The Modern Environment: The New Secondary Cause of Hypertension?
Abstract
:1. Introduction
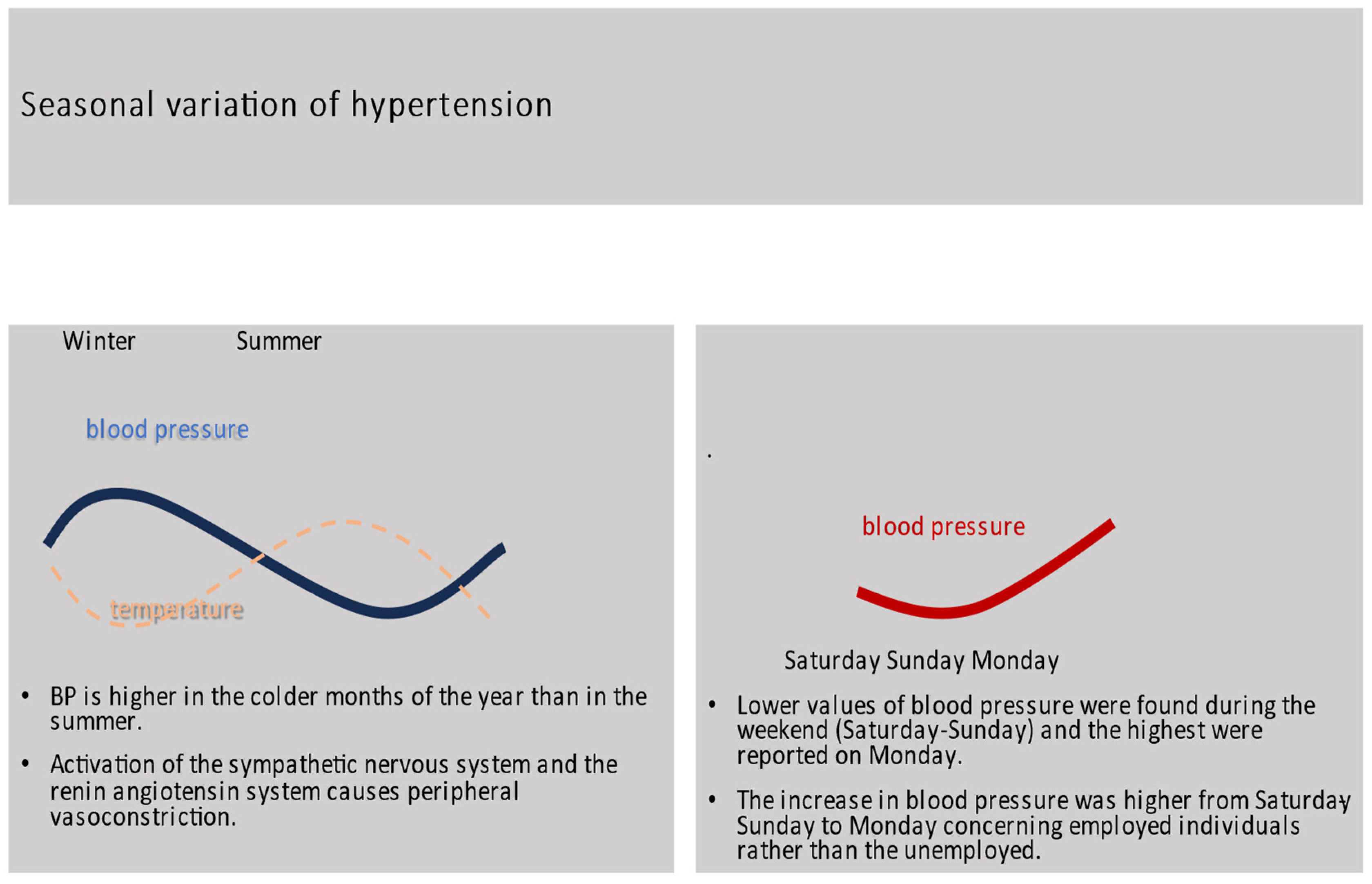
2. Seasonal Variation and Hypertension
3. Hypertension, Noise, and Air Pollution
4. Hypertension and Socioeconomic Status
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Centers for Disease Control and Prevention, 1999–2019. CDC WONDER Online Database Website; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. Available online: https://wonder.cdc.gov/ (accessed on 26 November 2023).
- World Health Organization. Hypertension. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 26 November 2023).
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence among Adults Aged 18 and over: United States, 2017–2018; NCHS Data Brief, no 364; National Center for Health Statistics: Hyattsville, MD, USA, 2020. [Google Scholar]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Poulter, N.R.; Borghi, C.; Castillo, R.R.; Charchar, F.J.; Ramirez, A.J.; Schlaich, M.P.; Schutte, A.E.; Stergiou, G.; Unger, T.; Wainford, R.D.; et al. May Measurement Month 2017: Results of 39 national blood pressure screening programmes. Eur. Heart J. Suppl. 2019, 21, D1–D4. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41. [Google Scholar] [CrossRef]
- Corrigendum to: Neglected tropical diseases and vitamin B12: A review of the current evidence. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 292. [CrossRef] [PubMed]
- Rossi, G.P.; Bisogni, V.; Rossitto, G.; Maiolino, G.; Cesari, M.; Zhu, R.; Seccia, T.M. Practice Recommendations for Diagnosis and Treatment of the Most Common Forms of Secondary Hypertension. High. Blood Press. Cardiovasc. Prev. 2020, 27, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Hahad, O.; Sørensen, M.; Lelieveld, J.; Duerr, G.D.; Nieuwenhuijsen, M.; Daiber, A. Environmental risk factors and cardiovascular diseases: A comprehensive expert review. Cardiovasc. Res. 2022, 118, 2880–2902. [Google Scholar] [CrossRef]
- El Sadat, M.M.H.A.E.S.; Naguib, T.A.; Farag, E.S.M.; Gad, M.M. Novel Overview of Seasonal Blood Pressure Variation: Review Article. Egypt. J. Hosp. Med. 2021, 85, 3949–3952. [Google Scholar] [CrossRef]
- Modesti, P.A.; Bamoshmoosh, M.; Rapi, S.; Massetti, L.; Al-Hidabi, D.; Al Goshae, H. Epidemiology of hypertension in Yemen: Effects of urbanization and geographical area. Hypertens. Res. 2013, 36, 711–717. [Google Scholar] [CrossRef]
- Goel, H.; Shah, K.; Kumar, A.; Hippen, J.T.; Nadar, S.K. Temperature, cardiovascular mortality, and the role of hypertension and renin-angiotensin-aldosterone axis in seasonal adversity: A narrative review. J. Hum. Hypertens. 2022, 36, 1035–1047. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Myrsilidi, A.; Kollias, A.; Destounis, A.; Roussias, L.; Kalogeropoulos, P. Seasonal variation in meteorological parameters and office, ambulatory and home blood pressure: Predicting factors and clinical implications. Hypertens. Res. 2015, 38, 869–875. [Google Scholar] [CrossRef]
- Stewart, S.; Moholdt, T.T.; Burrell, L.M.; Sliwa, K.; Mocumbi, A.O.; McMurray, J.J.; Keates, A.K.; Hawley, J.A. Winter Peaks in Heart Failure: An Inevitable or Preventable Consequence of Seasonal Vulnerability? Card. Fail. Rev. 2019, 5, 83–85. [Google Scholar] [CrossRef]
- Yu, B.; Jin, S.; Wang, C.; Yan, S.; Zhou, X.; Cui, X.; Tang, Z.; Luan, Q.; Guo, Y.; Bian, Z.; et al. The association of outdoor temperature with blood pressure, and its influence on future cardio-cerebrovascular disease risk in cold areas. J. Hypertens. 2020, 38, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Shahsanai, A.; Kalia, S.; Aliarzadeh, B.; Moineddin, R.; Bhatt, A.; Greiver, M. Seasonal variation in blood pressure recorded in routine primary care. medRxiv 2021. [Google Scholar] [CrossRef]
- Bauer, F.; Lindtke, J.; Seibert, F.; Rohn, B.; Doevelaar, A.; Babel, N.; Schlattmann, P.; Bertram, S.; Zgoura, P.; Westhoff, T.H. Impact of weather changes on hospital admissions for hypertension. Sci. Rep. 2022, 12, 5716. [Google Scholar] [CrossRef]
- Lin, S.; Luo, M.; Walker, R.J.; Liu, X.; Hwang, S.A.; Chinery, R. Extreme high temperatures and hospital admissions for respiratory and cardiovascular diseases. Epidemiology 2009, 20, 738–746. [Google Scholar] [CrossRef]
- Bai, L.; Li, Q.; Wang, J.; Lavigne, E.; Gasparrini, A.; Copes, R.; Yagouti, A.; Burnett, R.T.; Goldberg, M.S.; Villeneuve, P.J.; et al. Hospitalizations from Hypertensive Diseases, Diabetes, and Arrhythmia in Relation to Low and High Temperatures: Population-Based Study. Sci. Rep. 2016, 6, 30283. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, J.; Xu, Y.; Xu, T.; Yang, Y.; Wang, J. Novel insights into the association between seasonal variations, blood pressure, and blood pressure variability in patients with new-onset essential hypertension. BMC Cardiovasc. Disord. 2022, 22, 401. [Google Scholar] [CrossRef]
- Narita, K.; Kario, K. Management of seasonal variation in blood pressure through the optimal adjustment of antihypertensive medications and indoor temperature. Hypertens. Res. 2023, 46, 806–808. [Google Scholar] [CrossRef]
- Kinuta, M.; Hisamatsu, T.; Fukuda, M.; Taniguchi, K.; Komukai, S.; Nakahata, N.; Kanda, H. Associations of indoor and outdoor temperatures and their difference with home blood pressure: The Masuda Study. Hypertens. Res. 2023, 46, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Umishio, W.; Ikaga, T.; Kario, K.; Fujino, Y.; Hoshi, T.; Ando, S.; Suzuki, M.; Yoshimura, T.; Yoshino, H.; Murakami, S. Cross-Sectional Analysis of the Relationship Between Home Blood Pressure and Indoor Temperature in Winter: A Nationwide Smart Wellness Housing Survey in Japan. Hypertension 2019, 74, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Hanazawa, T.; Asayama, K.; Watabe, D.; Tanabe, A.; Satoh, M.; Inoue, R.; Hara, A.; Obara, T.; Kikuya, M.; Nomura, K.; et al. Association Between Amplitude of Seasonal Variation in Self-Measured Home Blood Pressure and Cardiovascular Outcomes: HOMED-BP (Hypertension Objective Treatment Based on Measurement By Electrical Devices of Blood Pressure) Study. J. Am. Heart Assoc. 2018, 7, e008509. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Hoshide, S.; Fujiwara, T.; Kanegae, H.; Kario, K. Seasonal Variation of Home Blood Pressure and Its Association With Target Organ Damage: The J-HOP Study (Japan Morning Surge-Home Blood Pressure). Am. J. Hypertens. 2020, 33, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lan, S.; Chen, C.; Zhang, X.; Zhang, Y.; Chen, S. Seasonal Variation: A Non-negligible Factor Associated With Blood Pressure in Patients Undergoing Hemodialysis. Front. Cardiovasc. Med. 2022, 9, 820483. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Kramer, A.; Szwarc, I.; Bieber, B.; Gayrard, N.; Jover, B.; Vetromile, F.; Massy, Z.A.; Combe, C.; Tentori, F.; et al. Geographical Variations in Blood Pressure Level and Seasonality in Hemodialysis Patients. Hypertension 2018, 71, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Argilés, A.; Mourad, G.; Mion, C. Seasonal changes in blood pressure in patients with end-stage renal disease treated with hemodialysis. N. Engl. J. Med. 1998, 339, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Wystrychowski, G.; Wystrychowski, W.; Zukowska-Szczechowska, E.; Tomaszewski, M.; Grzeszczak, W. Selected climatic variables and blood pressure in Central European patients with chronic renal failure on haemodialysis treatment. Blood Press. 2005, 14, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Juhanoja, E.P.; Puukka, P.J.; Johansson, J.K.; Niiranen, T.J.; Jula, A.M. The impact of the day of the week on home blood pressure: The Finn-Home study. Blood Press. Monit. 2016, 21, 63–68. [Google Scholar] [CrossRef]
- World Health Organization. Environmental Noise Guidelines for the European Region. 2019. Available online: https://www.who.int/europe/publications/i/item/9789289053563 (accessed on 26 November 2023).
- Münzel, T.; Sørensen, M.; Daiber, A. Transportation noise pollution and cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 619–636. [Google Scholar] [CrossRef]
- Bolm-Audorff, U.; Hegewald, J.; Pretzsch, A.; Freiberg, A.; Nienhaus, A.; Seidler, A. Occupational Noise and Hypertension Risk: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6281. [Google Scholar] [CrossRef]
- Maschke, C.; Rupp, T.; Hecht, K. The influence of stressors on biochemical reactions--a review of present scientific findings with noise. Int. J. Hyg. Environ. Health 2000, 203, 45–53. [Google Scholar] [CrossRef]
- Lu, S.Y.; Lee, C.L.; Lin, K.Y.; Lin, Y.H. The acute effect of exposure to noise on cardiovascular parameters in young adults. J. Occup. Health 2018, 60, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kupcikova, Z.; Fecht, D.; Ramakrishnan, R.; Clark, C.; Cai, Y.S. Road traffic noise and cardiovascular disease risk factors in UK Biobank. Eur. Heart J. 2021, 42, 2072–2084. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Wang, Y.; Wang, H.; Gao, Y.; Zhang, Y. Association of occupational noise exposure with hypertension: A cross-sectional study. J. Clin. Hypertens. 2023, 25, 158–164. [Google Scholar] [CrossRef]
- Wu, X.; Li, C.; Zhang, X.; Song, Y.; Zhao, D.; Lan, Y.; Zhou, B. The Impact of Occupational Noise on Hypertension Risk: A Case-Control Study in Automobile Factory Personnel. Front. Cardiovasc. Med. 2022, 9, 803695. [Google Scholar] [CrossRef]
- Kourieh, A.; Giorgis-Allemand, L.; Bouaoun, L.; Lefèvre, M.; Champelovier, P.; Lambert, J.; Laumon, B.; Evrard, A.S. Incident hypertension in relation to aircraft noise exposure: Results of the DEBATS longitudinal study in France. Occup. Environ. Med. 2022, 79, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.; Kannenkeril, D.; Jung, S.; Striepe, K.; Karg, M.V.; Ott, C.; Schmieder, R.E. The influence of aircraft noise exposure on the systemic and renal haemodynamics. Eur. J. Prev. Cardiol. 2022, 29, 116–124. [Google Scholar] [CrossRef]
- Petri, D.; Licitra, G.; Vigotti, M.A.; Fredianelli, L. Effects of Exposure to Road, Railway, Airport and Recreational Noise on Blood Pressure and Hypertension. Int. J. Environ. Res. Public Health 2021, 18, 9145. [Google Scholar] [CrossRef]
- Zaman, M.; Muslim, M.; Jehangir, A. Environmental noise-induced cardiovascular, metabolic and mental health disorders: A brief review. Environ. Sci. Pollut. Res. Int. 2022, 29, 76485–76500. [Google Scholar] [CrossRef]
- D’Souza, J.; Weuve, J.; Brook, R.D.; Evans, D.A.; Kaufman, J.D.; Adar, S.D. Long-Term Exposures to Urban Noise and Blood Pressure Levels and Control Among Older Adults. Hypertension 2021, 78, 1801–1808. [Google Scholar] [CrossRef]
- de Bont, J.; Jaganathan, S.; Dahlquist, M.; Persson, Å.; Stafoggia, M.; Ljungman, P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J. Intern. Med. 2022, 291, 779–800. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Jaganathan, S.; Jaacks, L.M.; Magsumbol, M.; Walia, G.K.; Sieber, N.L.; Shivasankar, R.; Dhillon, P.K.; Hameed, S.S.; Schwartz, J.; Prabhakaran, D. Association of Long-Term Exposure to Fine Particulate Matter and Cardio-Metabolic Diseases in Low- and Middle-Income Countries: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2541. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.K.; Tsang, H.; Wong, C.M. Meta-analysis of adverse health effects due to air pollution in Chinese populations. BMC Public Health 2013, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Vania, R.; Tondas, A.E.; Setianto, B.; Santoso, A. A time-to-event analysis on air pollutants with the risk of cardiovascular disease and mortality: A systematic review and meta-analysis of 84 cohort studies. J. Evid. Based Med. 2020, 13, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, B.; Ke, W.; Feng, B.; Lin, H.; Xiao, J.; Zeng, W.; Li, X.; Tao, J.; Yang, Z.; et al. Associations of Short-Term and Long-Term Exposure to Ambient Air Pollutants With Hypertension: A Systematic Review and Meta-Analysis. Hypertension 2016, 68, 62–70. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, M.; Liang, Q.; Wang, F.; Lin, L.; Li, T.; Duan, J.; Sun, Z. The relationship between long-term exposure to PM2.5 and hypertension in women: A meta-analysis. Ecotoxicol. Environ. Saf. 2021, 208, 111492. [Google Scholar] [CrossRef]
- Sacks, J.; Buckley, B.; Deflorio-Barker, S.; Jenkins, S.; Kirrane, E.; Krajewski, A.; Luben, T.; McDow, S.; Stewart, M.; Dubois, J.J.; et al. EPA IRIS Assessments. In Supplement to the 2019 Integrated Science Assessment for Particulate Matter; U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 2022. [Google Scholar]
- Dzau, V.J.; Balatbat, C.A. Future of Hypertension. Hypertension 2019, 74, 450–457. [Google Scholar] [CrossRef]
- Miao, B.; Liu, X.; Zhu, T. Automatic mental health identification method based on natural gait pattern. Psych. J. 2021, 10, 453–464. [Google Scholar] [CrossRef]
- Brunner, E.J.; Hemingway, H.; Walker, B.R.; Page, M.; Clarke, P.; Juneja, M.; Shipley, M.J.; Kumari, M.; Andrew, R.; Seckl, J.R.; et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: Nested case-control study. Circulation 2002, 106, 2659–2665. [Google Scholar] [CrossRef]
- Ghiadoni, L.; Donald, A.E.; Cropley, M.; Mullen, M.J.; Oakley, G.; Taylor, M.; O’Connor, G.; Betteridge, J.; Klein, N.; Steptoe, A.; et al. Mental stress induces transient endothelial dysfunction in humans. Circulation 2000, 102, 2473–2478. [Google Scholar] [CrossRef]
- Shah, R.M.; Doshi, S.; Shah, S.; Patel, S.; Li, A.; Diamond, J.A. Impacts of Anxiety and Depression on Clinical Hypertension in Low-Income US Adults. High. Blood Press. Cardiovasc. Prev. 2023, 30, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, L.S.; Tang, W.W.; Xu, F.; Xu, R.H.; Liu, X.; Liu, Y.; Liu, J.X.; Yi, Y.J.; Hu, T.S.; et al. High prevalence of obesity-related hypertension among adults aged 40 to 79 years in Southwest China. Sci. Rep. 2019, 9, 15838. [Google Scholar] [CrossRef]
- Jordan, J.; Yumuk, V.; Schlaich, M.; Nilsson, P.M.; Zahorska-Markiewicz, B.; Grassi, G.; Schmieder, R.E.; Engeli, S.; Finer, N. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: Obesity and difficult to treat arterial hypertension. J. Hypertens. 2012, 30, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef]
- Cutler, J.A.; Sorlie, P.D.; Wolz, M.; Thom, T.; Fields, L.E.; Roccella, E.J. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999–2004. Hypertension 2008, 52, 818–827. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.Q.; Tang, W.W.; Zhang, W.Y.; Liu, J.X.; Xu, R.H.; Wang, T.D.; Huang, X.B. The prevalence of obesity-related hypertension among middle-aged and older adults in China. Front. Public Health 2022, 10, 865870. [Google Scholar] [CrossRef]
- Zhang, W.; He, K.; Zhao, H.; Hu, X.; Yin, C.; Zhao, X.; Shi, S. Association of body mass index and waist circumference with high blood pressure in older adults. BMC Geriatr. 2021, 21, 260. [Google Scholar] [CrossRef]
- Wang, Y.; Howard, A.G.; Adair, L.S.; Wang, H.; Avery, C.L.; Gordon-Larsen, P. Waist Circumference Change is Associated with Blood Pressure Change Independent of BMI Change. Obesity 2020, 28, 146–153. [Google Scholar] [CrossRef]
- Te Vazquez, J.; Feng, S.N.; Orr, C.J.; Berkowitz, S.A. Food Insecurity and Cardiometabolic Conditions: A Review of Recent Research. Curr. Nutr. Rep. 2021, 10, 243–254. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Berkowitz, T.S.Z.; Meigs, J.B.; Wexler, D.J. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005–2012. PLoS ONE 2017, 12, e0179172. [Google Scholar] [CrossRef]
- Weigel, M.M.; Armijos, R.X. Food Insecurity, Cardiometabolic Health, and Health Care in U.S.-Mexico Border Immigrant Adults: An Exploratory Study. J. Immigr. Minor. Health 2019, 21, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Prieto-González, P.; Sánchez-Infante, J.; Fernández-Galván, L.M. Association between Adherence to the Mediterranean Diet and Anthropometric and Health Variables in College-Aged Males. Nutrients 2022, 14, 3471. [Google Scholar] [CrossRef]
- Duffey, K.J.; Gordon-Larsen, P.; Jacobs, D.R., Jr.; Williams, O.D.; Popkin, B.M. Differential associations of fast food and restaurant food consumption with 3-y change in body mass index: The Coronary Artery Risk Development in Young Adults Study. Am. J. Clin. Nutr. 2007, 85, 201–208. [Google Scholar] [CrossRef]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. Jama 2017, 317, 912–924. [Google Scholar] [CrossRef]
- Sakaki, J.R.; Gao, S.; Ha, K.; Chavarro, J.E.; Chen, M.H.; Sun, Q.; Hart, J.E.; Chun, O.K. Childhood beverage intake and risk of hypertension and hyperlipidaemia in young adults. Int. J. Food Sci. Nutr. 2022, 73, 954–964. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Seligman, H.K.; Choudhry, N.K. Treat or eat: Food insecurity, cost-related medication underuse, and unmet needs. Am. J. Med. 2014, 127, 303–310.e303. [Google Scholar] [CrossRef]
- Wilder, M.E.; Kulie, P.; Jensen, C.; Levett, P.; Blanchard, J.; Dominguez, L.W.; Portela, M.; Srivastava, A.; Li, Y.; McCarthy, M.L. The Impact of Social Determinants of Health on Medication Adherence: A Systematic Review and Meta-analysis. J. Gen. Intern. Med. 2021, 36, 1359–1370. [Google Scholar] [CrossRef]
- Silverman, J.; Krieger, J.; Kiefer, M.; Hebert, P.; Robinson, J.; Nelson, K. The Relationship Between Food Insecurity and Depression, Diabetes Distress and Medication Adherence Among Low-Income Patients with Poorly-Controlled Diabetes. J. Gen. Intern. Med. 2015, 30, 1476–1480. [Google Scholar] [CrossRef]
- Arenas, D.J.; Thomas, A.; Wang, J.; DeLisser, H.M. A Systematic Review and Meta-analysis of Depression, Anxiety, and Sleep Disorders in US Adults with Food Insecurity. J. Gen. Intern. Med. 2019, 34, 2874–2882. [Google Scholar] [CrossRef] [PubMed]
- Sadeghirad, B.; Duhaney, T.; Motaghipisheh, S.; Campbell, N.R.; Johnston, B.C. Influence of unhealthy food and beverage marketing on children’s dietary intake and preference: A systematic review and meta-analysis of randomized trials. Obes. Rev. 2016, 17, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, M.S.; Diez Roux, A.V.; Morenoff, J.D.; Raghunathan, T.E.; Cooper, R.S.; Ni, H.; Shea, S. Neighborhood characteristics and hypertension. Epidemiology 2008, 19, 590–598. [Google Scholar] [CrossRef]
- Levenstein, S.; Smith, M.W.; Kaplan, G.A. Psychosocial predictors of hypertension in men and women. Arch. Intern. Med. 2001, 161, 1341–1346. [Google Scholar] [CrossRef]
- Steptoe, A.; Brydon, L.; Kunz-Ebrecht, S. Changes in financial strain over three years, ambulatory blood pressure, and cortisol responses to awakening. Psychosom. Med. 2005, 67, 281–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossios, K.; Antza, C.; Kachtsidis, V.; Kotsis, V. The Modern Environment: The New Secondary Cause of Hypertension? Medicina 2023, 59, 2095. https://doi.org/10.3390/medicina59122095
Rossios K, Antza C, Kachtsidis V, Kotsis V. The Modern Environment: The New Secondary Cause of Hypertension? Medicina. 2023; 59(12):2095. https://doi.org/10.3390/medicina59122095
Chicago/Turabian StyleRossios, Konstantinos, Christina Antza, Vasileios Kachtsidis, and Vasilios Kotsis. 2023. "The Modern Environment: The New Secondary Cause of Hypertension?" Medicina 59, no. 12: 2095. https://doi.org/10.3390/medicina59122095
APA StyleRossios, K., Antza, C., Kachtsidis, V., & Kotsis, V. (2023). The Modern Environment: The New Secondary Cause of Hypertension? Medicina, 59(12), 2095. https://doi.org/10.3390/medicina59122095






