1. Introduction
Critically ill surgical patients are susceptible to various postoperative complications, including acute kidney injury (AKI) and multiorgan distress syndrome (MODS). These complications not only intensify patient suffering, but also significantly increase morbidity and mortality rates, compelling the medical community to explore innovative approaches for their early identification and management [
1,
2]. Biomarkers have emerged as promising candidates for addressing this clinical need.
Early detection of AKI and MODS is pivotal for informed clinical decision making and optimized patient care. Recently, the biological relevance of mitochondrial DNA (mtDNA) in predicting adverse outcomes has been increasingly recognized, particularly in patients in the intensive care unit (ICU) [
3,
4,
5,
6]. Mitochondrial integrity plays a pivotal role in AKI pathophysiology. In various forms of AKI, early pathological alterations are evident within the renal tubular epithelium, including reduced mitochondrial abundance, organelle swelling, and fragmentation [
7]. The impaired state of these mitochondria causes kidney damage by producing detrimental reactive oxygen species and releasing mtDNA. The release of mtDNA can activate innate immune responses via the Toll-like receptor-9 (TLR9) pathway [
8]. Moreover, mtDNA acts as a damage-associated molecular pattern initiator [
9] that can drive molecular processes leading to inflammatory responses and organ injuries [
9,
10,
11].
β2-microglobulin (β2-MG) has also been investigated recently as a predictive marker, in particular for AKI [
12,
13]. β2-MG is released into circulation at a constant rate, freely filtered by the glomeruli, and completely reabsorbed and catabolized in the renal tubules. Serum β2-MG levels are independent of muscle mass and start increasing early during kidney failure. Because of these properties, serum β2-MG has been proposed as a candidate marker to assess kidney function. Tubular injury leads to decreased reabsorption of β2-MG and tubular enzymes, leading to elevated urinary concentrations. In addition, a few studies reported that β2-MG was associated with severe inflammation [
14,
15].
Based on these findings, we hypothesized that urine mtDNA, circulating cell-free mtDNA levels, serum β2-MG, and urinary β2-MG would be associated with AKI and MODS and would improve risk prediction in critically ill surgical patients. Although many studies have investigated the effectiveness of mtDNA as a predictive marker, studies conducted on patients admitted to the intensive care unit because of acute surgical illnesses are rare.
Therefore, this study aimed to test whether circulating cell-free mtDNA, urinary mtDNA copy number (mtDNAcn), serum β2-MG, and urinary β2-MG are useful as biomarkers in critically ill surgical patients. We also investigated other well-known biomarkers, including the delta neutrophil index (DNI), white blood cell (WBC) count, and neutrophil count because of the scarcity of studies on critically ill surgical patients.
4. Discussion
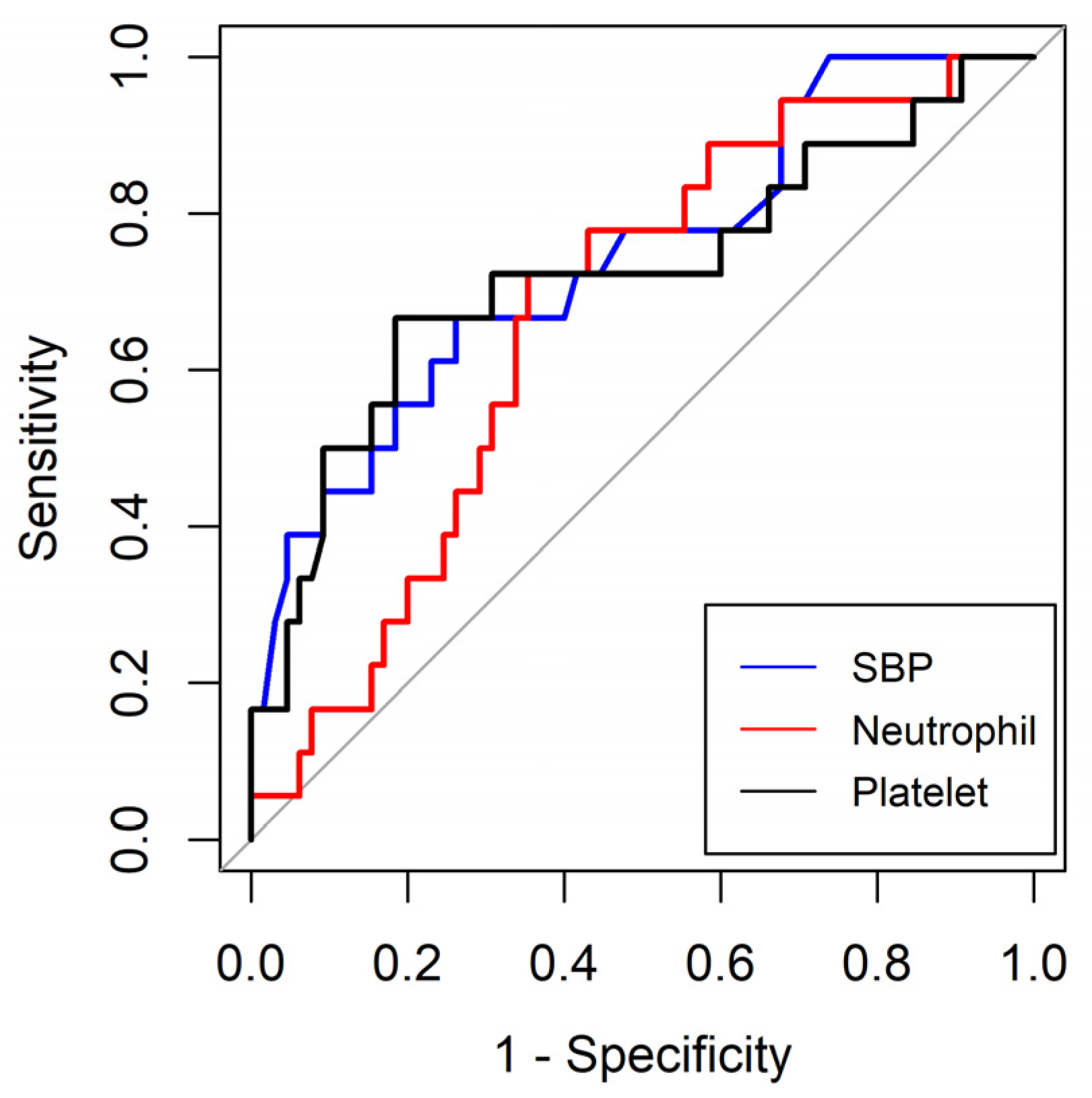
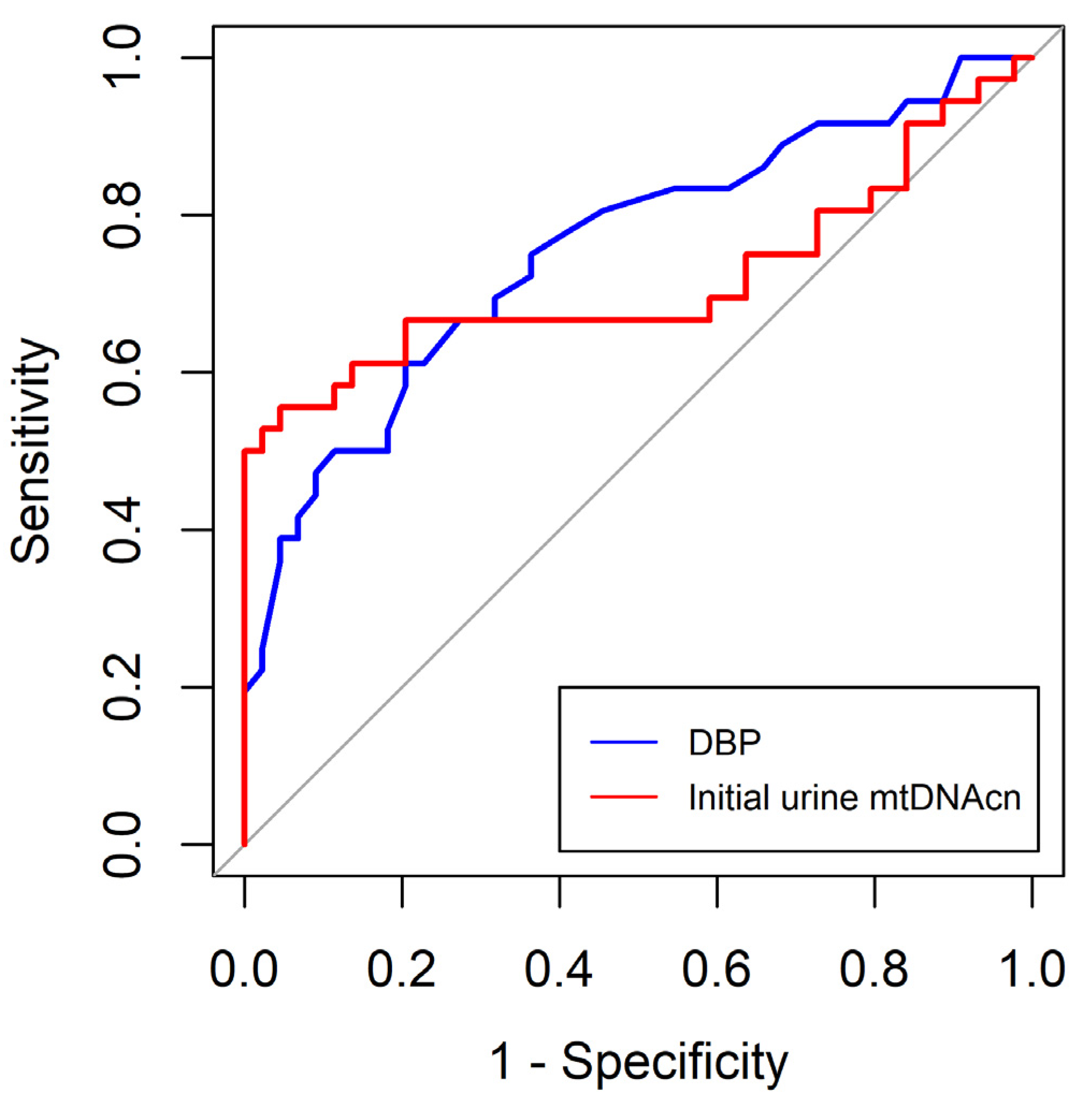
In this study, we found that SBP and initial neutrophil and platelet counts were independently associated with MODS in critically ill patients undergoing surgery. The optimal cut-off values for SBP and initial neutrophil and platelet counts were 113 mmHg, 8.65 (X3)(109/L), and 195.0 (X3)(109/L), respectively. In addition, DBP and initial urine mtDNAcn levels were independently associated with AKI in critically ill surgical patients. The optimal cut-off values for DBP and initial urine mtDNAcn were 68.5 mmHg and 1225.6 copies/μL, respectively.
Consistent with our findings, several studies have reported an established relationship between SBP and MODS occurrence across various clinical contexts [
20,
21]. This underscores the importance of maintaining optimal perfusion and effectively managing blood pressure in critically ill patients. Transient episodes of hypotension can precipitate inadequate oxygen delivery to vital organs, initiating a cascade of events that may ultimately culminate in MODS. Early recognition and prompt management of hypotension are imperative for preventing severe medical complications.
Polymorphonuclear leukocytes, monocytes, macrophages, dendritic cells, and natural killer cells are pivotal contributors to the cellular immune response following infection or trauma [
22,
23]. Neutrophils are attracted to the site of injury under the influence of cytokines, such as interleukin (IL)-8, where they actively participate in the immune response by combatting pathogens and assisting in the removal of damaged tissue. Additionally, these cells play a role in the initiation of molecules such as tumor necrosis factor (TNF)-α, IL-8, platelet-activating factor, and anaphylatoxin (C5a). This sequence of events leads to an increased inflammatory response, which triggers the activation and recruitment of polymorphonuclear leukocytes. This, in turn, contributes to the onset of the systemic inflammatory response syndrome and MODS [
22,
23,
24]. Recruitment of polymorphonuclear cells leads to neutrophilia. This period can be considered a “vulnerability window” during which a subsequent event, often referred to as a “second hit”, can trigger the onset of MODS [
24,
25]. In patients who develop MODS, initial neutrophilia is followed by neutropenia following trauma or infection [
26]. Therefore, patients with neutropenia at initial presentation may progress to MODS, as shown in our study.
Thrombocytopenia at initial presentation is most likely caused by the loss or consumption of platelets or sepsis in critically ill surgical patients. Thrombocytopenia in patients with sepsis occurs through several mechanisms. Platelets are activated during sepsis and adhere to the endothelial lining, leading to their sequestration and subsequent breakdown. Additionally, immune-related factors such as non-specific antibodies associated with platelets and cytokine-driven phagocytosis of platelets can also play a role in the development of thrombocytopenia in sepsis [
27]. Therefore, the development of thrombocytopenia indicates progression to more serious adverse events. Similar to our results, previous studies have reported that thrombocytopenia poses an independent risk for adverse events in ICU patients [
28,
29].
DBP serves as an indicator of the vascular tone. A study has illustrated that elevated levels of naturally occurring inflammatory mediators, such as nitrous oxide and TNF-α, are linked to the progression of severe sepsis, shock, or even mortality [
30]. These cytokines and autocrine hormones have vasodilatory properties. Patients with sepsis who exhibit a normal mean SBP and low DBP are in a state of compensated vasodilation, which precedes a more evident cardiovascular collapse. In patients with trauma, there can be an initial mild shock leading to an increase in systemic venous resistance and subsequently higher DBP. However, when severe and ongoing bleeding occurs, resulting in a loss of over 20–30% of the total blood volume, systemic venous resistance may decrease, leading to failure of vascular compensation [
31]. This, in turn, causes a decline in DBP. Therefore, a reduction in DBP in patients with trauma results in a substantial loss of blood volume. In other words, a lower DBP reflects a lower effective circulating volume at initial presentation and a high prevalence of AKI.
The mtDNA has a damage-associated molecular pattern (DAMP). In a landmark study in 2004, Collins et al. [
32]. found that injecting mtDNA into the joints of mice resulted in localized inflammation. Many studies have investigated the mechanism by which mtDNA affects the inflammatory cascade by acting as a DAMP. First, mtDNA is released into the cytoplasm or extracellular space via various mechanisms in various clinical settings. Various mitochondrial stresses, including bacterial and viral infections or trauma-induced cellular damage, can lead to the release of mtDNA associated with reactive oxygen species. Alternatively, activation of Bcl-2-associated X protein (BAX) and Bcl-2 homologous antagonist/killer (BAK) leads to outer mitochondrial membrane permeabilization (MOMP) and mtDNA release [
33]. Pathogen-infected cells often secrete IL-1β due to inflammasome activation. A recent report by Aarreberg et al. [
34] discovers a link between IL-1β secretion in infected cells, which can then activate a cyclic GMP-AMP synthase (cGAS) stimulator of interferon genes (STING)-dependent type-I interferon response in surrounding bystander cells. Interestingly, IL-1β stimulation of bystander cells increases mitochondrial mass, decreases mitochondrial membrane potential, and induces mtDNA release.
Once cytoplasmic, mtDNA released from mitochondria can also bind the DNA sensing protein cGAS that catalyzes the production of the secondary messenger 2030 cyclic GMP–AMP (2030cGAMP) from adenosine triphosphate (ATP) and guanosine-5′-triphosphate (GTP). Cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) binds to the adaptor molecule STING on the endoplasmic reticulum, leading to the activation of TANK-binding kinase 1 (TBK1). Active TBK1 phosphorylates the transcription factor interferon regulatory factor 3 (IRF3) initiating a type-I interferon response [
33]. Elevated levels of type-I interferons can hinder B-cell reactions, possibly inducing the generation of immunosuppressive substances. Additionally, these elevated concentrations diminish the ability of macrophages to respond effectively to interferon-γ activation [
35].
Cytoplasmic mtDNA can enter the extracellular environment through necroptosis or platelet aggregation [
36]. The released circulating mtDNA is detected by the TLR9 located on the neutrophil surface, which activates p38 mitogen-activated protein kinase (MAPK). Subsequently, diverse transcriptional factors including nuclear factor kappa-light-chain-enhancer of activated B cells is activated via MAPK, and thereby a cellular response is induced. Cellular responses include the expression of cytokines or adhesion molecules that accelerate inflammation and diapedesis of immune effector cells. In addition, released IL-1β also contributes to systemic inflammatory responses.
Therefore, we hypothesized that serum mtDNAcn level may be a predictor of MODS. However, we did not observe any difference in serum mtDNAcn levels between patients with and without MODS. Nakashira et al. [
5] reported that circulating mtDNA levels were associated with mortality in medical ICU patients. Harrington et al. [
36] reported that 11 out of 16 studies reported a significant association between circulating mtDNA levels and mortality among critically ill patients in their systematic review. These discrepancies between studies on critically ill patients, including that of our study, although our outcome was not mortality, may be due to the indirectness of the participants and small number of participants.
Mitochondria are pivotal contributors to AKI, serving a dual function as the primary energy source for cells and a crucial controller of cell death processes. Initially, alterations in the mitochondrial structure were detected in AKI cases. A primary cause of AKI is ischemia, which leads to a reduction in the number of mitochondria and causes structural changes in these organelles, characterized by swelling and loss of the inner mitochondrial membrane cristae. These changes occur because of diminished ATP levels and a decline in membrane potential [
37]. Furthermore, the opening of mitochondrial permeability transition pores (mPTP) is a pivotal event resulting from mitochondrial swelling and dysfunction. This event significantly contributes to AKI progression by releasing proapoptotic substances such as cytochrome c, which can trigger apoptosis in renal cells [
38]. Second, an imbalance in the regulation of mitochondrial fission and fusion has been linked to AKI progression. In this context, dynamin-related protein 1, which is responsible for controlling mitochondrial fission, exhibits swift activation, whereas mitofusin and optic atrophy 1, which oversee mitochondrial fusion, experience a reduction in their levels during AKI. This process leads to fragmentation of mitochondria [
39]. Third, the released mtDNA has the ability to bind to the DNA-sensing protein cGAS, triggering a sequence of events, as described earlier. Fourth, oxidative stress produces mitochondrial reactive oxygen species, which can destroy renal cells [
40]. Finally, the release of mtDNA as one of DAMPs from mitochondria into the extracellular space accelerates a vicious spiral of renal cellular damage [
41]. Urinary mtDNA levels can be elevated because of AKI-related mitochondrial dysfunction. Hu et al. [
41] reported that urinary mtDNA levels were associated with new-onset AKI in surgical ICU patients, which is consistent with our results. However, Whitaker et al. [
42] showed that urinary mtDNA levels did not differ between AKI and non-AKI groups after cardiac surgery. Additional research is needed to gain a more comprehensive understanding of the predictive potential of urinary mtDNA for AKI as variations and inconsistencies persist among studies.
Although serum β2-MG was not an independent predictor of AKI and MODS (
p = 0.053) in the multivariate analysis in this study, it could be a candidate of predictors for AKI and MODS. β2-MG is filtered by glomeruli and subsequently reabsorbed in the proximal tubule. Serum β2-MG is independent of muscle mass and starts increasing early in kidney failure. β2-MG is present in all nucleated cells [
43] and is not affected by sex or age [
44]. These properties may make β2-MG an ideal endogenous biomarker for estimating the glomerular filtration rate. Tubular injury leads to decreased reabsorption of β2-MG and tubular enzymes, leading to elevated urinary concentrations. Thus, these markers may act as functional markers of tubular damage and fibrosis. In addition, β2-MG may be related to inflammation. Although not fully understood, β2-MG may induce IL-1β and IL-18 release by macrophages in a caspase-1- and nod-like receptor family pyrin-domain-containing 3-dependent manner [
45]. Further investigation is necessary to determine the association between β2-MG and adverse outcomes.
Our study has several limitations. First, our sample size and the number of patients with MODS were small (n = 18). Second, several methodological factors may have influenced our results. For example, although we prepared samples within 2 h of collection, we cannot rule out the possibility that slight differences in the timing of sample collection could influence the associations within the groups (AKI vs. no AKI, MODS vs. no MODS). Moreover, slight differences in mtDNA measurements can occur throughout the process, from sample collection to qPCR. Third, although we considered many variables for the multivariate analysis, some possible factors could have been missed. Finally, our study included surgical patients admitted to the ICU, both trauma and non-trauma patients. The mechanism of MODS development may differ between patients, depending on whether it is related to infection or sterile inflammation. This may have influenced the results of this study.