, M.P.; Mehdian, H.; Webb, J.K. Access related complications in anterior lumbar surgery performed by spinal surgeons. Eur. Spine J. 2013, 22 (Suppl. 1), S16–S20. [Google Scholar] [CrossRef]
- Vazan, M.; Ryang, Y.M.; Gerhardt, J.; Zibold, F.; Janssen, I.; Ringel, F.; Gempt, J.; Meyer, B. L5 corpectomy-the lumbosacral segmental geometry and clinical outcome-a consecutive series of 14 patients and review of the literature. Acta Neurochir. 2017, 159, 1147–1152. [Google Scholar] [CrossRef]
- Yu, J.; Fridley, J.; Gokaslan, Z.; Telfeian, A.; Oyelese, A.A. Minimally invasive thoracolumbar corpectomy and stabilization for unstable burst fractures using intraoperative computed tomography and computer-assisted spinal navigation. World Neurosurg. 2019, 122, e1266–e1274. [Google Scholar] [CrossRef]
- Siasios, I.; Vakharia, K.; Khan, A.; Meyers, J.E.; Yavorek, S.; Pollina, J.; Dimopoulos, V. Bowel injury in lumbar spine surgery: A review of the literature. J. Spine Surg. 2018, 4, 130–137. [Google Scholar] [CrossRef]
- Shea, T.M.; Laun, J.; Gonzalez-Blohm, S.A.; Doulgeris, J.J.; Lee, W.E.; Aghayev, K.; Vrionis, F.D. Designs and techniques that improve the pullout strength of pedicle screws in osteoporotic vertebrae: Current status. Biomed Res. Int. 2014, 2014, 748393. [Google Scholar] [CrossRef]
- Tomé-Bermejo, F.; Piñera, A.R.; Alvarez-Galovich, L. Osteoporosis and the management of spinal degenerative disease (I). Arch. Bone Jt. Surg. 2017, 5, 272–282. [Google Scholar] [PubMed]
- Son, H.J.; Choi, S.H.; Heo, D.R.; Kook, I.; Lee, M.K.; Ahn, H.S.; Kang, C.N. Outcomes of the use of cement-augmented cannulated pedicle screws in lumbar spinal fusion. Spine J. 2021, 21, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Sumiya, S.; Fukushima, K.; Kurosa, Y.; Hirai, T.; Inose, H.; Yoshii, T.; Okawa, A. Comparative analysis of clinical factors associated with pedicle screw pull-out during or immediately after surgery between intraoperative cone-beam computed tomography and postoperative computed tomography. BMC Musculoskelet Disord. 2021, 22, 55. [Google Scholar] [CrossRef]
- Matthews, P.G.M.; Cadman, J.; Tomka, J.; Dabirrahmani, D.; Appleyard, R.; Kam, A. Pullout force of minimally invasive surgical and open pedicle screws-a biomechanical cadaveric study. J. Spine Surg. 2020, 6, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tohmeh, A.G.; Isaacs, R.E.; Dooley, Z.A.; Turner, A.W.L. Long Construct Pedicle Screw Reduction and Residual Forces are Decreased Using a Computer-assisted Rod Bending System. J. Spine Neurosurg. 2014, 14, S143–S144. [Google Scholar] [CrossRef]
- Tinelli, M.; Töpfer, F.; Kreinest, M.; Matschke, S.; Grützner, P.A.; Suda, A.J. Minimally invasive reduction and percutaneous posterior fixation of one-level traumatic thoraco-lumbar and lumbar spine fractures. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.F.; Huang, Y.X.; Chi, Y.L.; Xu, H.Z.; Lin, Y.; Wang, X.Y.; Huang, Q.S.; Mao, F.M. Percutaneous pedicle screw fixation for neurologic intact thoracolumbar burst fractures. J. Spinal Disord. Tech. 2010, 23, 530–537. [Google Scholar] [CrossRef]
- Defino, H.L.A.; Costa, H.R.T.; Nunes, A.A.; Barbosa, M.N.; Romero, V. Open versus minimally invasive percutaneous surgery for surgical treatment of thoracolumbar spine fractures- a multicenter randomized controlled trial: Study protocol. BMC Musculoskelet. Disord. 2019, 20, 397. [Google Scholar] [CrossRef]
- Masse, F.X. Dosimetry Report of the Medtronic O-Arm System; O-Arm Imaging System, Version 3.1; Medtronic: Littleton, MA, USA, 2009; pp. 4–6. [Google Scholar]
- Jones, D.P.G.; Robertson, P.A.; Lunt, B.; Phys, M.; Jackson, S.A. Radiation exposure during fluoroscopically assisted pedicle screw insertion in the lumbar spine. Spine 2000, 25, 1538–1541. [Google Scholar] [CrossRef]
- Orita, S.; Inage, K.; Furuya, T.; Koda, M.; Aoki, Y.; Kubota, G.; Nakamura, J.; Shiga, Y.; Matsuura, Y.; Maki, S.; et al. Oblique Lateral Interbody Fusion (OLIF): Indications and techniques. Oper. Tech. Orthop. 2017, 27, 223–230. [Google Scholar] [CrossRef]
- Woods, K.R.; Billys, J.B.; Hynes, R.A. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF 51) and evaluation of complication and fusion rates. Spine J. Off. J. North Am. Spine Soc. 2017, 17, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Lafage, V.; Shaffrey, C.I.; Schwab, F.; Lafage, R.; Hostin, R.; O’brien, M.; Boachie-Adjei, O.; Akbarnia, B.A.; Mundis, G.M.; et al. Outcomes of operative and nonoperative treatment for adult spinal deformity: A prospective, multicenter, propensity-matched cohort assessment with minimum 2-year follow-up. Neurosurgery 2016, 78, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Akıntürk, N.; Zileli, M.; Yaman, O. Complications of adult spinal deformity surgery: A literature review. J. Craniovertebr. Junction Spine. 2022, 13, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Dinizo, M.; Dolgalev, I.; Passias, P.G.; Errico, T.J.; Raman, T. Complications After Adult Spinal Deformity Surgeries: All Are Not Created Equal. Int. J. Spine Surg. 2021, 15, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Osorio, J.A.; Deviren, V.; Ames, C.P. The relationship of older age and perioperative outcomes following thoracolumbar three-column osteotomy for adult spinal deformity: An analysis of 300 consecutive cases. J. Neurosurg. Spine 2018, 28, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Mummaneni, P.V.; Park, P.; Shaffrey, C.I.; Wang, M.Y.; Uribe, J.S.; Fessler, R.G.; Chou, D.; Kanter, A.S.; Okonkwo, D.O.; Mundis, G.M.; et al. The MISDEF2 algorithm: An updated algorithm for patient selection in minimally invasive deformity surgery. J. Neurosurg. Spine 2019, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lykissas, M.G.; Giannoulis, D. Minimally invasive spine surgery for degenerative spine disease and deformity correction: A literature review. Ann. Transl. Med. 2018, 6, 99. [Google Scholar] [CrossRef]
- Lee, Y.P.; Sclafani, J. Lumbar iatrogenic spinal instability. Semin. Spine Surg. 2013, 25, 131–137. [Google Scholar] [CrossRef]
- Wong, K.W.; Ho, C.H.; Yu, T.C.; Wu, C.D.; Tsang, Y.S. Clinical Outcome of Minimally Invasive Decompression Without Discectomy in Contained Foraminal Disc Herniation: A Single-Center Study. World Neurosurg. 2018, 118, e367–e374. [Google Scholar] [CrossRef]
- Guha, D.; Jakubovic, R.; Gupta, S.; Fehlings, M.G.; Mainprize, T.G.; Yee, A.; Yang, V.X.D. Intraoperative Error Propagation in 3-Dimensional Spinal Navigation From Nonsegmental Registration: A Prospective Cadaveric and Clinical Study. Global Spine J. 2019, 5, 512–520. [Google Scholar] [CrossRef]
| Patients (54) | Female (50), Male (4) |
| Age (years) | 71.5 ± 6.2 |
| BMI (kg/m2) | 22.9 ± 4.1 |
| Follow-up period (months) | 29.2 ± 8.4 |
| OLIF51 | TLIF51 | ||
|---|---|---|---|
| Patients | Men (0), Women (13) | Men (4), Women (3) | 0.243 |
| Age (years) | 74.6 ± 3.2 | 70.5 ± 6.6 | 0.023 |
| BMI (kg/m2) | 22.7 ± 3.7 | 23.0 ± 4.2 | 0.715 |
| Postoperative L5-S1 angle gain (°) | 9.4 ± 4.7 | 1.6 ± 5.1 | 0.0001 |
| Postoperative L5-S1 height gain (mm) | 4.2 ± 2.9 | 0.8 ± 1.9 | 0.0002 |
| Reoperation | 2 | 8 | 0.570 |
| Preoperative Value | Postoperative Value | p Value | |
|---|---|---|---|
| ODI (%) | 46.0 ± 10.4 | 30.5 ± 18.9 | p < 0.01 |
| VAS (mm) | 52.9 ± 7.3 | 31.2 ± 6.9 | p < 0.01 |
| SVA (mm) | 96.5 ± 55.9 | 24.1 ± 39.0 | p < 0.01 |
| PT (degree) | 34.5 ± 11.0 | 17.1 ± 10.3 | p < 0.01 |
| LL (degree) | 13.3 ± 18.6 | 49.1 ± 9.7 | p < 0.01 |
| PI—LL (degree) | 39.3 ± 22.1 | 2.4 ± 12.6 | p < 0.01 |
| Advantages | Disadvantages |
|---|---|
| Less radiation hazard | Learning curve |
| Guidance to cage placement | Need of C-arm to verify dynamic changes |
| Contralateral release | Cost-effectiveness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zygogiannis, K.; Tanaka, M.; Sake, N.; Arataki, S.; Fujiwara, Y.; Taoka, T.; Uotani, K.; Askar, A.E.K.A.; Chatzikomninos, I. Our C-Arm-Free Minimally Invasive Technique for Spinal Surgery: The Thoracolumbar and Lumbar Spine—Based on Our Experiences. Medicina 2023, 59, 2116. https://doi.org/10.3390/medicina59122116
Zygogiannis K, Tanaka M, Sake N, Arataki S, Fujiwara Y, Taoka T, Uotani K, Askar AEKA, Chatzikomninos I. Our C-Arm-Free Minimally Invasive Technique for Spinal Surgery: The Thoracolumbar and Lumbar Spine—Based on Our Experiences. Medicina. 2023; 59(12):2116. https://doi.org/10.3390/medicina59122116
Chicago/Turabian StyleZygogiannis, Konstantinos, Masato Tanaka, Naveen Sake, Shinya Arataki, Yoshihiro Fujiwara, Takuya Taoka, Koji Uotani, Abd El Kader Al Askar, and Ioannis Chatzikomninos. 2023. "Our C-Arm-Free Minimally Invasive Technique for Spinal Surgery: The Thoracolumbar and Lumbar Spine—Based on Our Experiences" Medicina 59, no. 12: 2116. https://doi.org/10.3390/medicina59122116
APA StyleZygogiannis, K., Tanaka, M., Sake, N., Arataki, S., Fujiwara, Y., Taoka, T., Uotani, K., Askar, A. E. K. A., & Chatzikomninos, I. (2023). Our C-Arm-Free Minimally Invasive Technique for Spinal Surgery: The Thoracolumbar and Lumbar Spine—Based on Our Experiences. Medicina, 59(12), 2116. https://doi.org/10.3390/medicina59122116






