Herbal Melanin Inhibits Real-Time Cell Proliferation, Downregulates Anti-Apoptotic Proteins and Upregulates Pro-Apoptotic p53 Expression in MDA-MB-231 and HCT-116 Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture Preparation
2.3. Real-Time Cell Proliferation by the xCELLigence System
2.4. RNA Extraction and Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
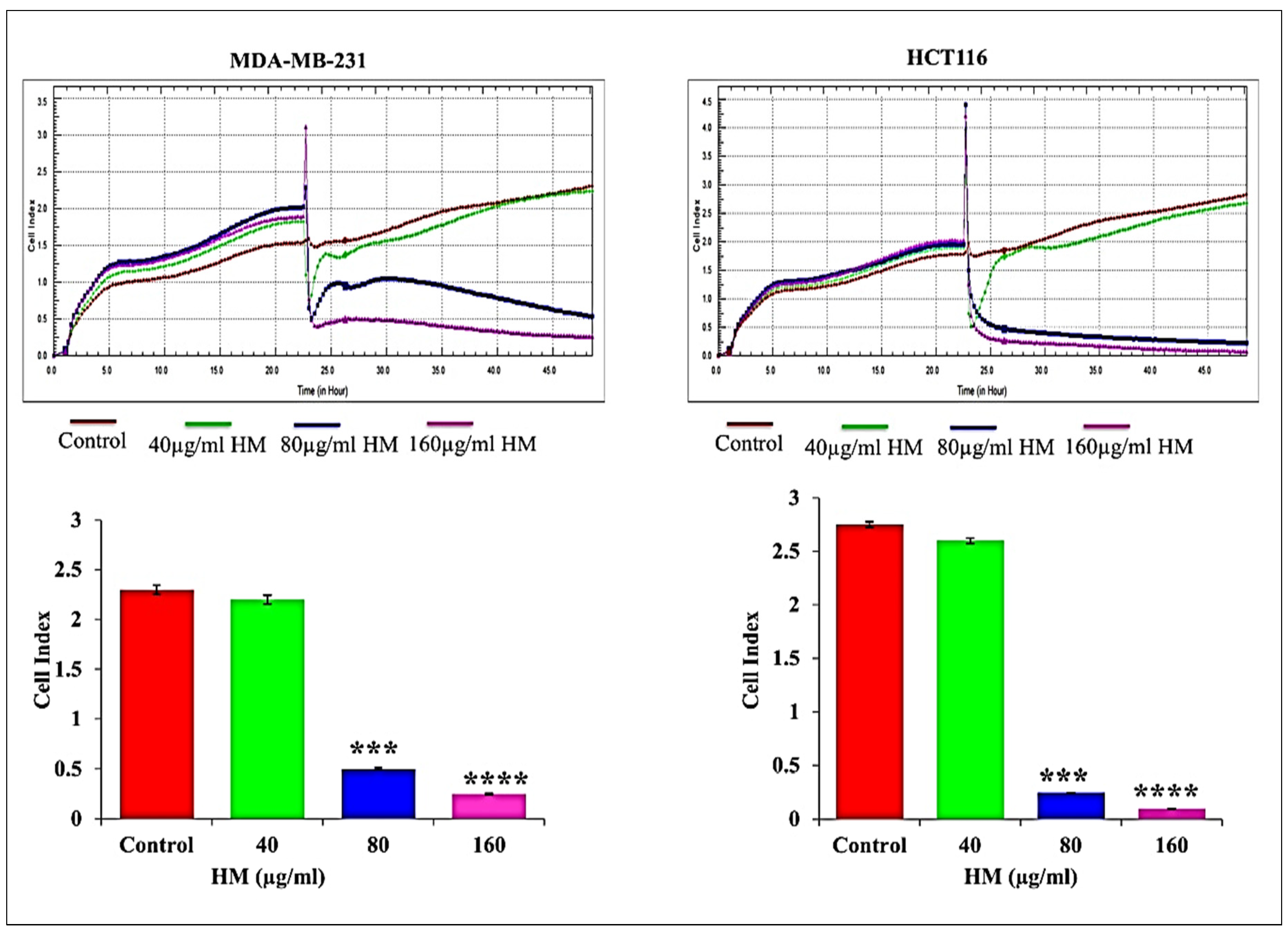
3.1. HM Reduced Real-Time Cell Proliferation in Breast Adenocarcinoma and Colorectal Cancer Cell Lines
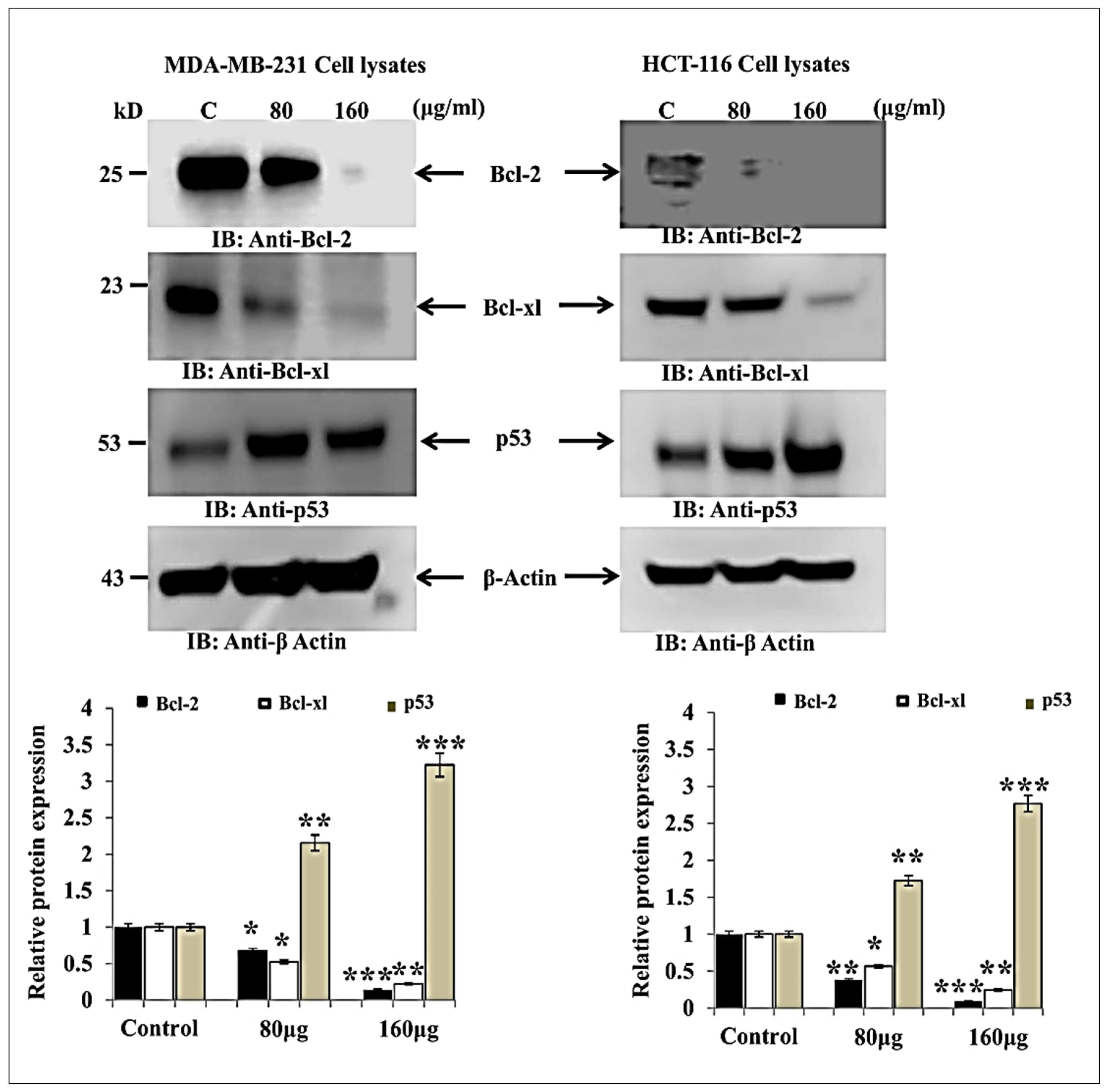
3.2. HM Induced Downregulated Anti-Apoptotic and Upregulated Pro-Apoptotic Proteins Expression in Both Cell Lines
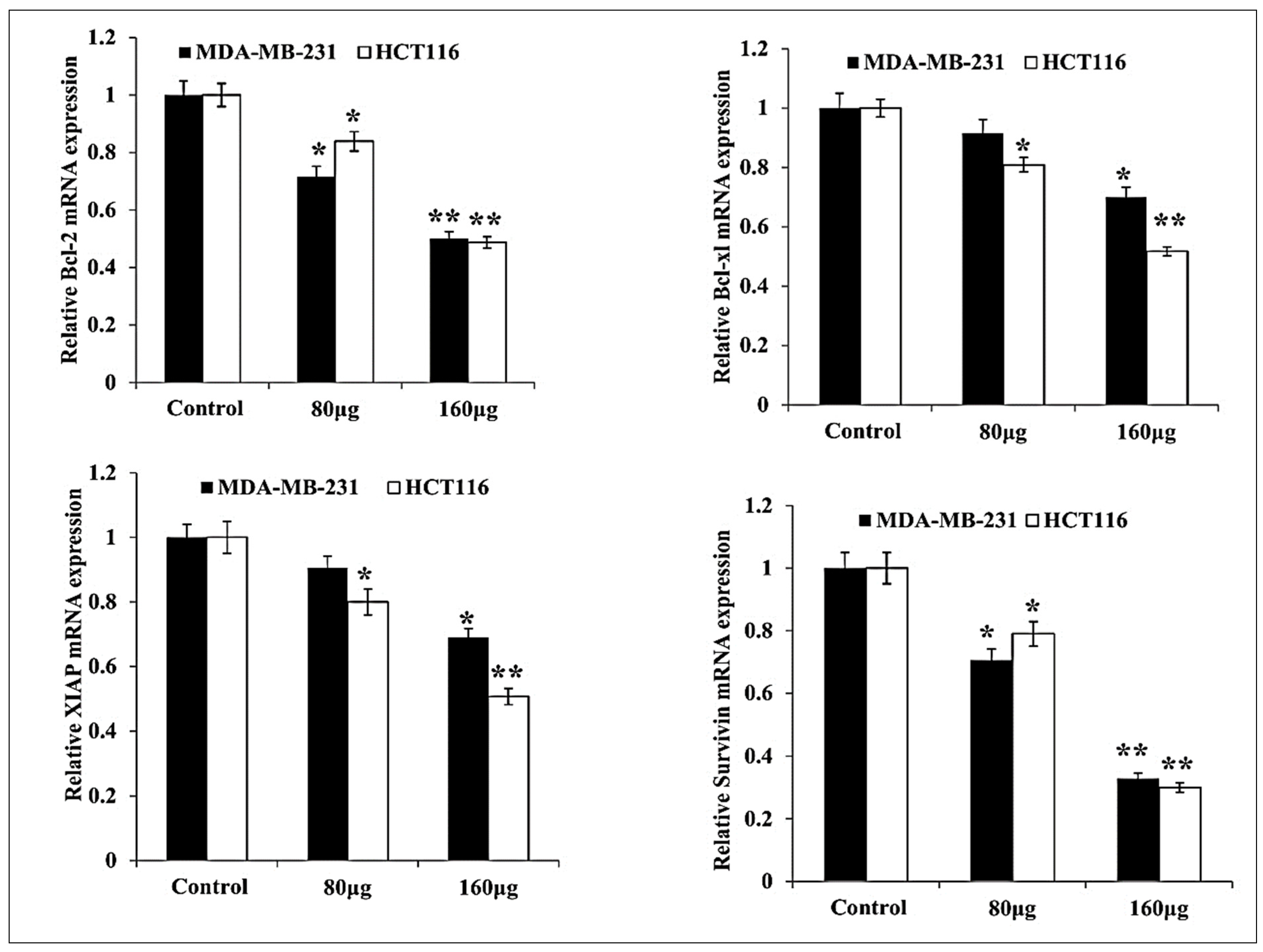
3.3. HM Induced Downregulated Anti-Apoptotic and Upregulated Pro-Apoptotic mRNA Expression in Both Cell Lines
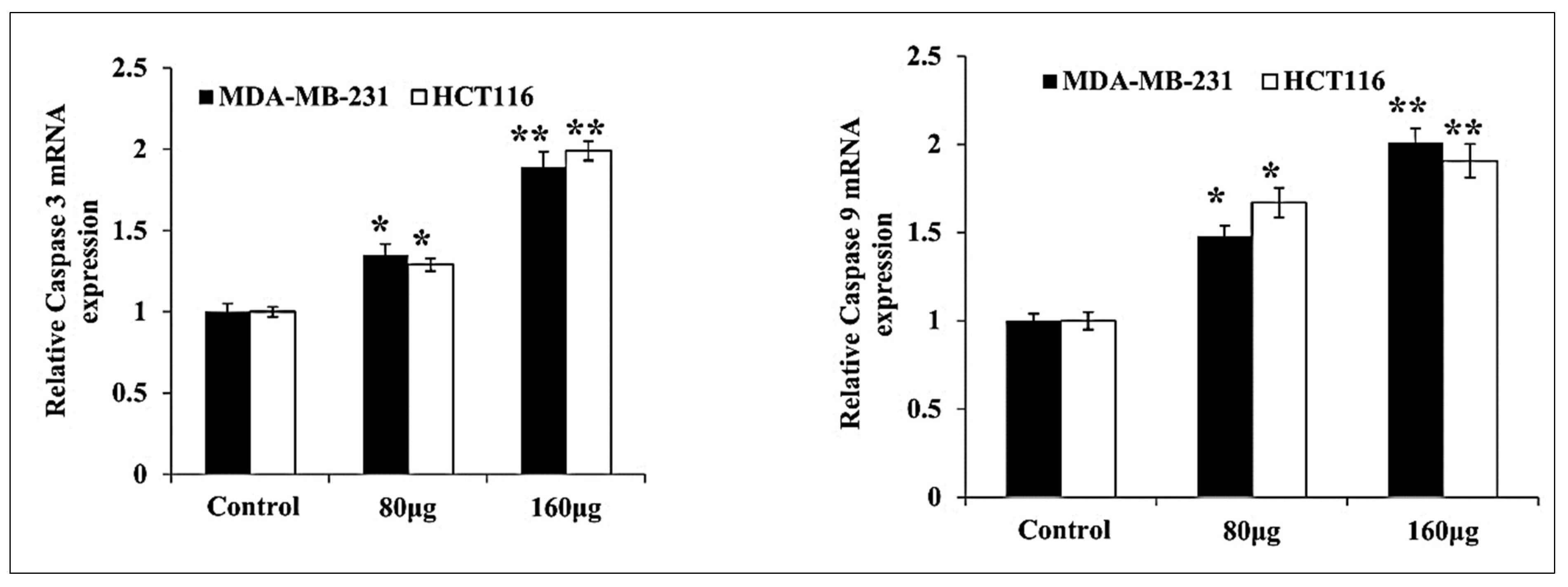
3.4. HM Induced Upregulated Caspase 3, 9 Expressions in Both Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Jemal, A.; Torre, L.A.; Forman, D.; Vineis, P. Long-term realism and cost-effectiveness: Primary prevention in combatting cancer and associated inequalities worldwide. J. Natl. Cancer Inst. 2015, 107, djv273. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Cancer Report; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Tan, G.; Gyllenhaal, C.; Soejarto, D. Biodiversity as a source of anticancer drugs. Curr. Drug Targets 2006, 7, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.F.J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Bray, F.; Ferlay, J.; Lortet-Tieulent, J.; Anderson, B.O.; Jemal, A. International variation in female breast cancer incidence and mortality rates. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1495–1506. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Cazzaniga, M.; Bonanni, B. Breast cancer chemoprevention: Old and new approaches. J. Biomed. Biotechnol. 2012, 2012, 985620. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Meng, X.; Gan, R.-Y.; Zhang, J.-J.; Li, H.-B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef]
- Al-Tayib, O.A.; El Tahir, K.E.; Idriss, M.H.; Eram, K.E.; Hassib, A.M. Nigella sativa L. seeds melanin: A new hypoglycemic agent. Comparison with insulin in alloxan-diabetic rats. Sch. Acad. J. Pharm. 2014, 3, 332–335. [Google Scholar]
- Eom, T.; Woo, K.; Cho, W.; Heo, J.E.; Jang, D.; Shin, J.I.; Martin, D.C.; Wie, J.J.; Shim, B.S. Nanoarchitecturing of natural melanin nanospheres by layer-by-layer assembly: Macroscale anti-inflammatory conductive coatings with optoelectronic tunability. Biomacromolecules 2017, 18, 1908–1917. [Google Scholar] [CrossRef]
- Harikrishnan, A.; Veena, V.; Kancharla, R.; Chavan, S.; Rajabathar, J.R.; Al-Lohedan, H.; Pandiaraj, S.; Karuppiah, P.; Arokiyaraj, S. Anti-breast cancer activity of bioactive metabolites from Andrographis paniculata through inhibition of PI3K activity in triple negative breast cancer (MDA-MB-231) cells. J. Mol. Struct. 2023, 1294, 136506. [Google Scholar] [CrossRef]
- Lampel, A.; McPhee, S.A.; Park, H.-A.; Scott, G.G.; Humagain, S.; Hekstra, D.R.; Yoo, B.; Frederix, P.W.J.M.; Li, T.-D.; Abzalimov, R.R.; et al. Polymeric peptide pigments with sequence-encoded properties. Science 2017, 356, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, C.; Zhang, X.; Hu, Q.; Zhang, Y.; Liu, Q.; Wen, D.; Milligan, J.; Bellotti, A.; Huang, L.; et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol. 2017, 2, eaan5692. [Google Scholar] [CrossRef]
- Rosas, Á.L.; MacGill, R.S.; Nosanchuk, J.D.; Kozel, T.R.; Casadevall, A. Activation of the alternative complement pathway by fungal melanins. Clin. Diagn. Lab. Immunol. 2002, 9, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Freitak, D.; Vanatoa, A.; Ots, I.; Rantala, M.J. Formation of melanin-based wing patterns is influenced by condition and immune challenge in Pieris brassicae. Entomol. Exp. Appl. 2005, 116, 237–243. [Google Scholar] [CrossRef]
- Al-Tayib, O.; El Tahir, K.; Hassib, A.; Idriss, M. The aqueous extracts of the Nigella sativa L. melanin: Experimental in vivo test and in vitro Hep-2 cell lines cytotoxicity effects. IOSR-JAVS 2016, 9, 84–90. [Google Scholar] [CrossRef]
- Al-Tayib, O.; ElBadwi, S.M.; Bakhiet, A.O. Cytotoxicity assay for herbal melanin derived from Nigella sativa seeds using in vitro cell lines. IOSR-JHSS 2017, 22, 43–51. [Google Scholar] [CrossRef]
- El-Obeid, A.; El-Tahir, K.; Elhag, H.; Haseeb, A. Anti-ulcerogenic effects of Nigella sativa L. Melanin World J. Pharm. Res. 2016, 5, 1579–1593. [Google Scholar]
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef]
- Dirin, M.M.; Mousavi, S.; Afshari, A.R.; Tabrizian, K.; Ashrafi, M.H. Potential drug-drug interactions in prescriptions dispensed in community and hospital pharmacies in East of Iran. J. Res. Pharm. Pract. 2014, 3, 104–107. [Google Scholar] [CrossRef]
- Spencer, D.S.; Puranik, A.S.; Peppas, N.A. Intelligent nanoparticles for advanced drug delivery in cancer treatment. Curr. Opin. Chem. Eng. 2015, 7, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Khumkhrong, P.; Piboonprai, K.; Chaichompoo, W.; Pimtong, W.; Khongkow, M.; Namdee, K.; Jantimaporn, A.; Japrung, D.; Asawapirom, U.; Suksamrarn, A.; et al. Crinamine Induces Apoptosis and Inhibits Proliferation, Migration, and Angiogenesis in Cervical Cancer SiHa Cells. Biomolecules 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Vaali-Mohammed, M.-A.; Abdulla, M.-H.; Matou-Nasri, S.; Eldehna, W.M.; Meeramaideen, M.; Elkaeed, E.B.; El-Watidy, M.; Alhassan, N.S.; Alkhaya, K.; Al Obeed, O. The Anticancer Effects of the Pro-Apoptotic Benzofuran-Isatin Conjugate (5a) Are Associated with p53 Upregulation and Enhancement of Conventional Chemotherapeutic Drug Efficiency in Colorectal Cancer Cell Lines. Front. Pharmacol. 2022, 13, 923398. [Google Scholar] [CrossRef] [PubMed]
- Ozturki, E.; Ayşe, K.K.A.; Alim, H.; Mükerrem, B. Real-time Analysis of Impedance Alterations by the Effects of Vanadium Pentoxide on Several Carcinoma Cell Lines. Turk. J. Pharm. Sci. 2018, 15, 1–6. [Google Scholar] [CrossRef]
- Baig, W.A.; Alwosaibai, K.; Al-Jubran, K.M.; Chaudhry, T.M.; Al-Dowish, N.; Alsaffar, F.; Alam, M.A. Synergistic anti-cancer effects of Nigella sativa seed oil and conventional cytotoxic agent against human breast cancer. Drug Metab. Pers. Ther. 2022, 37, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Vousden, K.H.; Norman, J.C. p53 and its mutants in tumor cell migration and invasion. J. Cell Biol. 2011, 192, 209–218. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Martinou, J.-C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef]
- Ramadan, A.M.; Shawkey, E.; Rabeh, A.M.; Ashraf, O.A. Expression of P53, BAX, and BCL-2 in human malignant melanoma and squamous cell carcinoma cells after tea tree oil treatment in vitro. Cytotechnology 2019, 71, 461–473. [Google Scholar] [CrossRef]
- Devi, G.R.; Finetti, P.; Morse, M.A.; Lee, S.; de Nonneville, A.; Van Laere, S.; Troy, J.; Geradts, J.; McCall, S.; Bertucci, F. Expression of X-Linked Inhibitor of Apoptosis Protein (XIAP) in Breast Cancer Is Associated with Shorter Survival and Resistance to Chemotherapy. Cancers 2021, 13, 2807. [Google Scholar] [CrossRef] [PubMed]
- Warrier, N.M.; Agarwal, P.; Kumar, P. Emerging Importance of Survivin in Stem Cells and Cancer: The Development of New Cancer Therapeutics. Stem Cell Rev. Rep. 2020, 16, 828–852. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Adila, S.; Sabine, M.; Mansoor, A.; Yazeid, A.; Mohammed, E.; Hamad, A.; Zeyad, A.; Khayal, A.; Adil, H.; et al. Herbal melanin inhibits colorectal cancer cellproliferation by altering redox balance, inducing apoptosis, and modulating MAPK signalling. Cancer Cell Int. 2020, 20, 126. [Google Scholar] [CrossRef]

| Target | Primer Sequences | Primary Antibody |
|---|---|---|
| genes | (dilution 1:1000) | |
| Bcl-2 | F: 5′-AAGATTGATGGGATCGTTGC-3′ | Bcl-2 (cat. no. sc-509) |
| R: 5′-GCGGAACACTTGATTCTGGT-3′ | ||
| Bcl-xl | F: 5′-CCCAGAAAGGATACAGCTGG-3′ | Bcl-xl (cat. no. sc-8392) |
| R: 5′-GCGATCCGACTCACCAATAC-3′ | ||
| XIAP | F: 5′-GACAGTATGCAAGATGACGTCAAGTCA-3′ | p53 (cat. no. sc-47698) |
| R: 5′-GCAAAGCTTCTCCTCTTGCAG-3′ | ||
| Survivin | F: 5′-ACCGCATCTCTACATTCAAG-3′ | β-Actin (cat. no. sc-47778) |
| R: 5′-CAAGTCTGGCTCGTTCTC-3′ | ||
| Bid | F: 5′-CCTTGCTCCGTGATGTCTTTC-3′ | Secondary Antibodies |
| R: 5′-GTAGGTGCGTAGGTTCTGGT-3′ | (dilution 1:10,000) | |
| Bax | F: 5′-GTGCACCAAGGTGCCGGAAC-3′ | Mouse anti-rabbit IgG-HRP (cat. no. sc-2357) |
| R: 5′-TCAGCCCATCTTCTTCCAGA--3′ | ||
| Cytochrome c | F: 5′-GGCTGCAGTGTAGCTGTGAT-3′ | (All Primary and secondary antibodies are purchased from Santa Cruz Biotechnology, Inc., Dallas, TX, USA.) |
| R: 5′-GATGGAGTTTCCTTTATCTGTTGC-3′ | ||
| PARP | F: 5′-GGAAAGGGATCTACTTTGCCG-3′ | |
| R: 5′-TCGGGTCTCCCTGAGATGTG-3′ | ||
| P53 | F: 5′-GAGGTTGGCTCTGACTGTACC-3′ | |
| R: 5′-TCCGTCCCAGTAGATTACCAC-3′ | ||
| Caspase 3 | F: 5′-AGAACTGGACTGTGGCATTGAG-3′ | |
| R: 5′-GCTTGTCGGCATACTGTTTCAG-3′ | ||
| Caspase 9 | F: 5′-TTCCCAGGTTTTGTTTCCTG-3′ | |
| GAPDH | R: 5′-CCTTTCACCGAAACAGCATT-3′ | |
| F: 5′-AATCCCATCACCATCTTCCAG-3′ | ||
| R: 5′-ATCAGCAGAGGGGGCAGAGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabathar, J.R.; Al-Lohedan, H.; Arokiyaraj, S.; Mohammed, F.; Al-Dhayan, D.M.; Faqihi, N.A.; Al-Saigh, H. Herbal Melanin Inhibits Real-Time Cell Proliferation, Downregulates Anti-Apoptotic Proteins and Upregulates Pro-Apoptotic p53 Expression in MDA-MB-231 and HCT-116 Cancer Cell Lines. Medicina 2023, 59, 2061. https://doi.org/10.3390/medicina59122061
Rajabathar JR, Al-Lohedan H, Arokiyaraj S, Mohammed F, Al-Dhayan DM, Faqihi NA, Al-Saigh H. Herbal Melanin Inhibits Real-Time Cell Proliferation, Downregulates Anti-Apoptotic Proteins and Upregulates Pro-Apoptotic p53 Expression in MDA-MB-231 and HCT-116 Cancer Cell Lines. Medicina. 2023; 59(12):2061. https://doi.org/10.3390/medicina59122061
Chicago/Turabian StyleRajabathar, Jothi Ramalingam, Hamad Al-Lohedan, Selvaraj Arokiyaraj, Fathima Mohammed, Dhaifallah M. Al-Dhayan, Norah A. Faqihi, and Hassan Al-Saigh. 2023. "Herbal Melanin Inhibits Real-Time Cell Proliferation, Downregulates Anti-Apoptotic Proteins and Upregulates Pro-Apoptotic p53 Expression in MDA-MB-231 and HCT-116 Cancer Cell Lines" Medicina 59, no. 12: 2061. https://doi.org/10.3390/medicina59122061
APA StyleRajabathar, J. R., Al-Lohedan, H., Arokiyaraj, S., Mohammed, F., Al-Dhayan, D. M., Faqihi, N. A., & Al-Saigh, H. (2023). Herbal Melanin Inhibits Real-Time Cell Proliferation, Downregulates Anti-Apoptotic Proteins and Upregulates Pro-Apoptotic p53 Expression in MDA-MB-231 and HCT-116 Cancer Cell Lines. Medicina, 59(12), 2061. https://doi.org/10.3390/medicina59122061








